Impact of Mediterranean Dietary Intervention on Reactive Oxygen Species Levels and Total Antioxidant Capacity During Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Sample and Research Design
2.2. Blood Collection
2.3. Reactive Oxygen Species (ROS) Analysis
2.4. Total Antioxidant Capacity (TAC) Analysis
2.5. Statistical Analysis
3. Results
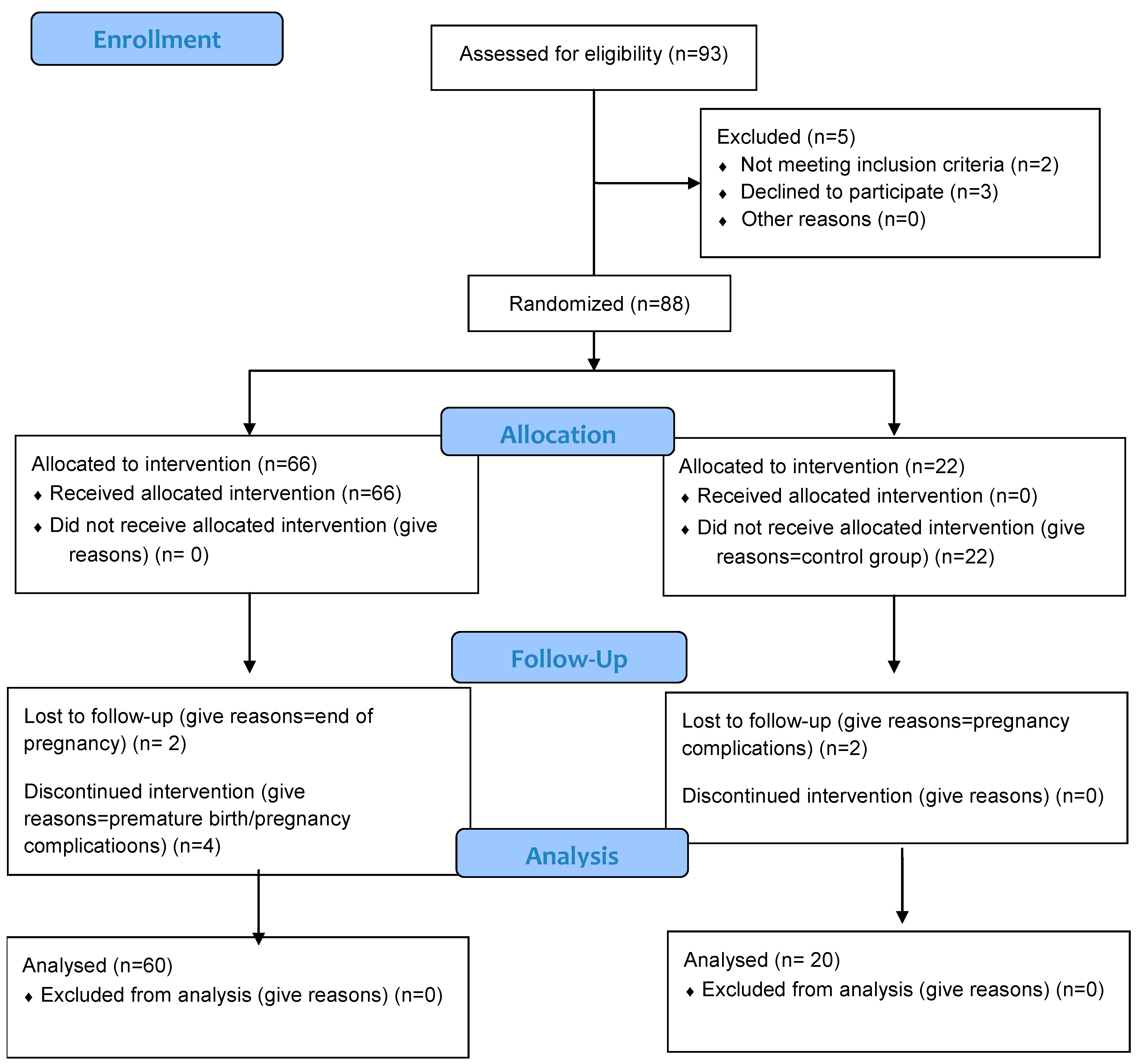
3.1. Recruitment and Enrollment
3.2. Subject Characteristics
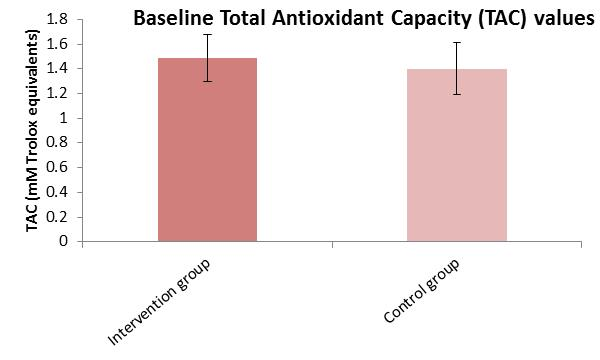
3.3. Baseline Comparison of Reactive Oxygen Species (ROS) and Total Antioxidant Capacity (TAC) Analysis
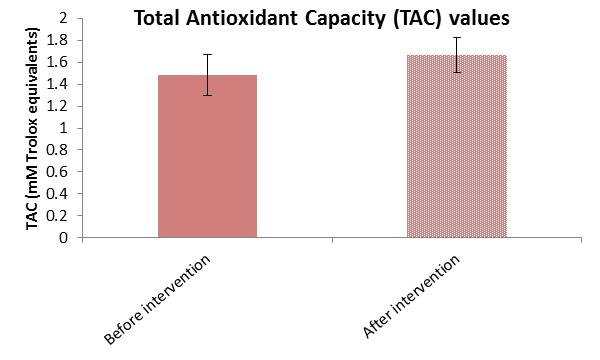
3.4. Within-Group Analysis of ROS and TAC Variables
3.5. Endpoint Comparison of Reactive Oxygen Species (ROS) and Total Antioxidant Capacity (TAC) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, A.C.; Martel, F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol. Toxicol. 2014, 30, 301–312. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Nightingale, P.; Kendall, M.J.; Brydon, P.; Dunne, F. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin. Sci. 2004, 106, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Dotsch, J.; Hogen, N.; Nyul, Z.; Hanze, J.; Knerr, I.; Kirschbaum, M. Increase in endothelial nitric oxide synthase and endothelin—1 mRNA expression in human placenta during gestation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 163–167. [Google Scholar] [CrossRef]
- Lymperaki, E.; Tsikopoulos, A.; Makedou, K.; Paliogianni, E.; Kiriazi, L.; Charisi, C.; Vagdatli, E. Impact of iron and folic acid supplementation on oxidative stress during pregnancy. J. ObstetGynaecol. 2015, 35, 803–806. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Mocatta, T.J.; Winterbourn, C.C.; Inder, T.E.; Darlow, B.A. The effect of gestational age and labour on markers of lipid and protein oxidation in cord plasma. Free Radic. Res. 2004, 38, 185–191. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Jovandaric, M.Z.; Babic, S.; Raus, M.; Medjo, B. The Importance of Metabolic and Environmental Factors in the Occurrence of Oxidative Stress during Pregnancy. Int. J. Mol. Sci. 2023, 24, 11964. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Leal, C.A.; Schetinger, M.R.; Leal, D.B.; Morsch, V.M.; da Silva, A.S.; Rezer, J.F.; de Bairros, A.V.; Jaques, J.A. Oxidative stress and antioxidant defenses in pregnant women. Redox Rep. 2011, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Skepper, J.N.; Charnock-Jones, D.S.; Burton, G.J. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ. Res. 2002, 90, 1274–1281. [Google Scholar] [CrossRef]
- Aramouni, K.; Assaf, R.; Shaito, A.; Fardoun, M.; Al-Asmakh, M.; Sahebkar, A.; Eid, A.H. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J. Cell Physiol. 2023, 238, 1951–1963. [Google Scholar] [CrossRef]
- Ademuyiwa, O.; Odusoga, O.L.; Adebawo, O.O.; Ugbaja, R. Endogenous antioxidant defenses in plasma and erythrocytes of pregnant women during different trimesters of pregnancy. Acta Obstet. Gynecol. Scand. 2007, 86, 1175–1182. [Google Scholar] [CrossRef]
- Morales, E.; García-Serna, A.M.; Larqué, E.; Sánchez-Campillo, M.; Serrano-Munera, A.; Martinez-Graciá, C.; Santaella-Pascual, M.; Suárez-Martínez, C.; Vioque, J.; Noguera-Velasco, J.A.; et al. Dietary Patterns in Pregnancy and Biomarkers of Oxidative Stress in Mothers and Offspring: The NELA Birth Cohort. Front. Nutr. 2022, 9, 869357. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Mihailović, M.; Cvetković, M.; Ljubić, A.; Kosanović, M.; Nedeljković, S.; Jovanović, I.; Pesut, O. Selenium and malondialdehyde content and glutathione peroxidase activity in maternal and umbilical cord blood and amniotic fluid. Biol. Trace Elem. Res. 2000, 73, 47–54. [Google Scholar] [CrossRef]
- Zachara, B.A.; Dobrzyński, W.; Trafikowska, U.; Szymański, W. Blood selenium and glutathione peroxidases in miscarriage. Br. J. Obstet. Gynaecol. 2001, 108, 244–247. [Google Scholar] [CrossRef]
- Maleki, A.; Fard, M.K.; Zadeh, D.H.; Mamegani, M.A.; Abasaizadeh, S.; Mazloomzadeh, S. The relationship between plasma level of Se and preeclampsia. Hypertens. Pregnancy 2011, 30, 180–187. [Google Scholar] [CrossRef]
- Rumiris, D.; Purwosunu, Y.; Wibowo, N.; Farina, A.; Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens. Pregnancy 2006, 25, 241–253. [Google Scholar] [CrossRef]
- Tara, F.; Rayman, M.P.; Boskabadi, H.; Ghayour-Mobarhan, M.; Sahebkar, A.; Yazarlu, O.; Ouladan, S.; Tavallaie, S.; Azimi-Nezhad, M.; Shakeri, M.T.; et al. Selenium supplementation and premature (pre-labour) rupture of membranes: A randomised double-blind placebo-controlled trial. J. Obstet. Gynaecol. 2010, 30, 30–34. [Google Scholar] [CrossRef]
- Izquierdo Alvarez, S.; Castañón, S.G.; Ruata, M.L.; Aragüés, E.F.; Terraz, P.B.; Irazabal, Y.G.; González, E.G.; Rodríguez, B.G. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J. Trace Elem. Med. Biol. 2007, 21, 49–52. [Google Scholar] [CrossRef]
- Han, M.H.; Po, L.L.; Da, Z.S.; Mei, G.Z. Hair and serum calcium, iron, copper, and zinc levels during normal pregnancy at three trimesters. Biol. Trace Elem. Res. 1999, 69, 111–120. [Google Scholar] [CrossRef]
- Gambling, L.; Andersen, H.S.; McArdle, H.J. Iron and copper, and their interactions during development. Biochem. Soc. Trans. 2008, 36, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Serdar, Z.; Gur, E.; Develioglu, O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem. Funct. 2006, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Broughton, F.; Pipkin. Risk factors for pre-eclampsia. N. Engl. J. Med. 2001, 344, 925–926. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development, Birth Defects Research Part B. Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef]
- Mistry, H.D.; Williams, P.J. The importance of antioxidant micronutrients in pregnancy. Oxid. Med. Cell Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef]
- Harley, K.; Eskenazi, B.; Block, G. The association of time in the US and diet during pregnancy in low-income women of Mexican descent. Paediatr. Perinat. Epidemiol. 2005, 19, 125–134. [Google Scholar] [CrossRef]
- King, J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1334S–1343S. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Tamura, T.; Neggers, Y.; Copper, R.L.; Johnston, K.E.; DuBard, M.B.; Hauth, J.C. The effect of zinc supplementation on pregnancy outcome. JAMA 1995, 274, 463–468. [Google Scholar] [CrossRef]
- Jin, S.; Hu, C.; Zheng, Y. Maternal serum zinc level is associated with risk of preeclampsia: A systematic review and meta-analysis. Front. Public Health 2022, 10, 968045. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Bayu, P.; Wibisono, J.J. Vitamin C and E antioxidant supplementation may significantly reduce pain symptoms in endometriosis: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0301867. [Google Scholar] [CrossRef]
- Xu, H.; Perez-Cuevas, R.; Xiong, X.; Reyes, H.; Roy, C.; Julien, P.; Smith, G.; von Dadelszen, P.; Leduc, L.; Audibert, F.; et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am. J. Obstet. Gynecol. 2010, 202, 239.e1–239.e10. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Thorne-Lyman, A.L.; Fawzi, W.W. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 36–54. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Haugen, M.; Rasmussen, S.E.; Alexander, J.; Samuelsen, S.O.; Meltzer, H.M. Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr. 2007, 10, 838–847. [Google Scholar] [CrossRef]
- Teixeira, J.; Chavarria, D.; Borges, F.; Wojtczak, L.; Wieckowski, M.R.; Karkucinska-Wieckowska, A.; Oliveira, P.J. Dietary Polyphenols and Mitochondrial Function: Role in Health and Disease. Curr. Med. Chem. 2019, 26, 3376–3406. [Google Scholar] [CrossRef]
- Croft, K.D. Dietary Polyphenols: Antioxidants or Not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Lymperaki, E.; Kazeli, K.; Tsamesidis, I.; Nikza, P.; Poimenidou, I.; Vagdatli, E. A Preliminary Study about the Role of Reactive Oxygen Species and Inflammatory Process after COVID-19 Vaccination and COVID-19 Disease. Clin. Pract. 2022, 12, 599–608. [Google Scholar] [CrossRef]
- Misan, N.; Paczkowska, K.; Szmyt, M.; Kapska, K.; Tomczak, L.; Bręborowicz, G.H.; Ropacka-Lesiak, M. Nutritional behavior in pregnancy. Ginekol. Pol. 2019, 90, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lipton, L.R.; Brunst, K.J.; Kannan, S.; Ni, Y.M.; Ganguri, H.B.; Wright, R.J.; Bosquet Enlow, M. Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J. Dev. Orig. Health Dis. 2017, 6, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Woodman, R.; Murphy, K.J. A Mediterranean Diet Reduces F2-Isoprostanes and Triglycerides among Older Australian Men and Women after 6 Months. J. Nutr. 2017, 147, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- El Sherbiny, S.; Squillacioti, G.; Colombi, N.; Ghelli, F.; Lenta, E.; Dalla Costa, C.; Bono, R. The Effect of Dietary Patterns and Nutrient Intake on Oxidative Stress Levels in Pregnant Women: A Systematic Review. Antioxidants 2023, 12, 1427. [Google Scholar] [CrossRef]
- Casas, R.; Urpi-Sardà, M.; Sacanella, E.; Arranz, S.; Corella, D.; Castañer, O.; Lamuela-Raventós, R.M.; Salas-Salvadó, J.; La-petra, J.; Portillo, M.P.; et al. Anti-Inflammatory Effects of the Mediterranean Diet in the Early and Late Stages of Atheroma Plaque Development. Mediat. Inflamm. 2017, 2017, 3674390. [Google Scholar] [CrossRef] [PubMed]
- Łakoma, K.; Kukharuk, O.; Śliż, D. The Influence of Metabolic Factors and Diet on Fertility. Nutrients 2023, 15, 1180. [Google Scholar] [CrossRef]
- Noli, S.A.; Ricci, E.; Cipriani, S.; Ferrari, S.; Castiglioni, M.; La Vecchia, I.; Somigliana, E.; Parazzini, F. Dietary Carbohydrate Intake, Dietary Glycemic Load and Outcomes of in Vitro Fertilization: Findings from an Observational Italian Cohort Study. Nutrients 2020, 12, 1568. [Google Scholar] [CrossRef]
- Fontana, R.; Della Torre, S. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients 2016, 8, 87. [Google Scholar] [CrossRef]
- Amjadi, A.; Abbasi Mobarakeh, K.; Doaei, S.; Dorosti, M.; Nami, S.; Mirshafaei, S.R.; Mirshafaei, M.A.; Ataei Kachooei, M.; Shamsi-Goushki, A.; Saeedirad, Z.; et al. Interactions of spontaneous abortion with FTO gene and dietary carotenoids; a case-control study. J. Nutr. Sci. 2024, 13, e75. [Google Scholar] [CrossRef]
- Vahid, F.; Rahmani, D.; Davoodi, S.H.; Hekmatdoost, A. The association among maternal index of nutritional quality, dietary antioxidant index, and odds of miscarriage incidence: Case-control study. J. Am. Nutr. Assoc. 2022, 41, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Toyoshima, M.; Watanabe, T.; Negishi, Y.; Kuwabara, Y.; Takeshita, T.; Suzuki, S. Associations of Nutrients and Dietary Preferences with Recurrent Pregnancy Loss and Infertility. J. Nippon. Med. Sch. 2024, 91, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Melo, P.; Pickering, O.; Dhillon-Smith, R.; Coomarasamy, A.; Devall, A. The association between dietary patterns and risk of miscarriage: A systematic review and meta-analysis. Fertil. Steril. 2023, 120, 333–357. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Rodríguez-Cano, A.M.; González-Ludlow, I.; Suárez-Rico, B.V.; Montoya-Estrada, A.; Piña-Ramírez, O.; Parra-Hernández, S.B.; Reyes-Muñoz, E.; Estrada-Gutierrez, G.; Calzada-Mendoza, C.C.; Perichart-Perera, O. Ultra-Processed Food Consumption during Pregnancy and Its Association with Maternal Oxidative Stress Markers. Antioxidants 2022, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Martín-Calvo, N. Mediterranean diet and life expectancy; beyond olive oil, fruits, and vegetables. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 401–407. [Google Scholar] [CrossRef]
- Martínez-González, M.A. Benefits of the Mediterranean diet beyond the Mediterranean Sea and beyond food patterns. BMC Med. 2016, 14, 157. [Google Scholar] [CrossRef]
- Bruić, M.; Pirković, A.; Vilotić, A.; Jovanović-Krivokuća, M.; Spremo-Potparević, B. Cytoprotective and genoprotective effects of taxifolin against oxidative damage in HTR-8/SVneo human trophoblast cells. Mutagenesis 2023, 38, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Bruić, M.; Pirković, A.; Borozan, S.; Nacka Aleksić, M.; Jovanović Krivokuća, M.; Spremo-Potparević, B. Antioxidative and anti-inflammatory effects of taxifolin in H2O2-induced oxidative stress in HTR-8/SVneo trophoblast cell line. Reprod. Toxicol. 2024, 126, 108585. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind Crops Prod. Ind. Crops. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Banerjee, S.; Mullick, H.I.; Banerjee, J.; Ghosh, A. Zingiber officinale: ‘A natural gold’. Int. J. Pharm. Bio-Sci. 2011, 2, 283–294. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Ji, K.; Fang, L.; Zhao, H.; Li, Q.; Shi, Y.; Xu, C.; Wang, Y.; Du, L.; Wang, J.; Liu, Q. Ginger oleoresin alleviated γ-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human mesenchymal stem cells. Oxid. Med. Cell Longev. 2017, 2017, 1480294. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Ojo, M.; Afolabi, T.T.; Arowoogun, M.D.; Nwawolor, D.; Farombi, E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. Biol. Interact. 2017, 270, 15–23. [Google Scholar] [CrossRef]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B 2016, 1011, 223–232. [Google Scholar] [CrossRef]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Yu, G.; Zhang, X.; Qi, X.; Zhang, J.; Zhang, L.; Wang, T. Dietary Curcumin Supplementation Ameliorates Placental Inflammation in Rats with Intra-Uterine Growth Retardation by Inhibiting the NF-KB Signaling Pathway. J. Nutr. Biochem. 2022, 104, 108973. [Google Scholar] [CrossRef]
- Cheng, K.; Ji, S.; Jia, P.; Zhang, H.; Wang, T.; Song, Z.; Zhang, L.; Wang, T. Resveratrol Improves Hepatic Redox Status and Lipid Balance of Neonates with Intrauterine Growth Retardation in a Piglet Model. Biomed. Res. Int. 2020, 2020, 7402645. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Baştemur, A.G.; Gezer, İ.S.; Kesikli, B.; Tanaçan, A.; Kara, Ö.; Yazıhan, N.; Şahin, D. The influence of dietary choices: Investigation of the relationship dietary inflammatory index and fetal growth restriction. BMC Pregnancy Childbirth 2025, 25, 569. [Google Scholar] [CrossRef]
- Parlapani, E.; Agakidis, C.; Karagiozoglou-Lampoudi, T.; Sarafidis, K.; Agakidou, E.; Athanasiadis, A.; Diamanti, E. The Mediterranean diet adherence by pregnant women delivering prematurely: Association with size at birth and complications of prematurity. J. Matern. Fetal Neonatal Med. 2019, 32, 1084–1091. [Google Scholar] [CrossRef]
- Sun, Y.; Kopp, S.; Strutz, J.; Gali, C.C.; Zandl-Lang, M.; Fanaee-Danesh, E.; Kirsch, A.; Cvitic, S.; Frank, S.; Saffery, R.; et al. Gestational diabetes mellitus modulates cholesterol homeostasis in human fetoplacental endothelium. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 968–979. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.; Wan, S.; Ma, X.; Long, Y.; Xu, Y.; Jiang, Z.Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef]
- Sun, C.; Shen, J.; Fang, R.; Huang, H.; Lai, Y.; Hu, Y.; Zheng, J. The impact of environmental and dietary exposure on gestational diabetes mellitus: A comprehensive review emphasizing the role of oxidative stress. Front. Endocrinol. 2025, 16, 1393883. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Gomez Ribot, D.; Diaz, E.; Fazio, M.V.; Gómez, H.L.; Fornes, D.; Macchi, S.B.; Gresta, C.A.; Capobianco, E.; Jawerbaum, A. An extra virgin olive oil-enriched diet improves maternal, placental, and cord blood parameters in GDM pregnancies. Diabetes Metab. Res. Rev. 2020, 36, e3349. [Google Scholar] [CrossRef]
- van der Pligt, P.; Wadley, G.D.; Lee, I.L.; Ebrahimi, S.; Spiteri, S.; Dennis, K.; Mason, S. Antioxidant Supplementation for Management of Gestational Diabetes Mellitus in Pregnancy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Curr. Nutr. Rep. 2025, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, E.M.; Pedroza, A.A.S.; Ferreira, D.J.S.; de Andrade, S.C.; Rozendo, A.; Fernandes, M.S.S.; Silva, T.L.; Fernandes, M.P.; Lagranha, C.J. The deleterious effects of maternal protein deprivation on the brainstem are minimized with moderate physical activity by offspring during early life. Appl. Physiol. Nutr. Metab. 2024, 49, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.N. Impact of Climate Change on Dietary Nutritional Quality and Implications for Asthma and Allergy. Immunol. Allergy Clin. N. Am. 2024, 44, 85–96. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R.; Kusanovic, J.P.; Hassan, S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 503.e1–503.e12. [Google Scholar] [CrossRef]
- Piirainen, T.; Isolauri, E.; Lagström, H.; Laitinen, K. Impact of dietary counselling on nutrient intake during pregnancy: A prospective cohort study. Br. J. Nutr. 2006, 6, 1095–1104. [Google Scholar] [CrossRef]
- Vähämiko, S.; Isolauri, E.; Poussa, T.; Laitinen, K. The impact of dietary counselling during pregnancy on vitamin intake and status of women and their children. Int. J. Food Sci. Nutr. 2013, 5, 551–560. [Google Scholar] [CrossRef]
- Kinnunen, T.; Liu, Y.; Koivisto, A.M.; Virtanen, S.; Luoto, R. Effects of dietary counselling on micronutrient intakes in pregnant women in Finland. Matern. Child. Nutr. 2021, 17, e13203. [Google Scholar] [CrossRef]
- Clark, J.; Craig, L.; McNeill, G.; Smith, N.; Norrie, J.; Devereux, G. A novel dietary intervention to optimize vitamin E intake of pregnant women to 15 mg/day. J. Acad. Nutr. Diet. 2012, 112, 297–301. [Google Scholar] [CrossRef]
- Scaife, A.R.; McNeill, G.; Campbell, D.M.; Martindale, S.; Devereux, G.; Seaton, A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br. J. Nutr. 2006, 95, 771–778. [Google Scholar] [CrossRef]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008, 2008, CD004227. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Q.; Dang, S.; Liu, X.; Zeng, L.; Yan, H. Dietary Quality during Pregnancy and Congenital Heart Defects. Nutrients 2022, 14, 3654. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Q.; Du, Q.; Dang, S.; Zeng, L.; Yan, H. Dietary Inflammatory Index during Pregnancy and Congenital Heart Defects. Nutrients 2023, 15, 2262. [Google Scholar] [CrossRef]
- Yang, J.; Du, Q.; Xiao, Z.; Guo, R.; Chang, Q.; Li, Y.H. Maternal Oxidative Balance Score during Pregnancy and Congenital Heart Defects. Nutrients 2024, 16, 1825. [Google Scholar] [CrossRef]
- Diniz, M.S.; Magalhães, C.C.; Tocantins, C.; Grilo, L.F.; Teixeira, J.; Pereira, S.P. Nurturing through Nutrition: Exploring the Role of Antioxidants in Maternal Diet during Pregnancy to Mitigate Developmental Programming of Chronic Diseases. Nutrients 2023, 15, 4623. [Google Scholar] [CrossRef]
- Poslusna, K.; Ruprich, J.; de Vries, J.H.; Jakubikova, M.; van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24-hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101, 73–85. [Google Scholar] [CrossRef]

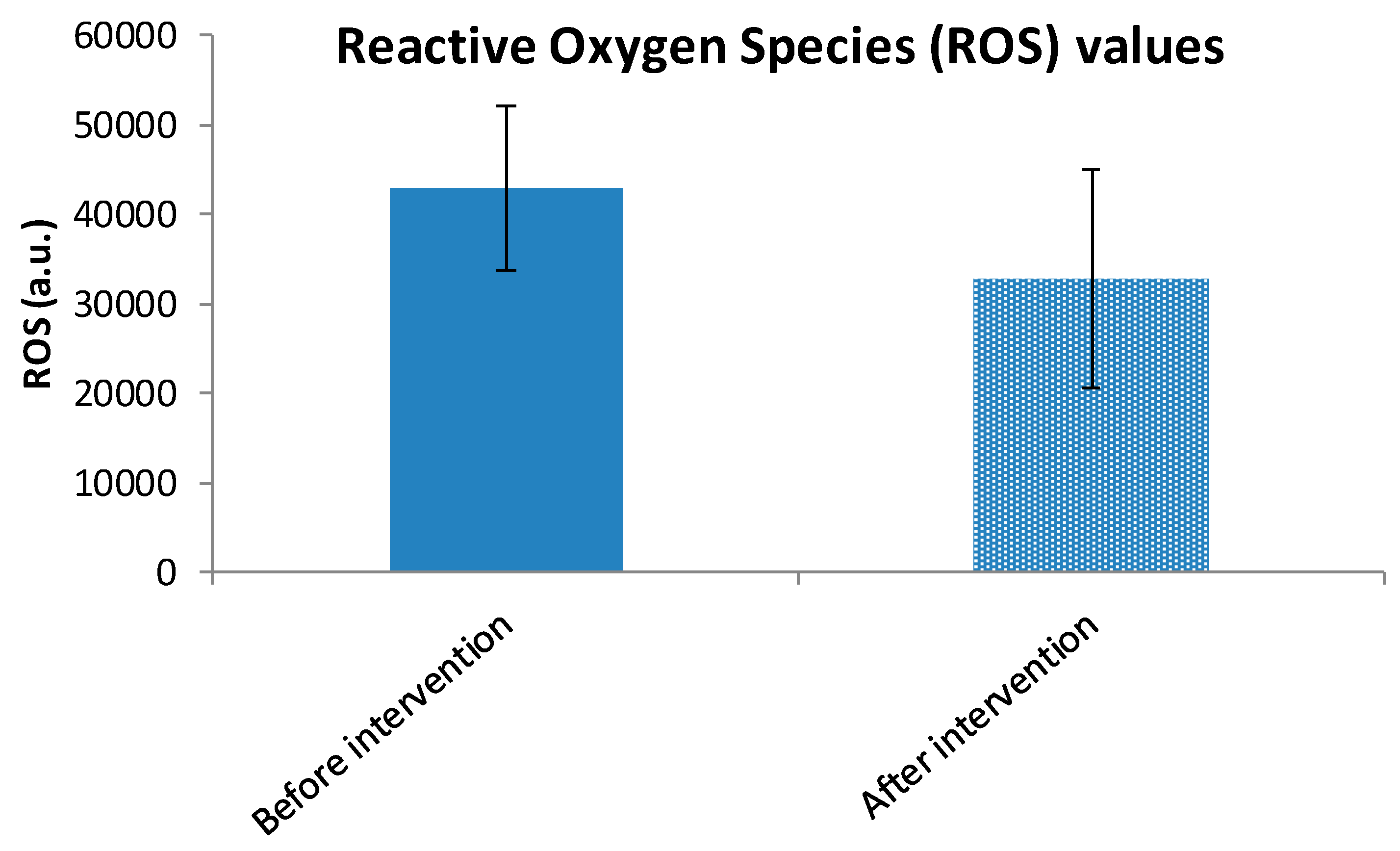
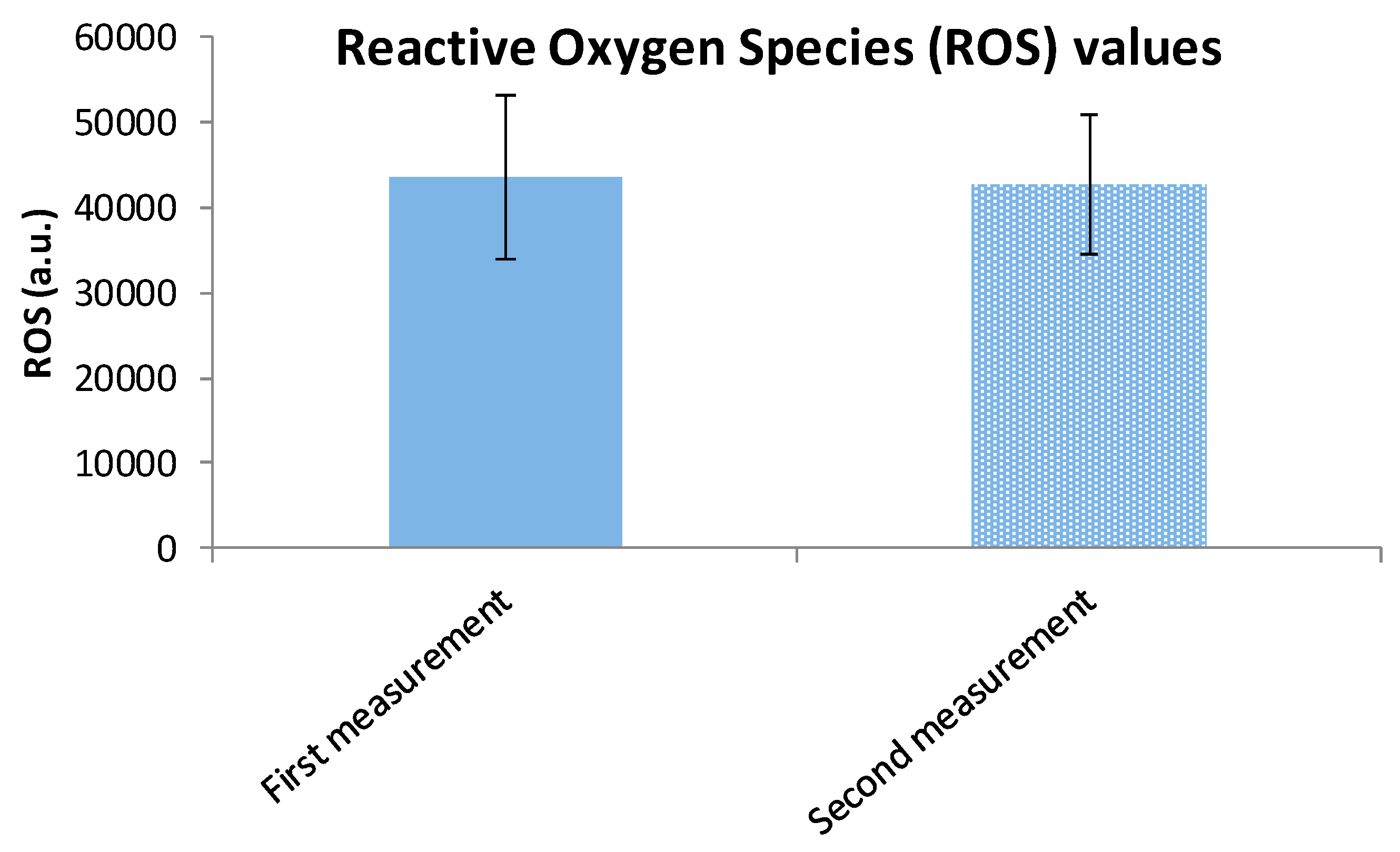
| Variable | Intervention Group (n = 60) | Control Group (n = 20) |
|---|---|---|
| Age (year) | 41 ± 4.01 | 34.2 ± 4.68 |
| Height (cm) | 165.57 ± 5.76 | 165.7 ± 5.00 |
| Weight before pregnancy (kg) | 62.37 ± 8.75 | 61.50 ± 10.34 |
| Weight at first trimester (kg) | 64.50 ± 8.31 | 66.72 ± 12.00 |
| BMI * (kg/m2) before pregnancy | 22.79 ± 3.35 | 22.36 ± 3.52 |
| BMI (kg/m2) at first trimester | 23.58 ± 3.26 | 24.27 ± 4.06 |
| Maternal education | ||
| High school [n (%)] | 6 (10) | 1 (5) |
| IVT * [n (%)] | 5 (8.3) | 6 (30) |
| Bachelor’s degree [n (%)] | 28 (46.7) | 7 (35) |
| Master’s degree [n (%)] | 17 (28.3) | 6 (30) |
| Ph.D. [n (%)] | 4 (6.7) | 0 (0) |
| Urban residence [n (%)] | 40 (66.7) | 16 (80) |
| Rural residence [n (%)] | 20 (33.3) | 4 (20) |
| Gravidity | ||
| First [n (%)] | 35 (58.3) | 11 (55) |
| Second [n (%)] | 20 (33.3) | 6 (30) |
| Third [n (%)] | 4 (6.7) | 2 (10) |
| Fourth [n (%)] | 1 (1.7) | 1 (5) |
| Maternal occupation | ||
| Private sector [n (%)] | 25 (41.7) | 7 (35) |
| Public sector [n (%)] | 8 (13.3) | 3 (15) |
| Freelancer [n (%)] | 18 (30) | 7 (35) |
| Household [n (%)] | 4 (6.7) | 2 (10) |
| Unemployed [n (%)] | 5 (8.3) | 1 (5) |
| Variable | Mean Rank/Mean | Sum of Ranks/SD * | Test Statistics | p-Value |
|---|---|---|---|---|
| ROS * Intervention Group | 42,939.40 a.u. | 9220.86 (SD) | t = −0.205 | 0.838 |
| ROS Control Group | 43,422.62 a.u. | 9589.19 (SD) | - | - |
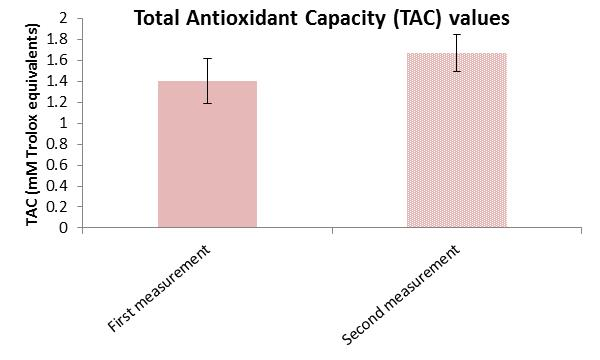
| TAC * Intervention Group | 43.82 | 2629 | Z = −1.823 | 0.068 |
| TAC Control Group | 32.95 | 692 | - | - |
| Variable | Direction | N | Mean Rank/Mean | Sum of Ranks/SD * | Test Statistics | p-Value |
|---|---|---|---|---|---|---|
| ROS * Intervention Group | Negative Ranks | 51 | 30.92 | 1577.00 | Z = −4.873 | <0.001 |
| Positive Ranks | 9 | 28.11 | 253.00 | |||
| TAC * Intervention Group | Negative Ranks | 13 | 22.77 | 296.00 | Z = -4.558 | <0.001 |
| Positive Ranks | 47 | 32.64 | 1534.00 | |||
| ROS Control Group | Negative Ranks | 11 | 9.67 | 116.00 | Z = −0.017 | 0.986 |
| Positive Ranks | 9 | 12.78 | 115.00 | |||
| TAC Control Group | Mean Difference | 20 | Pre: 1.401 | 0.212 (SD) | t = −5.046 | <0.001 |
| Post: 1.672 | 0.175 (SD) |
| Variable | Median/Mean | Test Statistics | p-Value |
|---|---|---|---|
| ROS * Intervention Group | 30,538 | Z = −4.873 | <0.001 |
| ROS Control Group | 42,857 | Z = −0.017 | 0.986 |
| TAC * Intervention Group | 1.71 | Z = −4.558 | <0.001 |
| TAC Control Group | 1.67 mmol/L | t = −5.046 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontopidou, E.; Tsamesidis, I.; Kourti, A.; Athanasiadis, A.; Itziou, A. Impact of Mediterranean Dietary Intervention on Reactive Oxygen Species Levels and Total Antioxidant Capacity During Pregnancy. Curr. Issues Mol. Biol. 2025, 47, 916. https://doi.org/10.3390/cimb47110916
Kontopidou E, Tsamesidis I, Kourti A, Athanasiadis A, Itziou A. Impact of Mediterranean Dietary Intervention on Reactive Oxygen Species Levels and Total Antioxidant Capacity During Pregnancy. Current Issues in Molecular Biology. 2025; 47(11):916. https://doi.org/10.3390/cimb47110916
Chicago/Turabian StyleKontopidou, Eirini, Ioannis Tsamesidis, Areti Kourti, Apostolos Athanasiadis, and Aikaterini Itziou. 2025. "Impact of Mediterranean Dietary Intervention on Reactive Oxygen Species Levels and Total Antioxidant Capacity During Pregnancy" Current Issues in Molecular Biology 47, no. 11: 916. https://doi.org/10.3390/cimb47110916
APA StyleKontopidou, E., Tsamesidis, I., Kourti, A., Athanasiadis, A., & Itziou, A. (2025). Impact of Mediterranean Dietary Intervention on Reactive Oxygen Species Levels and Total Antioxidant Capacity During Pregnancy. Current Issues in Molecular Biology, 47(11), 916. https://doi.org/10.3390/cimb47110916









