IKK/NF-κB Inactivation by Salidroside via Targeting TNF-α for the Treatment of LPS-Induced Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Culture and Treatment
2.3. Animals and Treatment
2.4. Western Blot
2.5. Histopathology and Immunohistochemistry (IHC)
2.6. Quantitative Real Time-PCR (qPCR)
2.7. Network Pharmacology and Molecular Docking
2.8. Plasmid Construction, Mutagenesis, and Transfection
2.9. Cellular Thermal Shift Assay (CETSA)
2.10. Immunoprecipitation (IP)
2.11. Statistical Analysis
3. Results
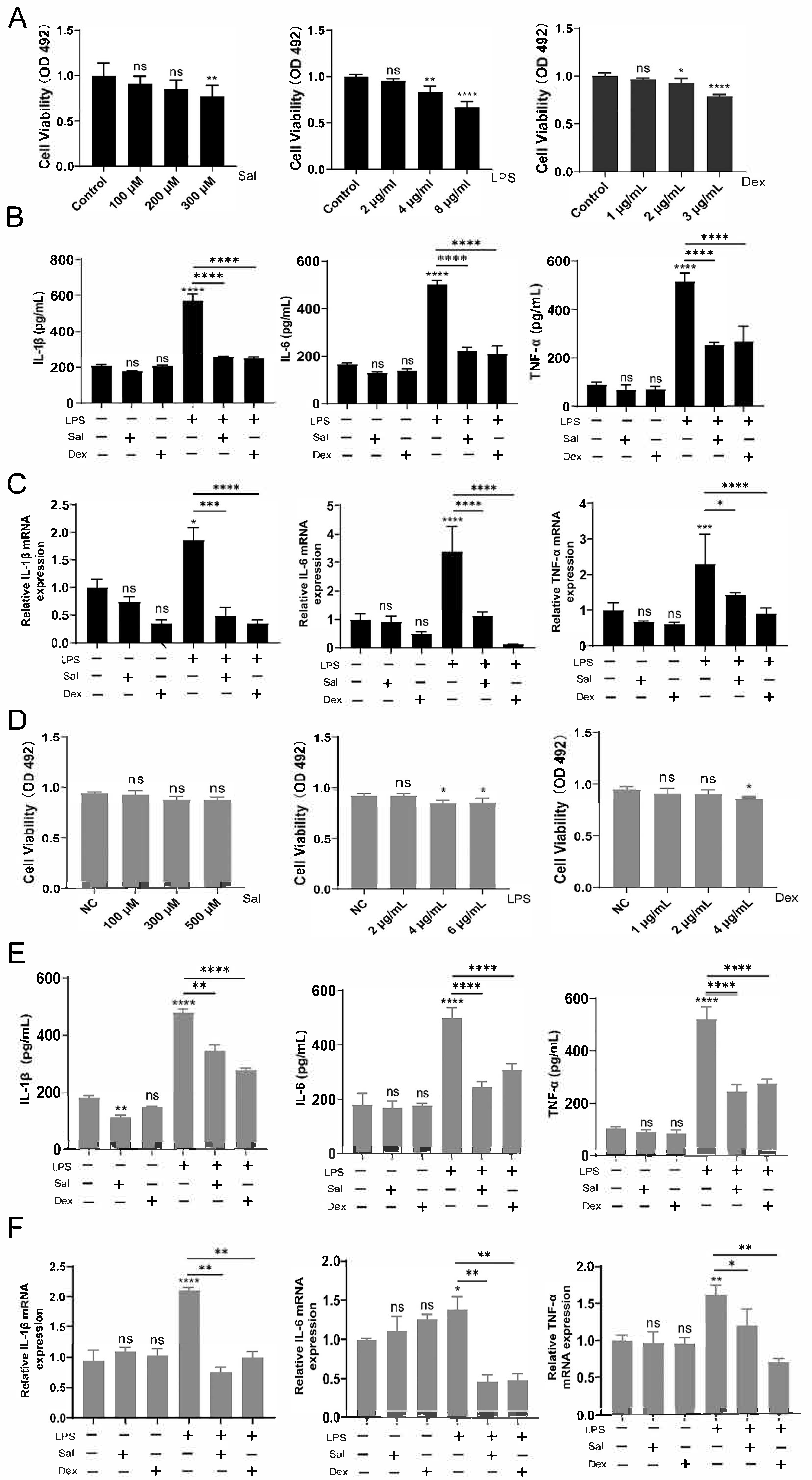
3.1. Salidroside Alleviates Inflammation on LPS-Induced Mice
3.2. Effects of Salidroside on NCM460 and RAW264.7 Cells Induced by LPS
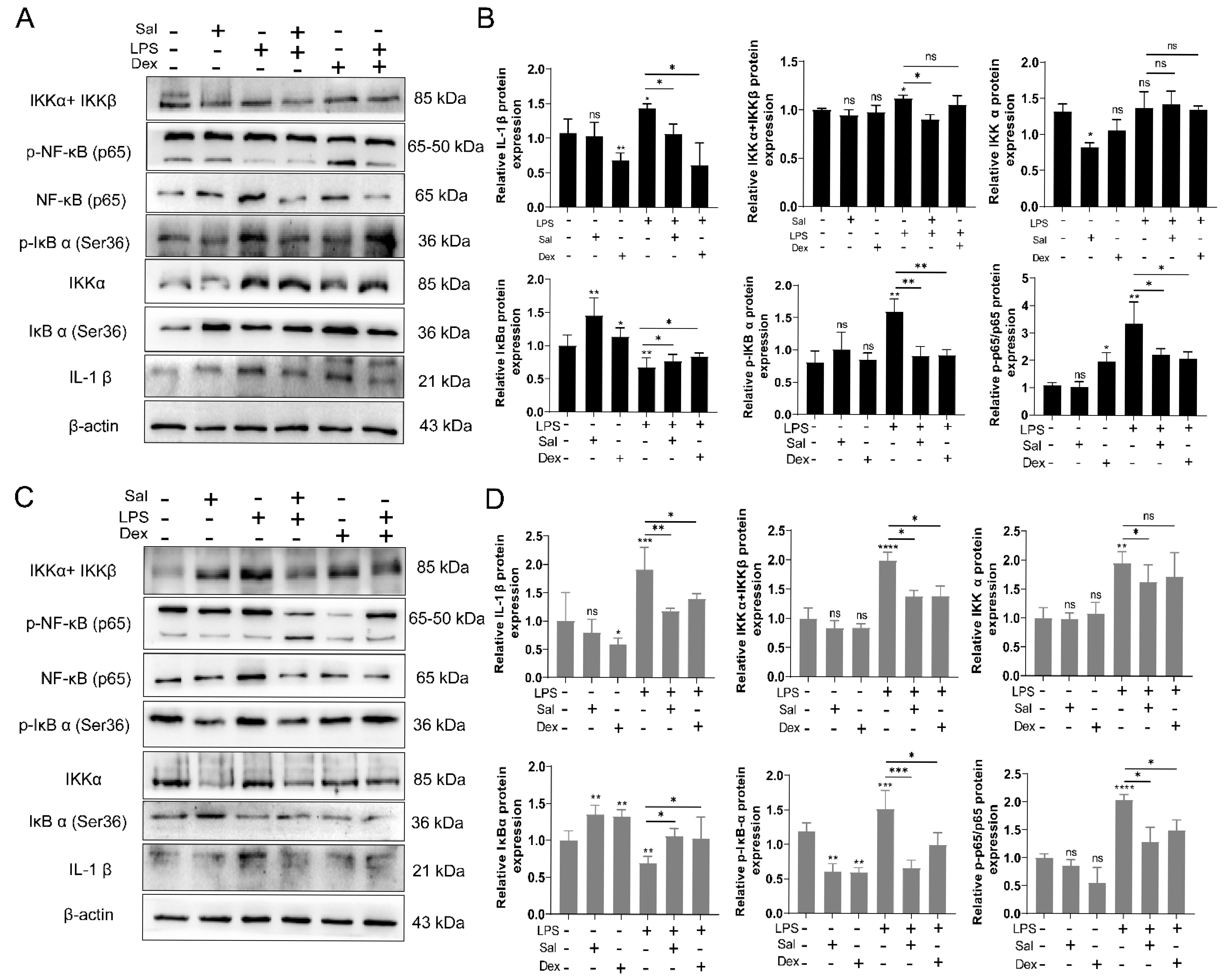
3.3. Salidroside Ameliorates Cell Inflammation by Inhibiting the IKK/NF-κB Signaling Pathway
3.4. Salidroside Ameliorates Colitis by Inhibiting the TNF-α Signaling Pathway
3.5. Arg179, Lys188 and Tyr191 Played Roles in the Binding of Sal with TNF-α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNF-α | Tumor necrosis factor-alpha |
| LPS | lipopolysaccharide |
| UC | ulcerative colitis |
| CETSA | cellular thermal shift assay |
| PAMPs | pathogen-associated molecular patterns |
| TCM | Traditional Chinese medicine |
| PVB | premature ventricular beats |
| IBD | inflammatory bowel disease |
| qPCR | Quantitative real Time-PCR |
| PPI | protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Sal | salidroside |
| IP | Immunoprecipitation |
| S.D. | Standard Deviation |
| H&E | hematoxylin and eosin |
| PAS | periodic acid–Schiff |
References
- Anderton, H.; Wicks, I.P.; Silke, J. Cell death in chronic inflammation: Breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 2020, 16, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Mahmud, F.; Zayas, J.; Kuzel, T.M.; Reiser, J.; Shafikhani, S.H. Reduced Bioactive Microbial Products (Pathogen-Associated Molecular Patterns) Contribute to Dysregulated Immune Responses and Impaired Healing in Infected Wounds in Mice with Diabetes. J. Investig. Dermatol. 2024, 144, 387–397.e311. [Google Scholar] [CrossRef]
- Siegmund, D.; Wajant, H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat. Rev. Rheumatol. 2023, 19, 576–591. [Google Scholar] [CrossRef]
- Temur, B.Z.; Timucin, A.C.; Atik, A.E.; Kocagoz, T.; Can, O. Peptide-Based Regulation of TNF-α-Mediated Cytotoxicity. Biomolecules 2025, 15, 559. [Google Scholar] [CrossRef]
- Tseng, H.C.; Tsai, P.M.; Chou, Y.H.; Lee, Y.C.; Lin, H.H.; Chen, J.H. In Vitro and In Vivo Protective Effects of Flavonoid-Enriched Lotus Seedpod Extract on Lipopolysaccharide-Induced Hepatic Inflammation. Am. J. Chin. Med. 2019, 47, 153–176. [Google Scholar] [CrossRef]
- Li, J.; Cui, Z.; Wei, M.; Almutairi, M.H.; Yan, P. Omics analysis of the effect of cold normal saline stress through gastric gavage on LPS induced mice. Front. Microbiol. 2023, 14, 1256748. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Souza, R.F.; Caetano, M.A.F.; Magalhães, H.I.R.; Castelucci, P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn′s Disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef]
- Bar-Yoseph, H.; Blatt, A.; Gerassy, S.; Pressman, S.; Mousa, A.; Sabo, E.; Waterman, M.; Ungar, B.; Ben-Horin, S.; Chowers, Y. Differential Serum-intestinal Dynamics of Infliximab and Adalimumab in Inflammatory Bowel Disease Patients. J. Crohns Colitis 2022, 16, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jiang, Y.; Wu, L.; Fu, J.; Du, J.; Luo, Z.; Guo, L.; Xu, J.; Liu, Y. Porphyromonas gingivalis aggravates colitis via a gut microbiota-linoleic acid metabolism-Th17/Treg cell balance axis. Nat. Commun. 2024, 15, 1617. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, S.; Zhang, W.; Cui, H.; Zhang, J.; Yin, X.; Zheng, X.; Shen, T.; Ying, H.; Chen, L.; et al. Cordycepin mitigates dextran sulfate sodium-induced colitis through improving gut microbiota composition and modulating Th1/Th2 and Th17/Treg balance. Biomed. Pharmacother. 2024, 180, 117394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Murugesan, S.; Ibrahim, N.; Elawad, M.; Al Khodor, S. Predictive biomarkers for anti-TNF alpha therapy in IBD patients. J. Transl. Med. 2024, 22, 284. [Google Scholar] [CrossRef]
- McPherson, M.J.; Hobson, A.D.; Hernandez, A., Jr.; Marvin, C.C.; Waegell, W.; Goess, C.; Oh, J.Z.; Shi, D.; Hayes, M.E.; Wang, L.; et al. An anti-TNF-glucocorticoid receptor modulator antibody-drug conjugate is efficacious against immune-mediated inflammatory diseases. Sci. Transl. Med. 2024, 16, eadd8936. [Google Scholar] [CrossRef] [PubMed]
- Moawadh, M.S. Molecular docking analysis of natural compounds as TNF-α inhibitors for Crohn's disease management. Bioinformation 2023, 19, 716–720. [Google Scholar] [CrossRef]
- Zhu, W.; Cremonini, E.; Mastaloudis, A.; Oteiza, P.I. Glucoraphanin and sulforaphane mitigate TNFα-induced Caco-2 monolayers permeabilization and inflammation. Redox Biol. 2024, 76, 103359. [Google Scholar] [CrossRef]
- Eldesoqui, M.; Ahmed, M.E.; Abdel-Kareem, M.A.; Badawy, M.M.; Dawood, A.F.; Mohamed, A.S.; Ibrahim, A.M.; El-Mansi, A.A.; El-Sherbiny, M.; Hendawy, M. Curcumin Mitigates Malathion-Induced Renal Injury: Suppression of Apoptosis and Modulation of NF-κβ/TNF-α and Nrf2, and HO-1 Signaling. Metabolites 2023, 13, 1117. [Google Scholar] [CrossRef]
- Gangwar, T.; Poonia, N.; Subudhi, R.N.; Arora, V. Therapeutic potential and underlying mechanisms of phytoconstituents: Emphasizing on resveratol, curcumin, quercetin, berberine, and hesperidin in ulcerative colitis. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 6579–6596. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Tong, Y.; Wu, L.; Xie, Y.; He, P.; Lin, S.; Hu, X. Integrating network pharmacology and animal experimental validation to investigate the action mechanism of oleanolic acid in obesity. J. Transl. Med. 2024, 22, 86. [Google Scholar] [CrossRef]
- Liang, K.; Ma, S.; Luo, K.; Wang, R.; Xiao, C.; Zhang, X.; Gao, Y.; Li, M. Salidroside: An Overview of Its Promising Potential and Diverse Applications. Pharmaceuticals 2024, 17, 1703. [Google Scholar] [CrossRef]
- Shuyuan, L.; Haoyu, C. Mechanism of Nardostachyos Radix et Rhizoma-Salidroside in the treatment of premature ventricular beats based on network pharmacology and molecular docking. Sci. Rep. 2023, 13, 20741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xin, J.; Zhang, T.; Zhang, Y.; Ji, C.; Kang, L.; Zhu, X.; Zhang, H.; Wang, W.; Liao, X. Assessing the potential impact of salidroside on Chikungunya virus-induced acute interstitial nephritis via network pharmacology, molecular docking and in vitro experiments. Front. Cell Infect. Microbiol. 2025, 15, 1623860. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, R.; Guo, Y.; Hu, B.; Xie, L.; An, Y.; Wen, J.; Liu, Z.; Zhou, M.; Kuang, W.; et al. Muscle-derived small extracellular vesicles induce liver fibrosis during overtraining. Cell Metab. 2025, 37, 824–841.e828. [Google Scholar] [CrossRef]
- Ouyang, Q.; Tian, S.; Zhou, H.; Mao, Y.; Li, X.; Yan, F.; Liu, A.; Hu, X.; You, C.; He, J. Salidroside inhibits melanin synthesis and melanoma growth via mTOR and PI3K/Akt pathways. Front. Oncol. 2025, 15, 1583580. [Google Scholar] [CrossRef]
- Xing, G.; Cui, Y.; Guo, Z.; Han, B.; Zhao, G. Progress on the mechanism of intestinal microbiota against colorectal cancer. Front. Cell Infect. Microbiol. 2025, 15, 1565103. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. Testing the predictive power of reverse screening to infer drug targets, with the help of machine learning. Commun. Chem. 2024, 7, 105. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, C.; Li, K.; Wang, J.; Wang, H.; Zhong, R.; Chen, L.; Ma, Q.; Zhang, H. Baicalin alleviates intestinal inflammation and microbial disturbances by regulating Th17/Treg balance and enhancing Lactobacillus colonization in piglets. J. Anim. Sci. Biotechnol. 2024, 15, 172. [Google Scholar] [CrossRef]
- Xie, X.; Chen, W.; Xu, M.; Chen, J.; Yang, T.; Wang, C.; Su, Y.; Zhao, J.; Xu, J.; Liu, Q. IKK/NF-κB and ROS signal axes are involved in Tenacissoside H mediated inhibitory effects on LPS-Induced inflammatory osteolysis. Cell Prolif. 2024, 57, e13535. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Sun, F.; Feng, W.; Ye, H.; Tian, T.; Lei, M. Salidroside alleviates LPS-Induced liver injury and inflammation through SIRT1- NF-κB pathway and NLRP3 inflammasome. Iran. J. Basic. Med. Sci. 2024, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Holak, T.A. Balinatunfib: A Clinical Oral Small Molecule TNFα Inhibitor. ChemMedChem 2025, 20, e202500258. [Google Scholar] [CrossRef]
- Oraby, M.A.; Abdel Mageed, S.S.; Amr Raouf, A.; Abdelshafy, D.A.; Ahmed, E.F.; Khalil, R.T.; Mangoura, S.A.; Fadaly, D.S. Remdesivir ameliorates ulcerative colitis-propelled cell inflammation and pyroptosis in acetic acid rats by restoring SIRT6/FoxC1 pathway. Int. Immunopharmacol. 2024, 137, 112465. [Google Scholar] [CrossRef]
- El-Dakroury, W.A.; Said, A.R.; Abdel Mageed, S.S.; Senbel, A.; Sohail, S.K.; Rizvi, S.F.; El-wakeel, H.S.; Abdellatif, H.; Nandi, P.; Nomier, Y.A.; et al. Rivaroxaban loaded lipid carriers integrating EPA oil: A novel paradigm in drug repurposing for navigating colon targeted oral treatment for ulcerative colitis. J. Drug Deliv. Sci. Technol. 2025, 109, 106985. [Google Scholar] [CrossRef]
- Duan, S.; Chen, P.; Liang, C.; Zhang, Y. Comparative Efficacy of Novel Biologics, Anti-tumor Necrosis Factor Agents, and Immunomodulators to Prevent Postoperative Recurrence in Crohn’s Disease: A Systematic Review and Network Meta-analysis. J. Crohns Colitis 2025, 19, jjae143. [Google Scholar] [CrossRef] [PubMed]
- Steenson, S.; Shojaee-Moradie, F.; Lovegrove, J.A.; Umpleby, A.M.; Jackson, K.G.; Fielding, B.A. Dose Dependent Effects of Fructose and Glucose on de novo Palmitate and Glycerol Synthesis in an Enterocyte Cell Model. Mol. Nutr. Food Res. 2022, 66, e2100456. [Google Scholar] [CrossRef] [PubMed]
- Paramita Pal, P.; Sajeli Begum, A.; Ameer Basha, S.; Araya, H.; Fujimoto, Y. New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies. Int. Immunopharmacol. 2023, 117, 109940. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Q.; Zhou, H.; Yu, Z.; Jiang, H.; Ji, C.; Sun, Y.; Zhou, F.; Xiang, S.; Hu, X. IKK/NF-κB Inactivation by Salidroside via Targeting TNF-α for the Treatment of LPS-Induced Colitis. Curr. Issues Mol. Biol. 2025, 47, 896. https://doi.org/10.3390/cimb47110896
Ouyang Q, Zhou H, Yu Z, Jiang H, Ji C, Sun Y, Zhou F, Xiang S, Hu X. IKK/NF-κB Inactivation by Salidroside via Targeting TNF-α for the Treatment of LPS-Induced Colitis. Current Issues in Molecular Biology. 2025; 47(11):896. https://doi.org/10.3390/cimb47110896
Chicago/Turabian StyleOuyang, Qi, Hao Zhou, Zixuan Yu, Hong Jiang, Chenhao Ji, Yijia Sun, Fang Zhou, Shuanglin Xiang, and Xiang Hu. 2025. "IKK/NF-κB Inactivation by Salidroside via Targeting TNF-α for the Treatment of LPS-Induced Colitis" Current Issues in Molecular Biology 47, no. 11: 896. https://doi.org/10.3390/cimb47110896
APA StyleOuyang, Q., Zhou, H., Yu, Z., Jiang, H., Ji, C., Sun, Y., Zhou, F., Xiang, S., & Hu, X. (2025). IKK/NF-κB Inactivation by Salidroside via Targeting TNF-α for the Treatment of LPS-Induced Colitis. Current Issues in Molecular Biology, 47(11), 896. https://doi.org/10.3390/cimb47110896





