Eleutheroside E Ameliorates D-Gal-Induced Senescence in Human Skin Fibroblasts Through PI3K/AKT Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Network Pharmacology Analysis
2.2.1. Collection and Screening of EE Targets
2.2.2. Collection and Screening of Targets Related to D-Gal-Induced Senescence
2.2.3. Target Prediction for Drug–Disease Associations
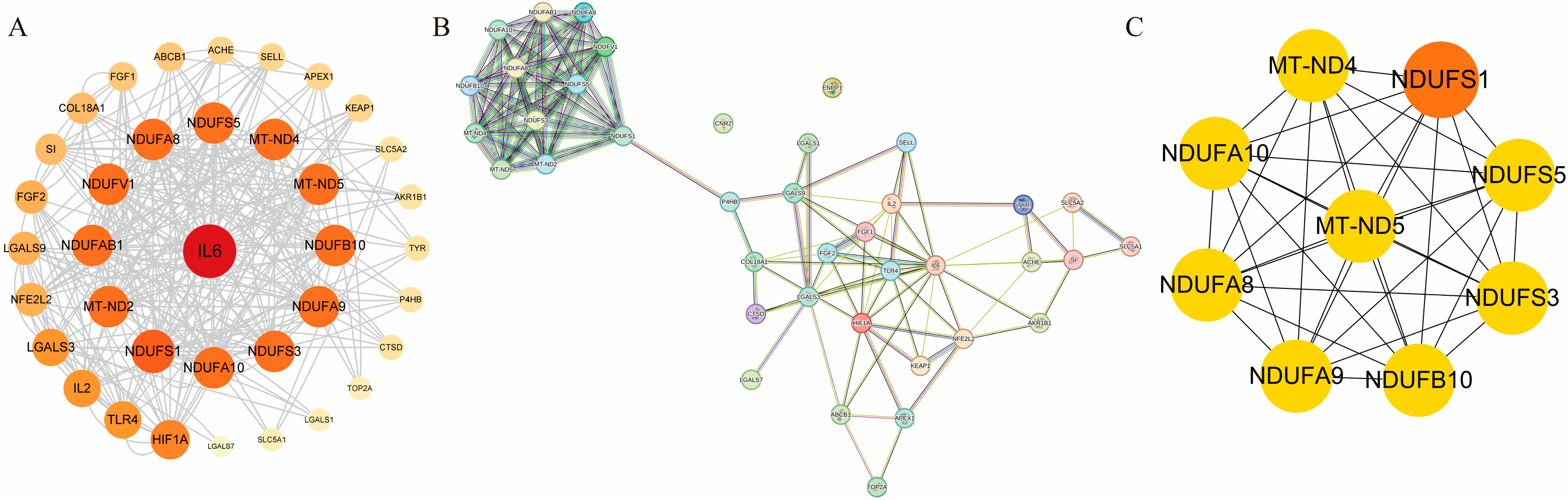
2.2.4. Protein–Protein Interaction (PPI) Network Construction and Hub Target Screening
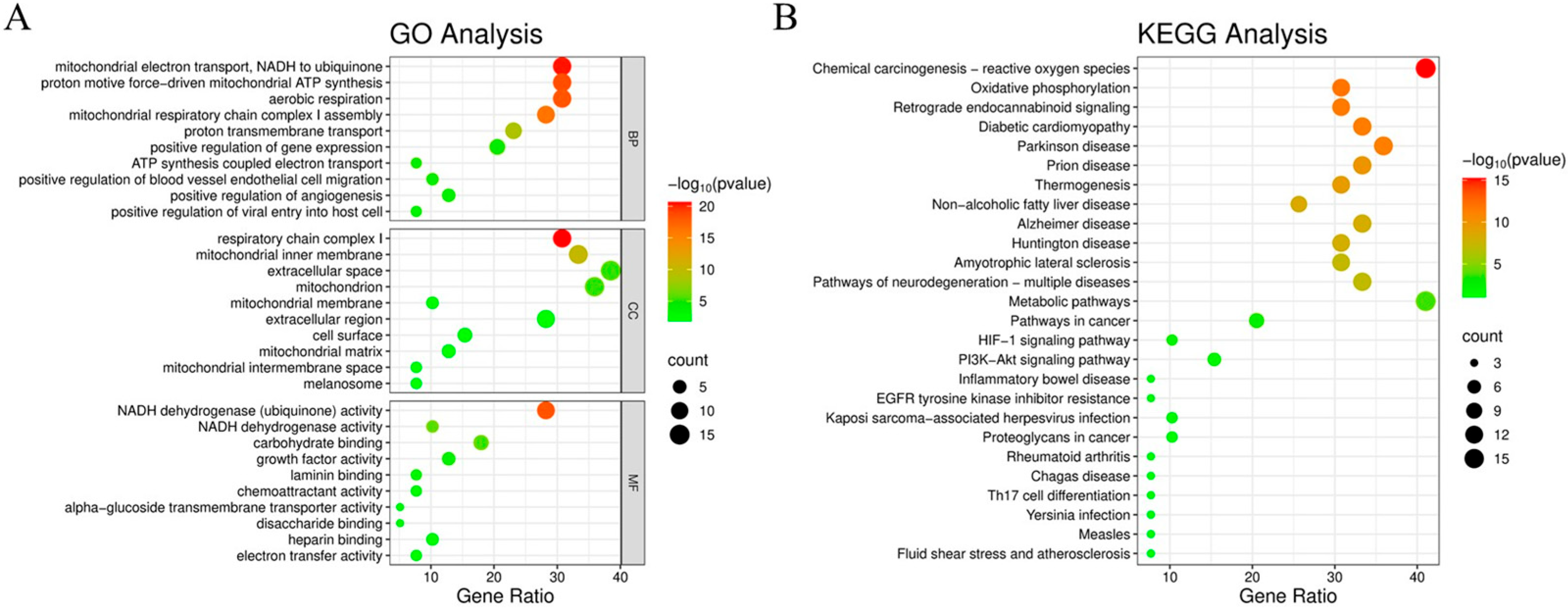
2.2.5. Gene Ontology (GO) Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.2.6. Molecular Docking
2.3. Experimental
2.3.1. Cell Culture and Treatment
2.3.2. Cell Grouping and Pharmacological Intervention
2.3.3. CCK-8 Assay
2.3.4. Measurement of SOD Activity, CAT Activity, and MDA Content in HSF Using Standardized Spectrophotometric Assays
2.3.5. Quantification of SA-β-Gal Positive Area in HSF Using Standardized Chromogenic Staining Protocol
2.3.6. Quantification of Intracellular ROS Levels in HSF Using 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA) Fluorescent Probe
2.3.7. Flow Cytometry Analysis of Apoptosis in HSF Cells
2.3.8. Western Blot Analysis of Senescence-Associated Protein Expression in HSF Cells
2.4. Statistical Analysis
2.5. Writing Assistance Disclosure
3. Results
3.1. Target Collection and Prediction
3.2. Construction of the PPI Network
3.3. GO and KEGG Pathway Enrichment Analysis
3.4. Molecular Docking Results
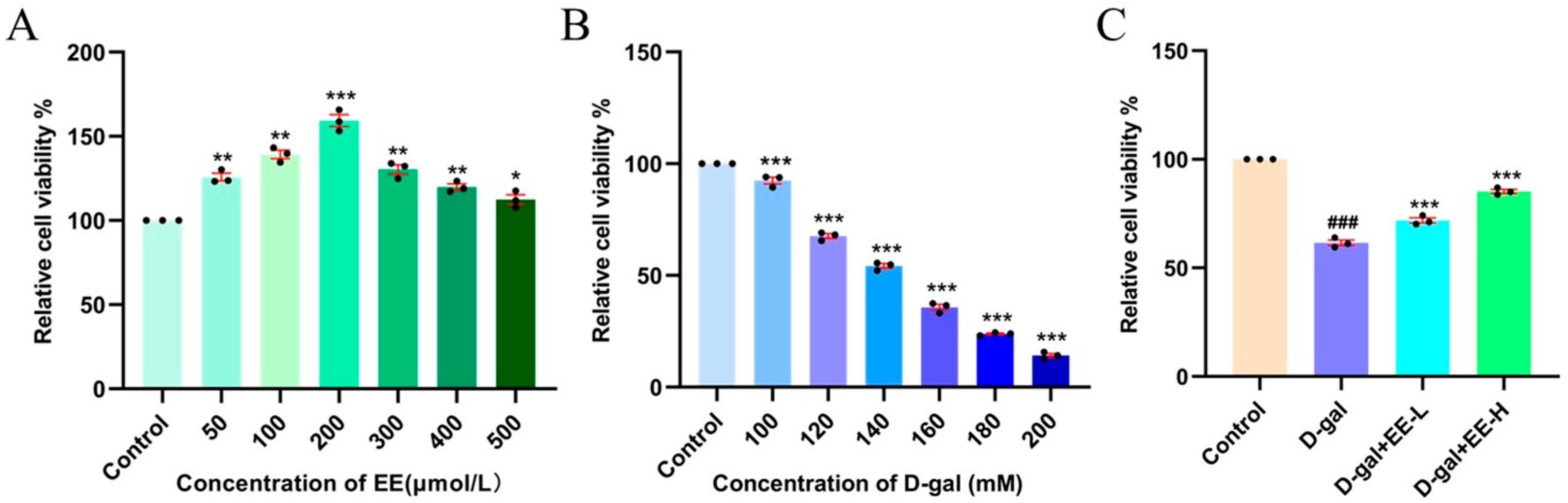
3.5. Cytotoxicity Evaluation of EE by CCK-8 Assay and Its Intervention in Oxidative Stress-Induced Senescence Model
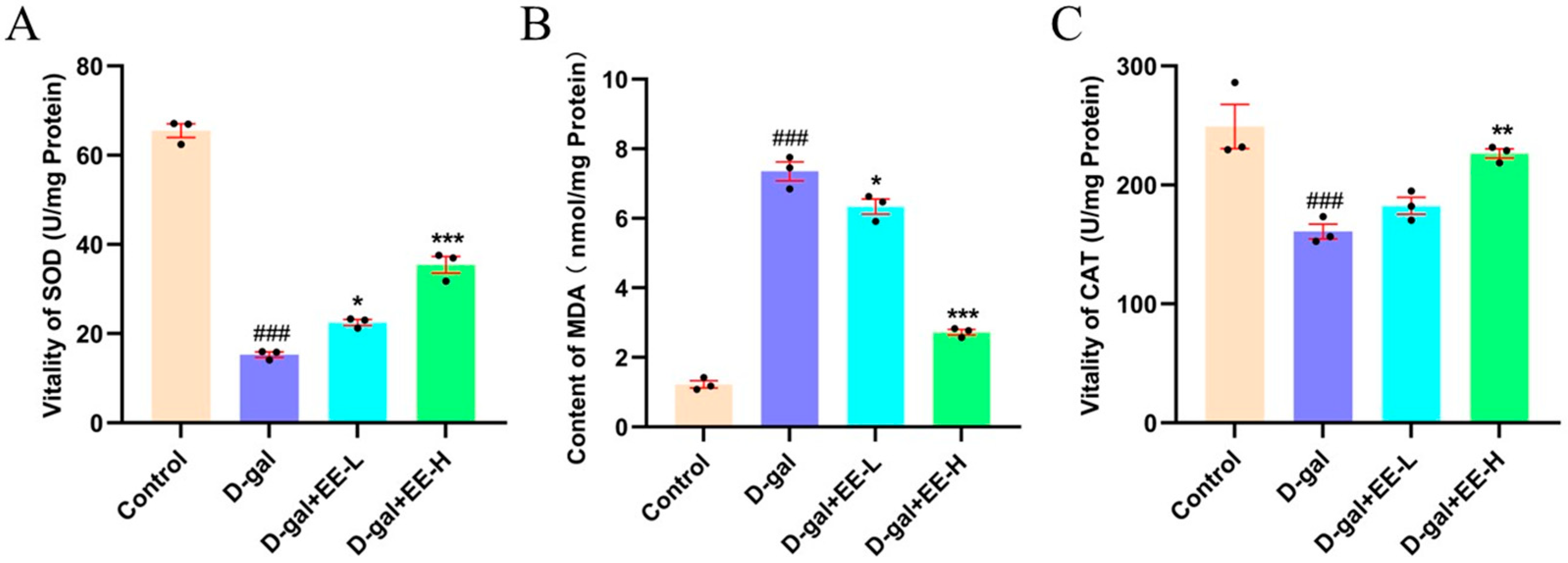
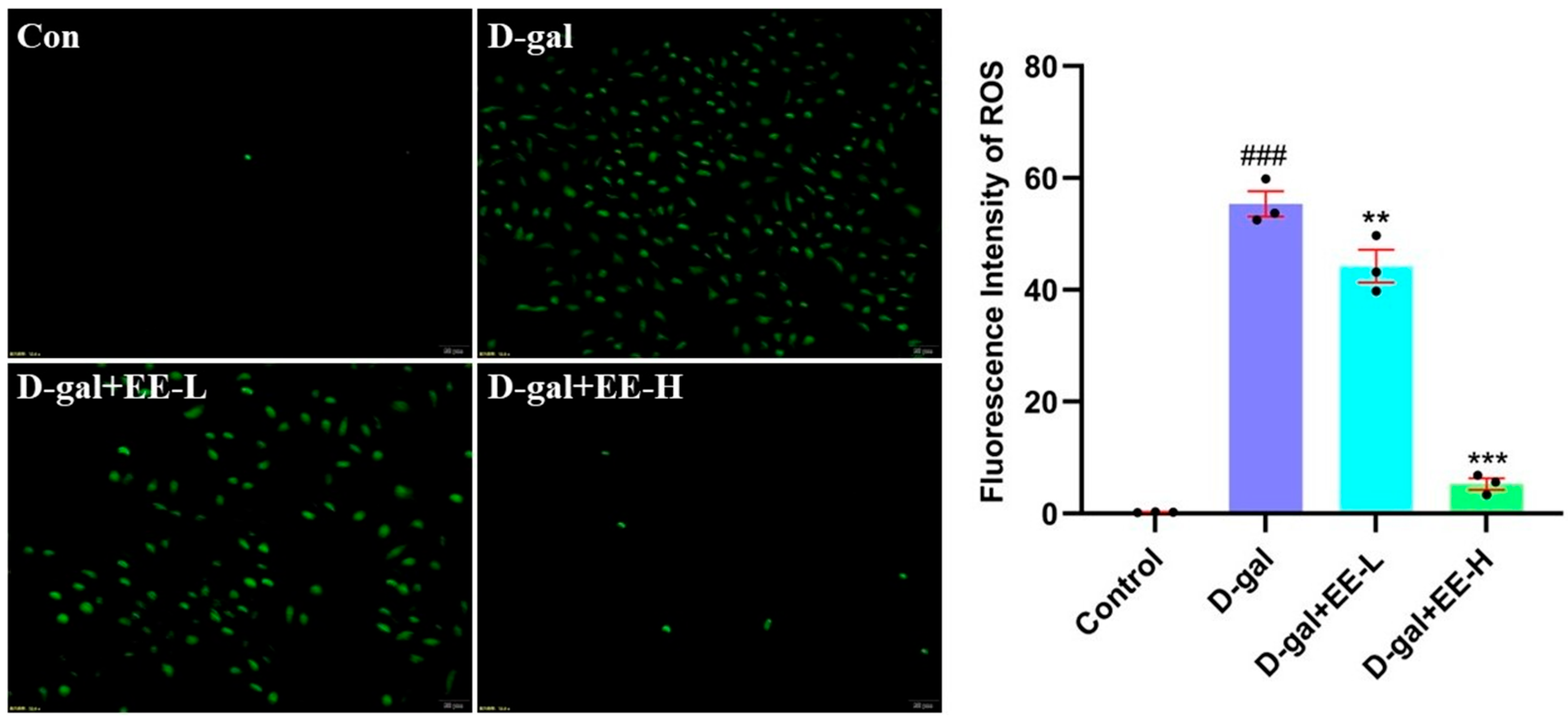
3.6. Effect of EE on Oxidative Stress Markers in HSF Cells
3.7. Measurement of Cellular Antioxidant Enzyme Activities
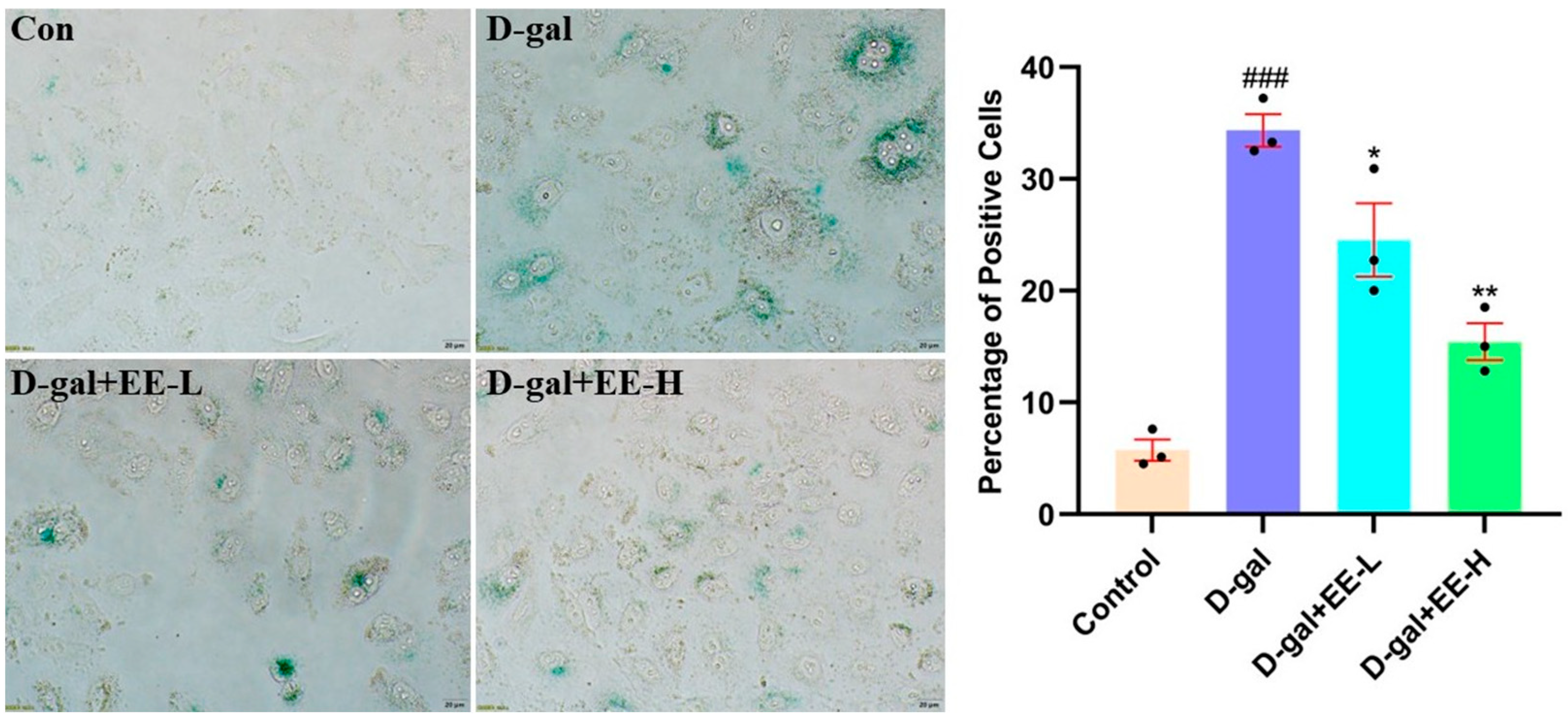
3.8. Effect of EE on D-Gal-Induced Cellular Senescence
3.9. Effect of EE on D-Gal-Induced Apoptosis
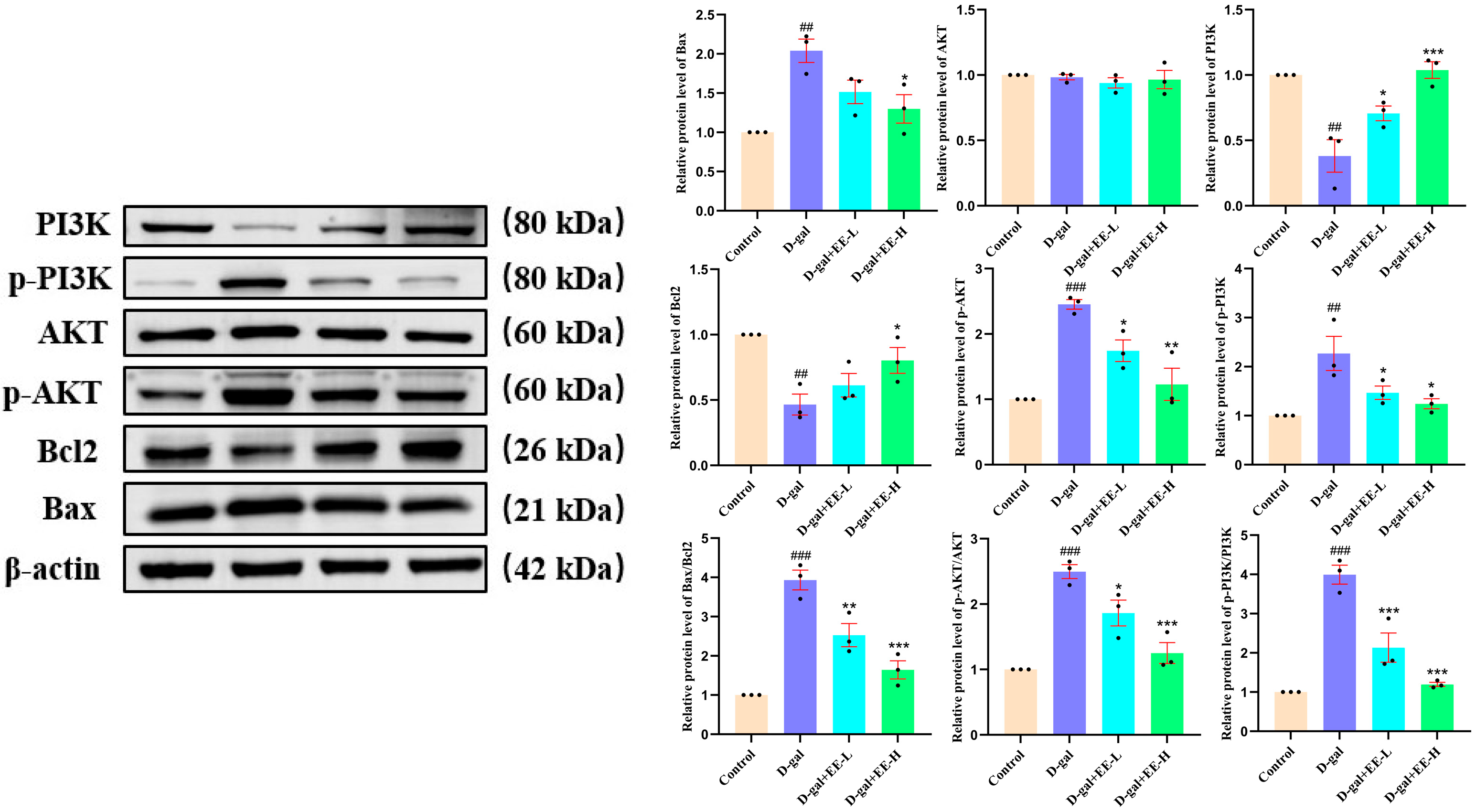
3.10. Western Blot Experiments Were Conducted to Investigate the Effects of EE on the Expression of PI3K/Akt Pathway-Related Proteins in HSF Cells
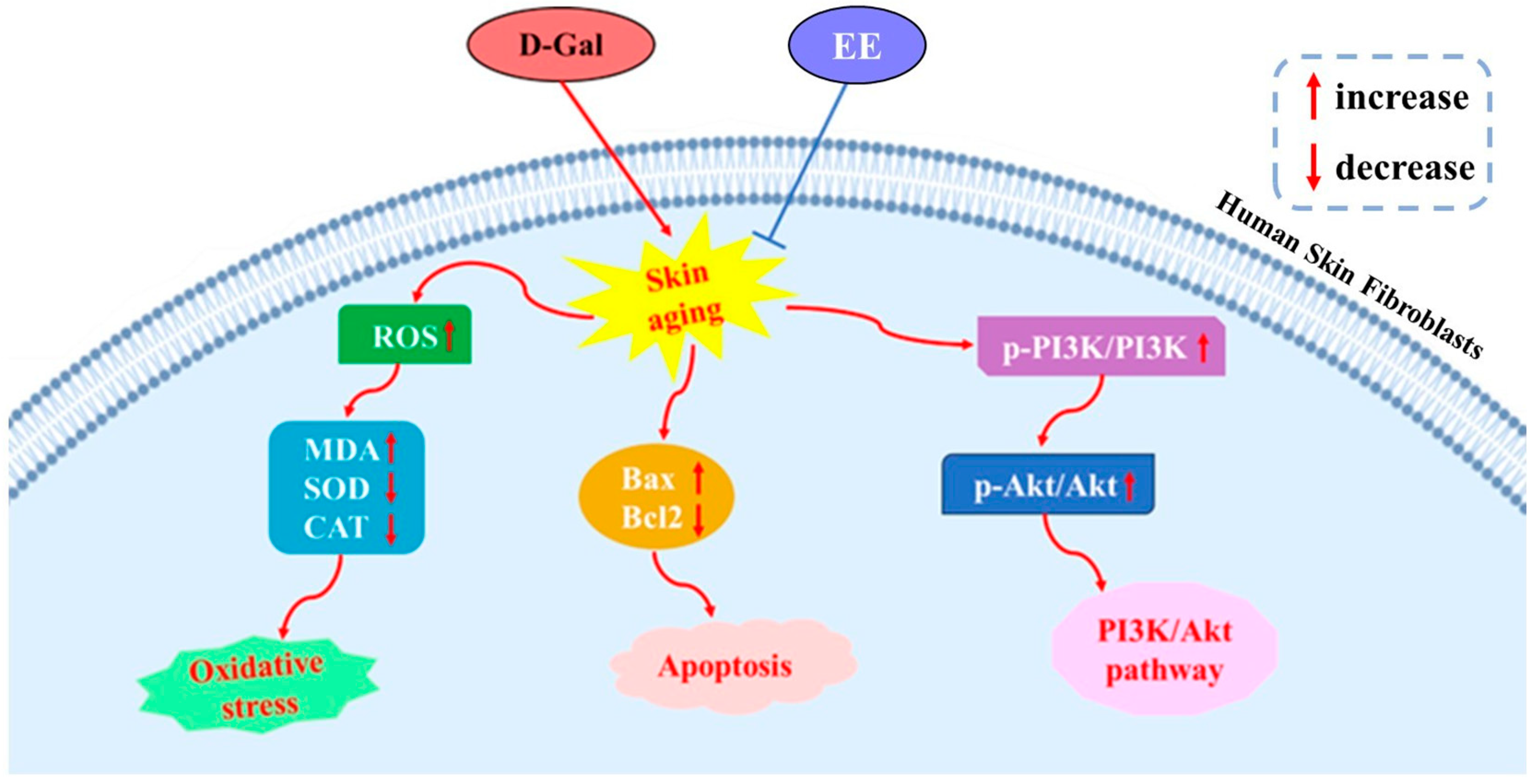
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EE | Eleutheroside E |
| HSF | Human Skin Fibroblast |
| BP | Biological Process |
| CC | Cellular Component |
| MF | Molecular Function |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| ROS | Reactive oxygen species |
| MDA | Malondialdehyde |
| PPI | protein–protein interaction |
References
- Gu, Y.P.; Han, J.X.; Jiang, C.P.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Stegemann, A.; Paus, R.; Kleszczyński, K.; Maity, P.; Wlaschek, M.; Scharffetter-Kochanek, K. Endocrine controls of skin aging. Endocr. Rev. 2025, 46, 349–375. [Google Scholar] [CrossRef]
- Tai, M.L.; Chen, J.L.; Chen, J.W.; Shen, X.Y.; Ni, J.H. Endoplasmic reticulum stress in skin aging induced by UVB. Exp. Dermatol. 2024, 33, e14956. [Google Scholar] [CrossRef]
- Ho, C.Y.; DrAEsen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, X.R.; Huang, J.Y.; Zhang, X.Y.; Bu, L.W.; Zhang, Y.R.; Liang, F.T.; Wu, S.H.; Zhang, M.; Zhang, L.; et al. CASIN exerts anti-aging effects through RPL4 on the skin of naturally aging mice. Aging Cell 2024, 23, e14333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Liu, L.; Yue, L.X.; Huang, Y.Z.; Wang, B.; Liu, P.F. Uncovering key mechanisms and intervention therapies in aging skin. Cytokine Growth Factor Rev. 2024, 79, 66–80. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef]
- Sun, J.C.; Xie, X.Y.; Song, Y.Y.; Sun, T.J.; Liu, X.Z.; Yuan, H.G.; Shen, C.N. Selenomethionine in gelatin methacryloyl hydrogels: Modulating ferroptosis to attenuate skin agin. Bioact. Mater. 2024, 35, 495–516. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Han, Y.Y.; Wang, S.; Lang, G.P. Anti-aging Effect of Rutin in Caenorhabditis elegans and D-Gal-Induced Aging Mouse Model. Dokl. Biochem. Biophys. 2023, 513, 350–354. [Google Scholar]
- Song, J.X.; Wu, Y.; Jiang, G.Q.; Feng, L.J.; Wang, Z.G.; Yuan, G.X.; Tong, H.B. Sulfated polysaccharides from Rhodiola sachalinensis reduce d-gal-induced oxidative stress in NIH 3T3 cells. Int. J. Biol. Macromol. 2019, 140, 288–293. [Google Scholar] [CrossRef]
- Liu, R.Y.; Meng, J.; Lou, D.F. Adiponectin inhibits D-gal-induced cardiomyocyte senescence via AdipoR1/APPL1. Mol. Med. Rep. 2021, 24, 719. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhu, H.W.; Zheng, Y.T.; Zhang, L.Y.; Wang, X.L.; Luo, Z.; Tang, J.; Lin, L.; Du, Z.Y.; Dong, C.Z. The effects and mechanism of collagen peptide and elastin peptide on skin aging induced by D-galactose combined with ultraviolet radiation. J. Photochem. Photobiol. B 2020, 210, 111964. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-induced skin aging: The role of oxidative stress. Oxid. Med. Cell Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.G.; Gheldiu, A.M.; Mates, L.; et al. Antioxidant effects of walnut (Juglans regia L.) kernel and walnut septum extract in a D-galactose-induced aging model and in naturally aged rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Hou, J.Y.; Hu, D.; Liu, J.; Zhao, M.J.; Ge, J.H.; Shen, Y.; Li, T.; Peng, Y.B.; Liu, P.; et al. Protective Effect ofPolygonatum odoratum Polysaccharides on A7r5Cell Senescence Induced by D-Galactose. Sci. Technol. Food Ind. 2022, 43, 380–388. [Google Scholar]
- Guan, J.; Ding, S.Y.; Wang, Y.Y.; Ge, S.Y.; Wang, J.M.; Zhu, H.Y.; Han, L.Q. Simultaneous Determination of Eleutheroside B and Eleutheroside E in Acanthopanacis Senticosi RadixEt Rhizoma Seu Caulis by Ultra-fast Liquid Chromatography. Tradit. Chin. Drug Res. Clin. Pharmacol. 2023, 34, 1608–1611. [Google Scholar]
- Shang, H.H.; Wang, M.; Liu, Y.; Zheng, Y.N.; Bai, R.; Liao, M.L. Determination of protocatechuic acid, syringing, chlorogenic acid, eleutherosideE, and isofraxidin in Acanthopanax senticosus from various of habitats by RP-HPLC. Mod. Pharm. Clin. 2018, 33, 1324–1328. [Google Scholar]
- Song, C.; Duan, F.Y.; Ju, T.; Qin, Y.; Zeng, D.Y.; Shan, S.; Shi, Y.D.; Zhang, Y.C.; Lu, W.H. Eleutheroside E supplementation prevents radiation-induced cognitive impairment and activates PKA signaling via gut microbiota. Commun. Biol. 2022, 5, 680. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yang, Y.J.; Chen, M.S.; Wang, Z.; Chen, Y.H.; Zhang, Y.F.; Shan, Y.M.; Yu, B. Anti-inflammatory and analgesic effects of eleutheroside E in alcoholic beverage. J. Biol. Regul. Homeost. Agents 2019, 33, 1815–1821. [Google Scholar]
- Bi, Y.X.; Liu, X.J.; Liu, Y.; Wang, M.Y.; Shan, Y.M.; Yin, Y.H.; Meng, X.L.; Sun, F.J.; Li, H.; Li, Z.D. Molecular and biochemical investigations of the anti-fatigue effects of tea polyphenols and fruit extracts of lycium ruthenicum murr. on mice with exercise-induced fatigue. Front. Mol. Biosci. 2023, 10, 1223411. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.W.; Xu, Y.Q. Eleutheroside E alleviates cerebral ischemia-reperfusion injury in a 5-hydroxytryptamine receptor 2C (Htr2c)-dependent manner in rats. Bioengineered 2022, 13, 11718–11731. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chai, X.Q. Eleutheroside E attenuates isoflurane-induced cognitive dysfunction by regulating the α7-nAChR-NMDAR pathway. Neuroreport 2019, 30, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.Y.; Cheon, Y.H.; Park, G.D.; Chung, C.H.; Lee, C.H.; Kim, J.Y.; Lee, M.S. Anti-Osteoporosis Effects of the Eleutherococcus senticosus, achyranthes japonica, and atractylodes japonica mixed extract fermented with nuruk. Nutrients 2021, 13, 3904. [Google Scholar] [CrossRef]
- Shen, Z.R.; Huang, D.M.; Jia, N.; Zhao, S.J.; Pei, C.X.; Wang, Y.L.; Wu, Y.C.; Wang, X.M.; Shi, S.H.; Wang, F.; et al. Protective effects of Eleutheroside E against high-altitude pulmonary edema by inhibiting NLRP3 inflammasome-mediated pyroptosis. Biomed. Pharmacother. 2023, 167, 115607. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.Z.; Li, X.; Jin, J.R.; Lu, Y.J.; Jiang, S.; Dong, X.P.; Qi, H. Interaction mechanism and binding mode of phycocyanin to lysozyme: Molecular docking and molecular dynamics simulation. Food Chem. 2024, 438, 138001. [Google Scholar] [CrossRef]
- Miao, W.; Hu, Y.Z. Aldo-Keto reductase 1C3 reduces myocardial cell damage after acute myocardial infarction by activating the Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2-antioxidant response element pathway to inhibit ferroptosis. J. Geriatr. Cardiol. 2024, 21, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.F.; Lai, Y.Y.; Xu, H.L.; Gao, L.; Fu, X.M.; Wang, X.; Wang, Q.; Shen, J.G.; Fang, J.S.; Fang, S.H. Bushen-Yizhi formula ameliorates mitochondrial dysfunction and oxidative stress via AMPK/Sirt1 signaling pathway in D-gal-induced aging rats. Chin. Med. 2023, 18, 53. [Google Scholar] [CrossRef]
- Xiong, X.C.; Wang, Y.P.; Wang, G.; Zhang, T.; Bao, Y.L.; Ainiwaer, D.N.; Sun, Z. Ferrostatin-1 delay D-gal induced cardiomyocyte senescence by inhibiting ferroptosis. Tianjin Med. J. 2023, 51, 19–23. [Google Scholar]
- Hou, J.Y.; Li, M.H.; Xu, M.R.; Ge, J.H.; Shen, Y.; Li, T.; Sun, X. Protective effect of Bupleurum chinense polysaccharide onoxidative damage of HK-2 cells induced by D-gal. J. Jilin Univ. (Med. Ed.) 2022, 48, 65–73. [Google Scholar]
- Chen, Q.N.; Fan, Z.; Lyu, A.K.; Wu, J.; Guo, A.; Yang, Y.F.; Chen, J.L.; Xiao, Q. Effect of sarcolipin-mediated cell transdifferentiation in sarcopenia-associated skeletal muscle fibrosis. Exp. Cell Res. 2020, 389, 111890. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.; Davies, M.J.; Dean, R.T. Protein oxidation and ageing. Exp. Gerontol. 2001, 36, 1503–1518. [Google Scholar] [CrossRef]
- Ran, K.J.; Wang, J.; Li, D.W.; Jiang, Z.J.; Ding, B.Y.; Yu, F.N.; Hu, S.K.; Wang, L.F.; Sun, W.W.; Xu, H.L. Sustained-release of SOD from multivesicular liposomes accelerated the colonic mucosal healing of colitis mice by inhibiting oxidative stress. Colloids Surf. B Biointerfaces 2024, 243, 114143. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. Nox4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, L.; Huang, S.X.; Ke, J.F.; Wang, Q.; Zhou, Z.W.; Chang, W. Lutein attenuates angiotensin II- induced cardiac remodeling by inhibiting AP-1/IL-11 signaling. Redox Biol. 2021, 44, 102020. [Google Scholar] [CrossRef]
- Xu, M.R.; Wang, J.Q.; Gao, J.W.; Ge, J.H.; Shen, Y.; Li, T.; Sun, X. Optimization of Extraction Technology of Schisandra chinensis Polysaccharide and Its Protective Effect on LPS Induced Mitochondrial Membrane Potential in Macrophages. Sci. Technol. Food Ind. 2020, 41, 33–40. [Google Scholar]
- Xie, D.L.; Hu, J.C.; Yang, Z.H.; Wu, T.; Xu, W.; Meng, Q.Y.; Cao, K.L.; Luo, X.G. Vitamin supplementation protects against nanomaterial-induced oxidative stress and inflammation damages: A meta-analysis of in vitro and in vivo studies. Nutrients 2022, 14, 2214. [Google Scholar] [CrossRef] [PubMed]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Fan, C.Y.; Han, J.; Liu, X.J.; Zhang, F.; Long, Y.S.; Xie, Q.T. Modulation of hypoxia-inducible factor-1 α/cyclo-oxygenase-2 pathway associated with attenuation of intestinal mucosa inflammatory damage by Acanthopanax senticosus polysaccharides in lipopolysaccharide-challenged piglets. Br. J. Nutr. 2019, 122, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.R.; Tian, L.L.; Kou, W.Z.; Cao, G.Z.; Wang, L.J.; Xia, Y.F.; Lin, Y.D.; Tang, S.H.; Zhang, J.J.; Yang, H.J. Xiyangshen Sanqi Danshen granules attenuated D-gal-induced C57BL/6J mouse aging through the AMPK/SIRT1 signaling pathway. Phytomedicine 2025, 136, 156213. [Google Scholar] [CrossRef]
- Zou, M.; Wang, D.; Chen, Y.Y.; Yang, C.; Xu, S.J.; Dai, Y. Dajianzhong decoction ameliorated D-gal-induced cognitive aging by triggering mitophagy in vivo and in vitro. J. Ethnopharmacol. 2024, 319, 117212. [Google Scholar] [CrossRef]
- Venditti, P.; Di, M.S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef] [PubMed]
- Gitego, N.; Agianian, B.; Mak, O.W.; Kumar, M.V.; Cheng, E.H.; Gavathiotis, E. Chemical modulation of cytosolic BAX homodimer potentiates BAX activation and apoptosis. Nat. Commun. 2023, 14, 8381. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, J.L.; Wang, W.; Mao, Z.B.; Wang, D.D.; Zhang, T.; Zhang, P.X. Oleanolic Acid Slows Down Aging Through IGF-1 Affecting the PI3K/AKT/mTOR Signaling Pathway. Molecules 2025, 30, 740. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.Y.; Yan, H.Y.; Zhou, T.L.; Zheng, L.M.; Wen, F.; Guo, G.H.; Zhang, Z.W. Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging and mitochondrial dysfunction via PI3K/Akt/mTOR signaling pathway. Phytomedicine 2025, 136, 156316. [Google Scholar] [CrossRef]
- Liu, S.F.; Liu, T.T.; Li, J.W.; Hong, J.; Moosavi-Movahedi, A.A.; Wei, J.S. Moosavi-Movahedi, A.A.; Wei, J. Type 2 Diabetes Mellitus Exacerbates Pathological Processes of Parkinson’s Disease: Insights from Signaling Pathways Mediated by Insulin Receptors. Neurosci. Bull. 2025, 41, 676–690. [Google Scholar] [CrossRef]
- Zhang, R.A.; Wang, P.; Jin, Y.; Xie, Q.Y.; Xiao, P.X. Imperatorin’s Effect on Myocardial Infarction Based on Network Pharmacology and Molecular Docking. Cardiovasc. Ther. 2025, 2025, 7551459. [Google Scholar] [CrossRef]

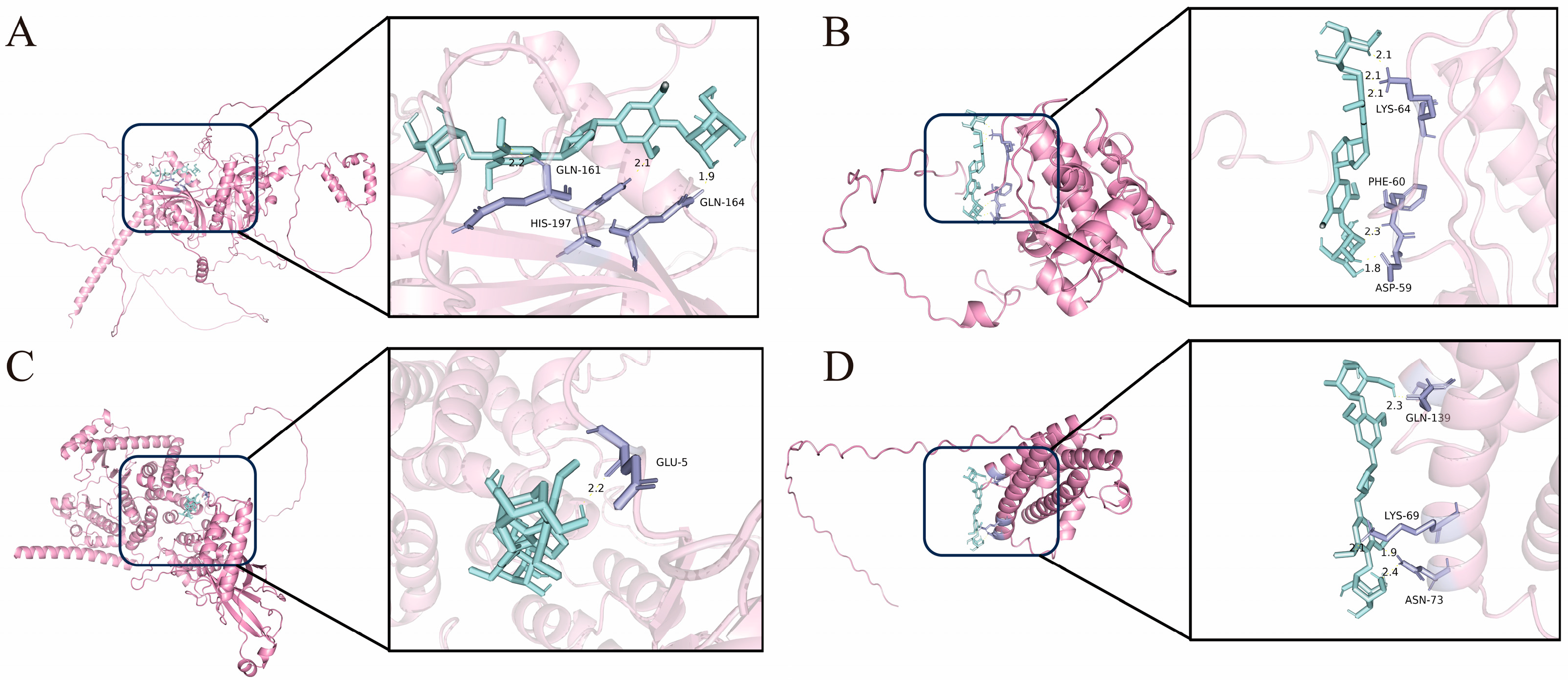

| Ingredients | Target Point | Binding Energy (kJ/mol) |
|---|---|---|
| EE | HIF1A | −4.62 |
| AKT1 | −5.92 | |
| PI3Kγ | −5.13 | |
| IL-6 | −5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Han, L.; Xu, M.; Feng, Y.; Liu, C.; Zhao, Y.; Zhang, M.; Xu, G.; Sun, X. Eleutheroside E Ameliorates D-Gal-Induced Senescence in Human Skin Fibroblasts Through PI3K/AKT Signaling. Curr. Issues Mol. Biol. 2025, 47, 895. https://doi.org/10.3390/cimb47110895
Ma X, Han L, Xu M, Feng Y, Liu C, Zhao Y, Zhang M, Xu G, Sun X. Eleutheroside E Ameliorates D-Gal-Induced Senescence in Human Skin Fibroblasts Through PI3K/AKT Signaling. Current Issues in Molecular Biology. 2025; 47(11):895. https://doi.org/10.3390/cimb47110895
Chicago/Turabian StyleMa, Xiangyu, Liu Han, Mengran Xu, Yuling Feng, Changsheng Liu, Yida Zhao, Min Zhang, Guanghua Xu, and Xin Sun. 2025. "Eleutheroside E Ameliorates D-Gal-Induced Senescence in Human Skin Fibroblasts Through PI3K/AKT Signaling" Current Issues in Molecular Biology 47, no. 11: 895. https://doi.org/10.3390/cimb47110895
APA StyleMa, X., Han, L., Xu, M., Feng, Y., Liu, C., Zhao, Y., Zhang, M., Xu, G., & Sun, X. (2025). Eleutheroside E Ameliorates D-Gal-Induced Senescence in Human Skin Fibroblasts Through PI3K/AKT Signaling. Current Issues in Molecular Biology, 47(11), 895. https://doi.org/10.3390/cimb47110895






