Myocardium miRNA Analysis Reveals Potential Biomarkers of Sudden Coronary Death in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Modeling
2.3. Sample Extraction and Preparation
2.4. RNA Isolation and Sequencing
2.5. HE Staining
2.6. MiRNA Data Preprocessing
- (1)
- The joint was removed.
- (2)
- Sequences shorter than 18 nucleotides or longer than 30 nucleotides were removed.
- (3)
- Reads with more than 10% unknown bases were removed.
- (4)
- Sequences with a low quality value for each sample were removed.
2.7. Identification of miRNAs
2.8. Bioinformation Analysis
2.9. Statistical Analysis
3. Results
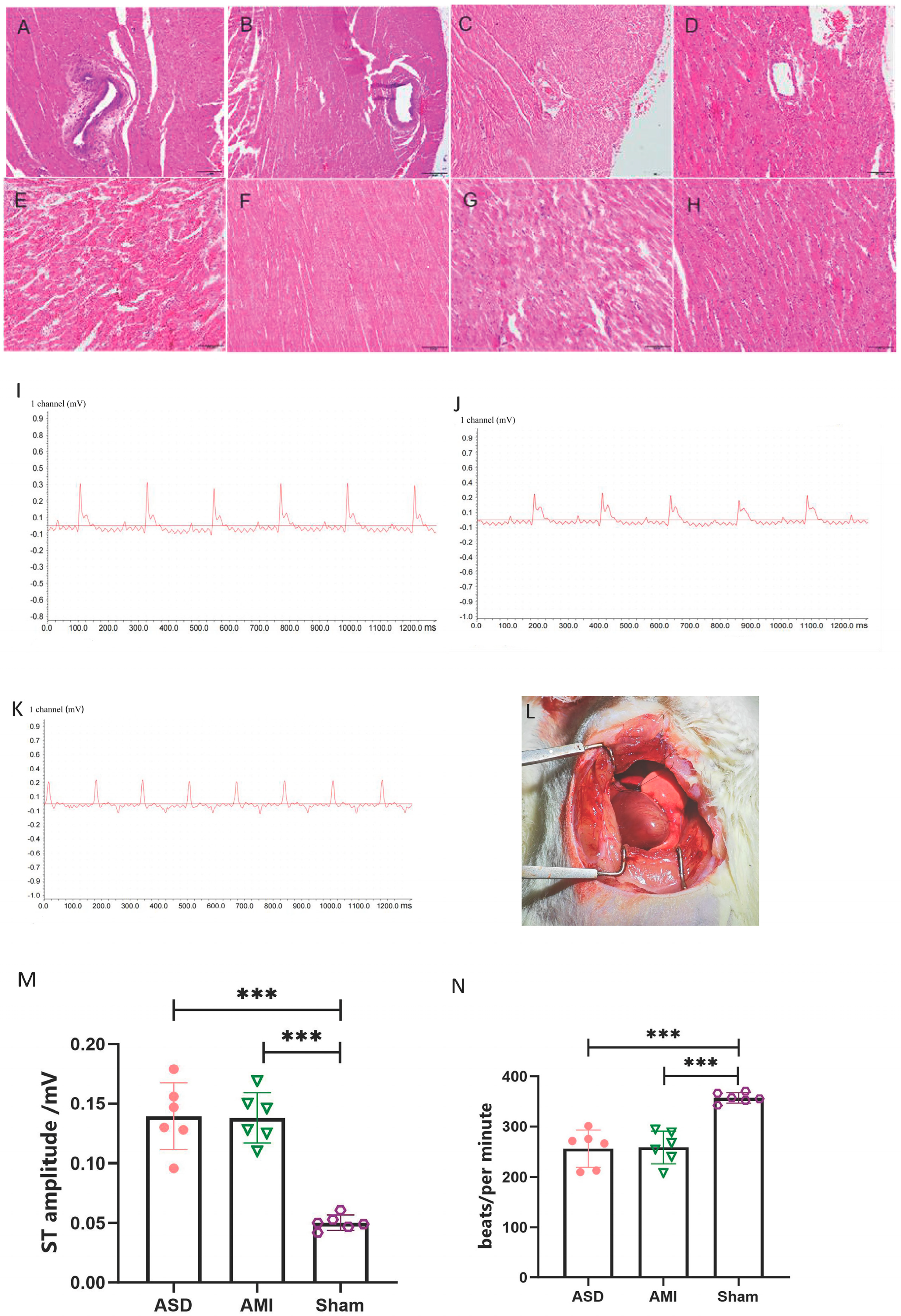
3.1. Successful Development of Rat SCD Model
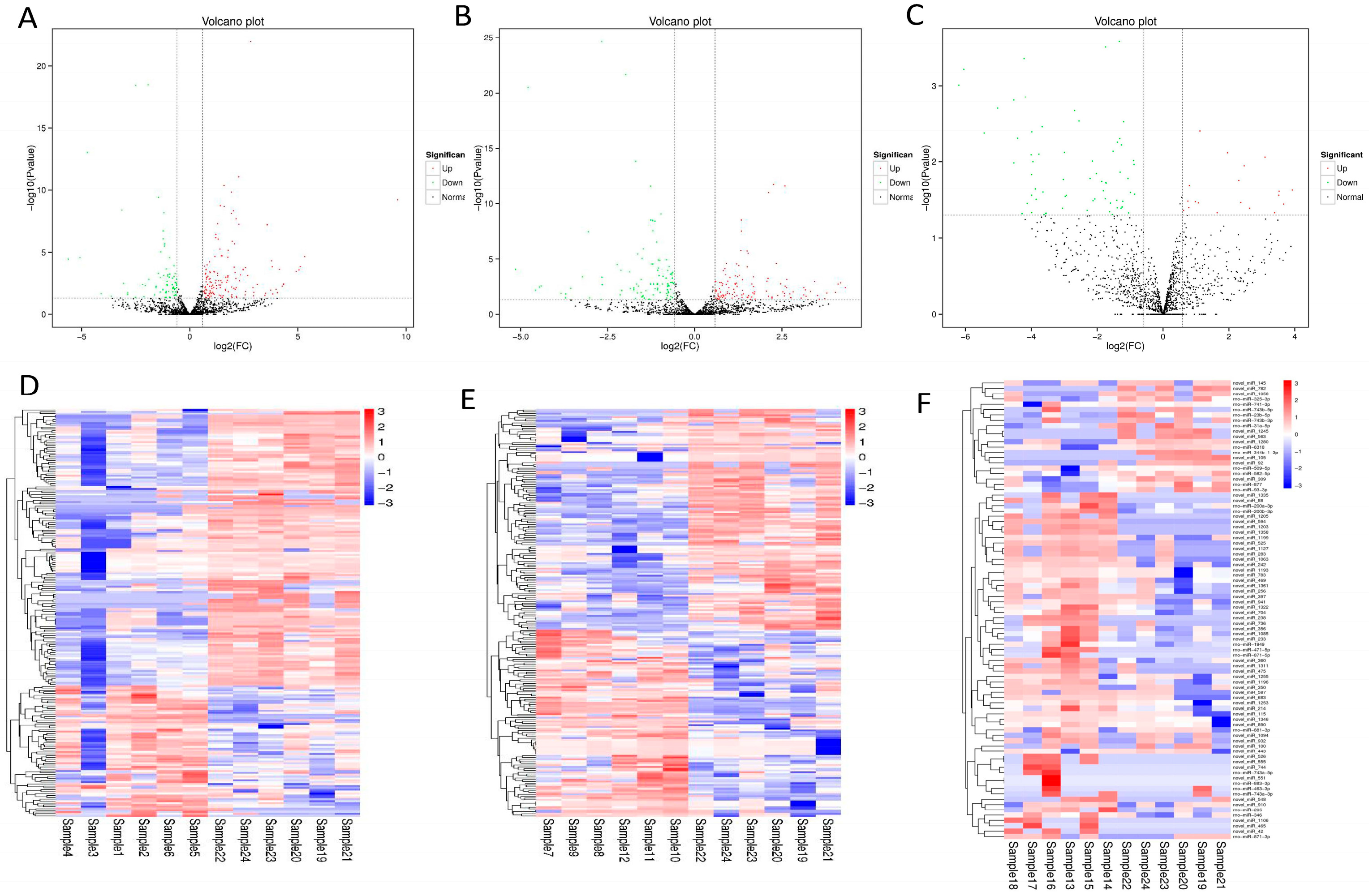
3.2. Differential Expression of miRNA in Rat Myocardium
3.3. Target Gene Analysis of Differential miRNA
3.4. miRNA-mRNA Regulatory Network and Key miRNA or Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Semsarian, C.; Ingles, J. Molecular autopsy in victims of inherited arrhythmias. J. Arrhythm. 2016, 32, 359–365. [Google Scholar] [CrossRef]
- Kumar, A.; Avishay, D.M.; Jones, C.R.; Shaikh, J.D.; Kaur, R.; Aljadah, M.; Kichloo, A.; Shiwalkar, N.; Keshavamurthy, S. Sudden cardiac death: Epidemiology, pathogenesis and management. Rev. Cardiovasc. Med. 2021, 22, 147–158. [Google Scholar] [CrossRef]
- Zipes, D.P.; Wellens, H.J.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Ooi, E.L.; Rajendran, S. Obstructive Sleep Apnea in Coronary Artery Disease. Curr. Probl. Cardiol. 2023, 48, 101178. [Google Scholar] [CrossRef] [PubMed]
- Cohle, S.D.; Sampson, B.A. The negative autopsy: Sudden cardiac death or other? Cardiovasc. Pathol. 2001, 10, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Osman, J.; Tan, S.C.; Lee, P.Y.; Low, T.Y.; Jamal, R. Sudden Cardiac Death (SCD)–risk stratification and prediction with molecular biomarkers. J. Biomed. Sci. 2019, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Santisukwongchote, K.; Amornlertwatana, Y.; Sastraruji, T.; Jaikang, C. Possible Use of Blood Tryptophan Metabolites as Biomarkers for Coronary Heart Disease in Sudden Unexpected Death. Metabolites. 2019, 10, 6. [Google Scholar] [CrossRef]
- Burke, A.P.; Tracy, R.P.; Kolodgie, F.; Malcom, G.T.; Zieske, A.; Kutys, R.; Pestaner, J.; Smialek, J.; Virmani, R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: Association with different pathologies. Circulation 2002, 105, 2019–2023. [Google Scholar] [CrossRef]
- Fasanaro, P.; Greco, S.; Ivan, M.; Capogrossi, M.C.; Martelli, F. microRNA: Emerging therapeutic targets in acute ischemic diseases. Pharmacol. Ther. 2010, 125, 92–104. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, D.; Jia, D.; Zhang, X.; Wu, A.; Lou, L.; Zhao, M.; Zhao, M.; Gao, Y.; Wang, M.; et al. Bioinformatics prediction and experimental verification of a novel microRNA for myocardial fibrosis after myocardial infarction in rats. PeerJ 2023, 11, e14851. [Google Scholar] [CrossRef]
- Jung, M.; Schaefer, A.; Steiner, I.; Kempkensteffen, C.; Stephan, C.; Erbersdobler, A.; Jung, K. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin. Chem. 2010, 56, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Hanson, E.K.; Ballantyne, J. Circulating microRNA for the identification of forensically relevant body fluids. Methods Mol. Biol. 2013, 1024, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.; Zeng, Y.; Lv, Y.; Tao, L.; Ma, J.; Xu, H.; Ma, K.; Shi, Q.; Xiao, B.; et al. Identification of the miRNA-3185/CYP4A11 axis in cardiac tissue as a biomarker for mechanical asphyxia. Forensic Sci. Int. 2020, 311, 110293. [Google Scholar] [CrossRef]
- Lv, Y.H.; Ma, J.L.; Pan, H.; Zeng, Y.; Tao, L.; Zhang, H.; Li, W.C.; Ma, K.J.; Chen, L. Estimation of the human postmortem interval using an established rat mathematical model and multi-RNA markers. Forensic Sci. Med. Pathol. 2017, 13, 20–27. [Google Scholar] [CrossRef]
- Soh, J.; Iqbal, J.; Queiroz, J.; Fernandez-Hernando, C.; Hussain, M.M. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat. Med. 2013, 19, 892–900. [Google Scholar] [CrossRef]
- Guo, X.J.; Li, H.; Bai, Y.Q.; Wu, P.; Zhao, C.M.; Dong, Y.M.; Chen, N.N.; Yun, K.M.; Gao, C.R. Screening of biomarkers related to sudden coronary death based on mRNA expression profile in rat myocardial tissue. J. Forensic Med. 2022, 38, 443–451. [Google Scholar]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.S.; Anderson, N.N.; Wagschal, A.; de Cabo, R.; et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; deLemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Sacco, J.; Adeli, K. MicroRNAs: Emerging roles in lipid and lipoprotein metabolism. Curr. Opin. Lipidol. 2012, 23, 220–225. [Google Scholar] [CrossRef]
- Vickers, K.C.; Landstreet, S.R.; Levin, M.G.; Shoucri, B.M.; Toth, C.L.; Taylor, R.C.; Palmisano, B.T.; Tabet, F.; Cui, H.L.; Rye, K.A.; et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 14518–14523. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Christenson, R.H.; Kandil, H.; El-Khazragy, N.; Rashed, L.; Yacoub, B.; Eldeeb, H.; Ali, M.; Sharafieh, R.; Klueh, U.; et al. Nourin-Dependent miR-137 and miR-106b: Novel Early Inflammatory Diagnostic Biomarkers for Unstable Angina Patients. Biomolecules 2021, 11, 368. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Christenson, R.H.; Kandil, H.; Ibrahim, M.; Rizk, H.; El-Khazragy, N.; Rashed, L.; Yacoub, B.; Eldeeb, H.; Ali, M.M.; et al. Nourin-Dependent miR-137 and miR-106b: Novel Biomarkers for Early Diagnosis of Myocardial Ischemia in Coronary Artery Disease Patients. Diagnostics 2021, 11, 703. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wang, X.; Song, H. LncRNA UCA1, miR-26a, and miR-195 in coronary heart disease patients: Correlation with stenosis degree, cholesterol levels, inflammatory cytokines, and cell adhesion molecules. J. Clin. Lab. Anal. 2022, 36, e24070. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Wang, Y.; Sun Phd, L.; Liu, C.; Zhang, H.; Qin, W.; Liu, J.; Han, L.; Shan, W. miR-195-3p/BDNF axis regulates hypoxic injury by targeting P-ERK1/2 expression. Medicine 2022, 101, e31586. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, P. MiR-33 may be a Biological Marker for Coronary Heart Disease. Clin. Lab. 2018, 64, 1755–1760. [Google Scholar] [CrossRef]
- Pilbrow, A.P.; Cordeddu, L.; Cameron, V.A.; Frampton, C.M.; Troughton, R.W.; Doughty, R.N.; Whalley, G.A.; Ellis, C.J.; Yandle, T.G.; Richards, A.M.; et al. Circulating miR-323-3p and miR-652: Candidate markers for the presence and progression of acute coronary syndromes. Int. J. Cardiol. 2014, 176, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Y.; Wang, F.; Qin, M.; Ren, L. MiRNA-205-5p regulates the ERBB4/AKT signaling pathway to inhibit the proliferation and migration of HAVSMCs induced by ox-LDL. Pathol. Res. Pract. 2022, 233, 153858. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.T.; Liu, Y.X.; Wang, X.C.; Xu, B.L.; Chen, Z.C.; Lu, H.A.; Zhang, M. LncRNA HOXA-AS2 accelerates the proliferation and migration and inhibits the apoptosis of vascular smooth muscle cells by absorbing miRNA-877-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 362–368. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, B.J.; Ma, M.; Yu, K.; Zhang, Q.; Zhang, X.W. MicroRNA-325-3p protects the heart after myocardial infarction by inhibiting RIPK3 and programmed necrosis in mice. BMC Mol. Biol. 2019, 20, 17, Erratum in BMC Mol. Biol. 2019, 20, 18. [Google Scholar]
- Yan, D.; Zhao, L.L.; Yue, B.W.; Qian, H.; Zhang, Z.H.; Wang, N.; Yan, S.H.; Qian, Y.L. Granule of BU-XIN RUAN-MAI Attenuates the Patients’ Angina Pectoris of Coronary Heart Disease via Regulating miR-542-3p/GABARAP Signaling. Evid.-Based Complement. Altern. Med. 2019, 2019, 1808419. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Wang, D.; He, K. MicroRNA-520d-3p alleviates hypoxia/reoxygenation-induced damage in human cardiomyocytes by targeting ATG-12. J. Thromb. Thrombolysis. 2021, 52, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tong, Y.; Zhou, Y.; Han, Z.; Wang, X.; Ding, T.; Qu, Y.; Zhang, Z.; Chang, C.; Zhang, X.; et al. LncRNA KCNQ1OT1 as a miR-26a-5p sponge regulates ATG12-mediated cardiomyocyte autophagy and aggravates myocardial infarction. Int. J. Cardiol. 2021, 338, 14–23. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kato, H.; Fujita, T. Physiological functions of RIG-I-like receptors. Immunity 2024, 57, 731–751. [Google Scholar] [CrossRef]
- Chu, C.; Liu, S.; Nie, L.; Hu, H.; Liu, Y.; Yang, J. The interactions and biological pathways among metabolomics products of patients with coronary heart disease. Biomed. Pharmacother. 2024, 173, 116305. [Google Scholar] [CrossRef]
- Zhao, L.L.; Qiu, X.J.; Wang, W.B.; Li, R.M.; Wang, D.S. NMR Metabolomics and Random Forests Models to Identify Potential Plasma Biomarkers of Blood Stasis Syndrome With Coronary Heart Disease Patients. Front. Physiol. 2019, 10, 1109. [Google Scholar] [CrossRef]
- Volodko, O.; Volinsky, N.; Yarkoni, M.; Margalit, N.; Kusniec, F.; Sudarsky, D.; Elbaz-Greener, G.; Carasso, S. Amir OCharacterization of Systemic Culprit-Coronary Artery miR-483-5p Expression in Chronic CAD, Acute Myocardial Infarction Male Patients. Int. J. Mol. Sci. 2023, 24, 8551. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; He, M.; Li, J.; Pessentheiner, A.; Wang, C.; Zhang, J.; Sun, Y.; Wang, W.T.; Zhang, Y.; Liu, J.; et al. microRNA-483 ameliorates hypercholesterolemia by inhibiting PCSK9 production. JCI Insight 2020, 5, e143812. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Harrison, B.C.; Jeong, M.Y.; Reid, B.G.; Wempe, M.F.; Wagner, F.F.; Holson, E.B.; McKinsey, T.A. Signal-dependent repression of DUSP5 by class I HDACs controls nuclear ERK activity and cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2013, 110, 9806–9811. [Google Scholar] [CrossRef]
- Peng, M.; Sun, R.; Hong, Y.; Wang, J.; Xie, Y.; Zhang, X.; Li, J.; Guo, H.; Xu, P.; Li, Y.; et al. Extracellular vesicles carrying proinflammatory factors may spread atherosclerosis to remote locations. Cell. Mol. Life Sci. 2022, 79, 430. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Han, W. Identification of necroptosis-related diagnostic biomarkers in coronary heart disease. Heliyon 2024, 10, e30269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, S.; Yang, L.; Xiang, D. microRNA-363-3p reduces endothelial cell inflammatory responses in coronary heart disease via inactivation of the NOX4-dependent p38 MAPK axis. Aging 2021, 13, 11061–11082. [Google Scholar] [CrossRef] [PubMed]


| AS/ASD Group | AMI/Sham Group | |
|---|---|---|
| TC (mmol/L) | 3.93 ± 0.56 * | 1.96 ± 0.14 |
| TG (mmol/L) | 2.17 ± 0.24 * | 1.52 ± 0.65 |
| LDL (mmol/L) | 1.07 ± 0.22 * | 0.18 ± 0.07 |
| HDL (mmol/L) | 0.69 ± 0.12 * | 0.5 ± 0.03 |
| AI (mmol/L) | 4.77 ± 0.47 * | 2.92 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhou, X.; Bai, Y.; Zhao, Z.; Zhang, H.; Gao, C.; Yun, K.; Guo, X. Myocardium miRNA Analysis Reveals Potential Biomarkers of Sudden Coronary Death in Rats. Curr. Issues Mol. Biol. 2025, 47, 889. https://doi.org/10.3390/cimb47110889
Zhao C, Zhou X, Bai Y, Zhao Z, Zhang H, Gao C, Yun K, Guo X. Myocardium miRNA Analysis Reveals Potential Biomarkers of Sudden Coronary Death in Rats. Current Issues in Molecular Biology. 2025; 47(11):889. https://doi.org/10.3390/cimb47110889
Chicago/Turabian StyleZhao, Chunmei, Xinyu Zhou, Yaqin Bai, Zhenxiang Zhao, Huaping Zhang, Cairong Gao, Keming Yun, and Xiangjie Guo. 2025. "Myocardium miRNA Analysis Reveals Potential Biomarkers of Sudden Coronary Death in Rats" Current Issues in Molecular Biology 47, no. 11: 889. https://doi.org/10.3390/cimb47110889
APA StyleZhao, C., Zhou, X., Bai, Y., Zhao, Z., Zhang, H., Gao, C., Yun, K., & Guo, X. (2025). Myocardium miRNA Analysis Reveals Potential Biomarkers of Sudden Coronary Death in Rats. Current Issues in Molecular Biology, 47(11), 889. https://doi.org/10.3390/cimb47110889






