HMGB1 and Its Signaling Pathway in Osteosarcoma: Current Advances in Targeted Therapy
Abstract
1. Introduction
2. HMGB1: From Structural Features to Tumorigenic Signaling
2.1. Molecular Structure of HMGB1
2.2. Biological Effects of HMGB1 in Tumors
3. Osteosarcoma: Clinical Hallmarks and Pivotal Signaling Pathways
3.1. Clinical Characteristics and Therapeutic Dilemmas
3.2. Osteosarcoma-Associated Core Signaling Pathways
| Research Object | Target/Pathway | Function | Reference |
|---|---|---|---|
| ZIP10 | PI3K/AKT | Promotes OS proliferation and chemotherapy resistance | [24] |
| MARK2 | PI3K/AKT/NF-kB | Leads to resistance to cisplatin chemotherapy | [25] |
| EGFR | PI3K/AKT | Promotes OS progression and leads to gemcitabine resistance | [26] |
| Serglycin | JAK/STAT | Promotes OS proliferation, migration and invasion | [27] |
| miR-181a-5p | PTEN-AKT | Promotes OS progress | [28] |
| miR-210-5p | PIK3R5, AKT/mTOR | Promotes OS EMT and oncogenic autophagy | [29] |
| miR-134-5p | ITGB1/MMP2/PI3K/AKT | Inhibits malignant OS phenotype | [30] |
| lncRNA SNHG10 | Wnt/β-catenin | Promotes proliferation and invasion of OS | [31] |
| USP3 | PI3K/AKT | Promotes OS proliferation and metastasis | [32] |
| microRNA-101 | PI3K/AKT, JAK/STAT | Inhibits OS tumor growth and metastasis | [33] |
| GABPB1-AS1 | SP1/Wnt/β-catenin | Competitive binding and inhibition of miR-199a-3p promote OS progression | [34] |
| COL5A2 | TGF-β, Wnt/β-catenin | Inhibits OS cell invasion and metastasis | [35] |
| CCR9 | Wnt/β-catenin | Promotes OS transfer | [36] |
| JAG1 | Notch | Promotes metastasis and recurrence of OS | [37] |
| YAP/TAZ | Hippo | Regulates cell proliferation and cell survival to stop organ overgrowth | [38] |
| ZFP36L1 | SDC4-TGF-β | Inhibits OS lung metastasis | [39] |
| ICSBP | PD-L1 | Promotes OS growth in vitro and in vivo | [40] |
| miR-138 | DEC2 | Inhibits OS proliferation and invasion | [41] |
| Loxl2 | Wnt | Promotes invasiveness of OS | [42] |
| Curcumin | Nrf2/GPX4 | Induces iron death and apoptosis in OS cells | [43] |
4. Therapeutic Strategies for Osteosarcoma Targeting HMGB1 and Its Signaling Pathway
4.1. Advances in HMGB1 and Its Signaling Pathway in OS
4.1.1. HMGB1/RAGE Pathway
4.1.2. HMGB1/TLR4 Pathways
4.1.3. Integrin-Dependent Signaling Pathway
4.1.4. Non-Coding RNA Regulatory Network
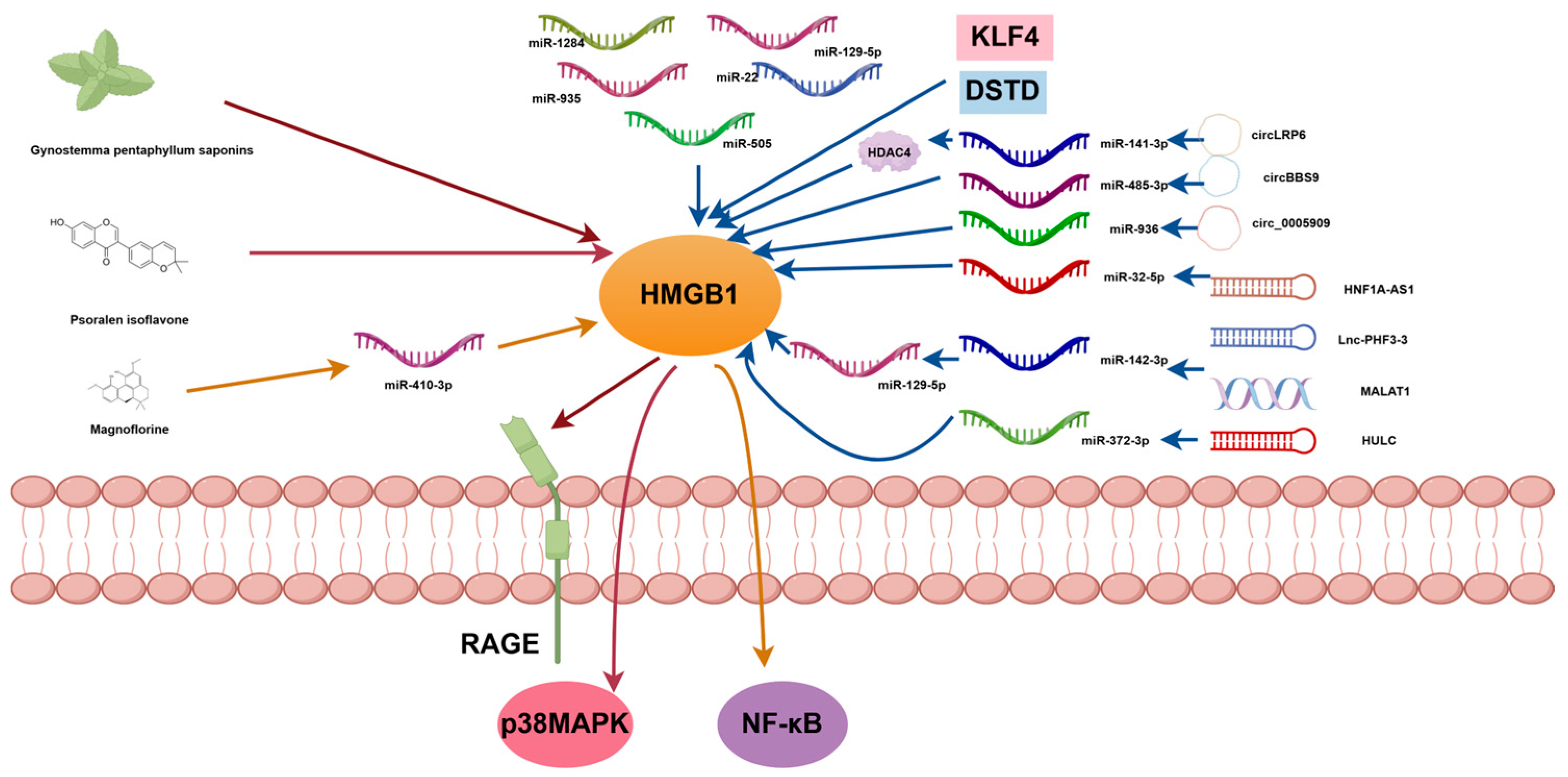
4.2. Therapeutic Strategies Targeting HMGB1 and Its Signaling Pathways
4.2.1. Inhibition of HMGB1 Expression in OS
4.2.2. Target Downstream Signaling Pathways of HMGB1 in OS
| Research Object | Target/Pathway | Function | Reference |
|---|---|---|---|
| miR-505 | HMGB1 | Inhibits OS cell proliferation and invasion and promotes cell apoptosis | [61] |
| Gynostemma pentaphyllum saponins | HMGB1-RAGE | Inhibits malignant biological behavior of OS cells | [59] |
| Psoralen isoflavone | p38 MAPK/HMGB1 | Inhibits OS cell migration and invasion | [67] |
| Magnoflorine | miR-410-3p/HMGB1/NF-κB | Inhibits the malignant phenotype of OS cells and increases sensitivity to cisplatin | [72] |
| miR-1284 | HMGB1 | Inhibits OS cell proliferation and migration | [62] |
| miR-129-5p | HMGB1 | Inhibits OS cell proliferation and migration and promotes cell apoptosis | [60] |
| miR-935 | HMGB1 | Inhibits OS cell proliferation and migration | [63] |
| miR-22 | HMGB1 | Inhibits OS cell proliferation and migration | [64] |
| KLF4 | HMGB1 | Leads to OS chemotherapy resistance | [65] |
| DSTD | HMGB1 | Enhances OS chemotherapy sensitivity | [66] |
| circLRP6 | miR-141-3p/HDAC4/HMGB1 | Promotes OS progress | [45] |
| circBBS9 | miR-485-3p/HMGB1 | Promotes OS progress | [73] |
| circ_0005909 | miR-936/HMGB1 | Inhibits OS progress | [55] |
| HNF1A-AS1 | miR-32-5p/HMGB1 | Inhibits OS progress | [69] |
| MALAT1 | miR-142-3p/miR-129-5p/HMGB1 | Promotes OS progress | [70] |
| Lnc-PHF3-3 | miR-142-3p/HMGB1 | Leads to OS chemotherapy resistance | [6] |
| HULC | miR-372-3p/HMGB1 | Promotes OS progress | [71] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OS | Osteosarcoma |
| HMGB1 | High mobility group protein B1 |
| ncRNA | Non-coding RNA |
| MMP-9 | Matrix metalloproteinase-9 |
| RAGE | Receptor for advanced glycosylation end products |
| TLR4 | Toll-like Receptor 4 |
| miRNAs | MicroRNAs |
| MDSCs | Myeloid-derived suppressor cells |
References
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef]
- Yu, S.; Yao, X. Advances on immunotherapy for osteosarcoma. Mol. Cancer 2024, 23, 192. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Gao, B.; Ma, M.; Chen, M.; Wu, Y.; Zhang, W.; Sun, Y.; Liu, S.; Shen, H. KDM6B-mediated histone demethylation of LDHA promotes lung metastasis of osteosarcoma. Theranostics 2021, 11, 3868–3881. [Google Scholar] [CrossRef]
- Yan, P.; Wang, J.; Yue, B.; Wang, X. Unraveling molecular aberrations and pioneering therapeutic strategies in osteosarcoma. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189171. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Lu, M.; Lei, Z.; Dai, S.; Chen, W.; Du, S.; Jin, Q.; Zhou, Z.; Li, H. HMGB1 Positive Feedback Loop Between Cancer Cells and Tumor-Associated Macrophages Promotes Osteosarcoma Migration and Invasion. Lab. Investig. 2023, 103, 100054. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, M.; Zhao, W.; Zhang, H.; Cao, L.; Li, Q.; Wang, G. Lnc-PHF3-3 aggravates the chemoresistance of osteosarcoma cells to doxorubicin via the miR-142-3p/HMGB1 axis. Transl. Oncol. 2025, 53, 102328. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Forveille, S.; Zhao, L.; Sauvat, A.; Cerrato, G.; Leduc, M.; Doffe, F.; Pan, Y.; Liu, P.; Kroemer, G.; Kepp, O. Patritumab deruxtecan induces immunogenic cell death. Oncoimmunology 2025, 14, 2514050. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Manfredi, A.A.; Bianchi, M.E.; Rovere-Querini, P. HMGB1: An immmune odyssey. Discov. Med. 2005, 5, 388–392. [Google Scholar]
- Kwak, M.S.; Kim, H.S.; Lee, B.; Kim, Y.H.; Son, M.; Shin, J.S. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front. Immunol. 2020, 11, 1189. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Jiang, S.; Yang, T.; Lan, J.; Lei, Y.; Tan, H.; Pan, K. HMGB1 mediates lipopolysaccharide-induced inflammation via interacting with GPX4 in colon cancer cells. Cancer Cell. Int. 2020, 20, 205. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef]
- Zaitsu, M.; Takeuchi, T.; Zaitsu, M.; Tonooka, A.; Uekusa, T.; Miyake, Y.; Kobayashi, Y.; Kobashi, G.; Kawachi, I. Occupational disparities in tumor grade and cytosolic HMGB1 expression in renal cell cancer. J. Occup. Health 2022, 64, e12340. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Lyu, Q.; Yi, R.; Sun, Y.; Zhang, W.; Wei, T.; Zhang, Y.; Shi, J.; Zhang, J. HMGB1 promotes chemoresistance in small cell lung cancer by inducing PARP1-related nucleophagy. J. Adv. Res. 2024, 66, 165–180. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, W.; Wang, J.; Chen, H.; Li, H.; Yin, Z.; Hao, J.; Chen, K. Nuclear HMGB1 is critical for CD8 T cell IFN-gamma production and anti-tumor immunity. Cell. Rep. 2024, 43, 114591. [Google Scholar] [CrossRef]
- Lv, D.J.; Song, X.L.; Huang, B.; Yu, Y.Z.; Shu, F.P.; Wang, C.; Chen, H.; Zhang, H.B.; Zhao, S.C. HMGB1 Promotes Prostate Cancer Development and Metastasis by Interacting with Brahma-Related Gene 1 and Activating the Akt Signaling Pathway. Theranostics 2019, 9, 5166–5182. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, Z.; Fan, T.M.; Irudayaraj, J.M.K. Osteosarcoma mechanobiology and therapeutic targets. Br. J. Pharmacol. 2022, 179, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex But Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Ning, B.; Liu, Y.; Huang, T.; Wei, Y. Autophagy and its role in osteosarcoma. Cancer Med. 2023, 12, 5676–5687. [Google Scholar] [CrossRef]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef]
- Wei, Z.; Xia, K.; Zheng, D.; Gong, C.; Guo, W. RILP inhibits tumor progression in osteosarcoma via Grb10-mediated inhibition of the PI3K/AKT/mTOR pathway. Mol. Med. 2023, 29, 133. [Google Scholar] [CrossRef]
- Synoradzki, K.J.; Bartnik, E.; Czarnecka, A.M.; Fiedorowicz, M.; Firlej, W.; Brodziak, A.; Stasinska, A.; Rutkowski, P.; Grieb, P. TP53 in Biology and Treatment of Osteosarcoma. Cancers 2021, 13, 4284. [Google Scholar] [CrossRef]
- Li, H.; Shen, X.; Ma, M.; Liu, W.; Yang, W.; Wang, P.; Cai, Z.; Mi, R.; Lu, Y.; Zhuang, J.; et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 2021, 40, 340. [Google Scholar] [CrossRef]
- Wei, X.; Xu, L.; Jeddo, S.F.; Li, K.; Li, X.; Li, J. MARK2 enhances cisplatin resistance via PI3K/AKT/NF-kappaB signaling pathway in osteosarcoma cells. Am. J. Transl. Res. 2020, 12, 1807–1823. [Google Scholar] [PubMed]
- Wang, S.; Wei, H.; Huang, Z.; Wang, X.; Shen, R.; Wu, Z.; Lin, J. Epidermal growth factor receptor promotes tumor progression and contributes to gemcitabine resistance in osteosarcoma. Acta Biochim. Biophys. Sin. 2021, 53, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Gao, G.; Guo, Y.; Zhang, Z.; Liu, R.; Dai, Z.; Ju, C.; Liang, Y.; Tang, X.; Tang, M.; et al. Serglycin promotes proliferation, migration, and invasion via the JAK/STAT signaling pathway in osteosarcoma. Aging 2021, 13, 21142–21154. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, C.; Chen, Z.; Guo, J.; Yu, Z.H.; Qian, W.; Ai, F.; Xiao, L.; Guo, X. MicroRNA-181a-5p Promotes Osteosarcoma Progression via PTEN/AKT Pathway. Anal. Cell. Pathol. 2022, 2022, 3421600. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, D.; Gong, F.; Huang, Y.; Luo, Y.; Rong, Y.; Wang, J.; Ge, X.; Ji, C.; Fan, J.; et al. miR-210-5p promotes epithelial-mesenchymal transition by inhibiting PIK3R5 thereby activating oncogenic autophagy in osteosarcoma cells. Cell Death Dis. 2020, 11, 93. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, R.; Feng, Y.; Li, R.; Zhang, L.; Pan, Y.; Qiao, X.; Li, P.; Wei, X.; Xu, C.; et al. MiR-134-5p inhibits the malignant phenotypes of osteosarcoma via ITGB1/MMP2/PI3K/Akt pathway. Cell Death Discov. 2024, 10, 193. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Wang, X.; Wang, J.; Xi, G. lncRNA SNHG10 Promotes the Proliferation and Invasion of Osteosarcoma via Wnt/β-Catenin Signaling. Mol. Ther. Nucleic Acids 2020, 22, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, S.; Nie, J.; Xiao, S.; Xie, X.; Zhang, Y.; Tong, W.; Yao, G.; Liu, N.; Dan, F.; et al. USP3 promotes osteosarcoma progression via deubiquitinating EPHA2 and activating the PI3K/AKT signaling pathway. Cell Death Dis. 2024, 15, 235. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am. J. Cancer Res. 2017, 7, 88–97. [Google Scholar]
- Chen, J.; Bian, M.; Pan, L.; Liu, C.; Yang, H. GABPB1-AS1 Promotes the Development of Osteosarcoma by Targeting SP1 and Activating the Wnt/beta-Catenin Pathway. J. Oncol. 2022, 2022, 8468896. [Google Scholar] [CrossRef]
- Han, Y.L.; Luo, D.; Habaxi, K.; Tayierjiang, J.; Zhao, W.; Wang, W.; Aikebaier, W.; Wang, L. COL5A2 Inhibits the TGF-beta and Wnt/beta-Catenin Signaling Pathways to Inhibit the Invasion and Metastasis of Osteosarcoma. Front. Oncol. 2022, 12, 813809. [Google Scholar] [CrossRef]
- Kong, H.; Yu, W.; Chen, Z.; Li, H.; Ye, G.; Hong, J.; Xie, Z.; Chen, K.; Wu, Y.; Shen, H. CCR9 initiates epithelial-mesenchymal transition by activating Wnt/beta-catenin pathways to promote osteosarcoma metastasis. Cancer Cell Int. 2021, 21, 648. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The role of Notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J. Orthop. Surg. Res. 2021, 16, 226. [Google Scholar] [CrossRef]
- Kovar, H.; Bierbaumer, L.; Radic-Sarikas, B. The YAP/TAZ Pathway in Osteogenesis and Bone Sarcoma Pathogenesis. Cells 2020, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhuang, J.; Li, H.; Mi, R.; Song, Y.; Yang, W.; Lu, Y.; Shen, X.; Wu, Y.; Shen, H. Low expression of ZFP36L1 in osteosarcoma promotes lung metastasis by inhibiting the SDC4-TGF-beta signaling feedback loop. Oncogene 2024, 43, 47–60. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, J.H.; Kang, H.G.; Park, J.W.; Park, S.Y.; Park, B.K.; Kim, Y.N. ICSBP-induced PD-L1 enhances osteosarcoma cell growth. Front. Oncol. 2022, 12, 918216. [Google Scholar] [CrossRef]
- Jiang, B.; Mu, W.; Wang, J.; Lu, J.; Jiang, S.; Li, L.; Xu, H.; Tian, H. MicroRNA-138 functions as a tumor suppressor in osteosarcoma by targeting differentiated embryonic chondrocyte gene 2. J. Exp. Clin. Cancer Res. 2016, 35, 69. [Google Scholar] [CrossRef]
- Matsuoka, K.; Bakiri, L.; Wolff, L.I.; Linder, M.; Mikels-Vigdal, A.; Patino-Garcia, A.; Lecanda, F.; Hartmann, C.; Sibilia, M.; Wagner, E.F. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020, 30, 885–901. [Google Scholar] [CrossRef]
- Yuan, C.; Fan, R.; Zhu, K.; Wang, Y.; Xie, W.; Liang, Y. Curcumin induces ferroptosis and apoptosis in osteosarcoma cells by regulating Nrf2/GPX4 signaling pathway. Exp. Biol. Med. 2023, 248, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Sroga, G.; Jaouni, S.K.A.; Mousa, S.A. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dong, G.; Li, Z.; Zheng, Y.; Shi, Z.; Wang, G. circ-LRP6 contributes to osteosarcoma progression by regulating the miR-141-3p/HDAC4/HMGB1 axis. Int. J. Oncol. 2022, 60, 38. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Gao, M.; Tang, X.; Jiao, M.; Wang, C.; Wei, Y.; Gong, Q.; Zhong, J. HMGB1/RAGE axis in tumor development: Unraveling its significance. Front. Oncol. 2024, 14, 1336191. [Google Scholar] [CrossRef]
- Chu, C.; Tang, C.; Xu, L.; Li, C.; Wang, Y. Association between Toll-like receptor 4 and pancreatic cancer. J. Clin. Hepatol. 2021, 37, 485–488. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Kang, Y.; Gunassekaran, G.R.; Lee, S.M.; Park, M.S.; Jo, D.G.; Kim, S.K.; Lee, H.; Kim, W.J.; Lee, B. IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis. Theranostics 2024, 14, 2605–2621. [Google Scholar] [CrossRef]
- Tachibana, M. The Immunosuppressive Function of Myeloid-derived Suppressor Cells Is Regulated by the HMGB1-TLR4 Axis. Yakugaku Zasshi 2018, 138, 143–148. [Google Scholar] [CrossRef]
- Pistoia, V.; Pezzolo, A. Involvement of HMGB1 in Resistance to Tumor Vessel-Targeted, Monoclonal Antibody-Based Immunotherapy. J. Immunol. Res. 2016, 2016, 3142365. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Tazawa, H.; Demiya, K.; Kure, M.; Kondo, H.; Komatsubara, T.; Sugiu, K.; Hasei, J.; Yoshida, A.; Kunisada, T.; et al. Telomerase-specific oncolytic immunotherapy for promoting efficacy of PD-1 blockade in osteosarcoma. Cancer Immunol. Immunother. 2021, 70, 1405–1417. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Y.; Wang, J.; Han, Y.; Ren, T.; Huang, Y.; Chen, C.; Huang, Q.; Wang, W.; Niu, J.; et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021, 21, 192. [Google Scholar] [CrossRef]
- Yang, D.; Liu, K.; Fan, L.; Liang, W.; Xu, T.; Jiang, W.; Lu, H.; Jiang, J.; Wang, C.; Li, G.; et al. LncRNA RP11-361F15.2 promotes osteosarcoma tumorigenesis by inhibiting M2-Like polarization of tumor-associated macrophages of CPEB4. Cancer Lett 2020, 473, 33–49. [Google Scholar] [CrossRef]
- Hu, S.; Yi, T.; Wang, J.; Liao, Q.; Ye, M. Research progress on long noncoding RNA in tumor immune microenvironment. Chin. Bull. Life Sci. 2022, 34, 212–219. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, G.; Gao, Y.; Chen, S.; Cao, C. Circular RNA hsa_circ_0005909 modulates osteosarcoma progression via the miR-936/HMGB1 axis. Cancer Cell Int. 2020, 20, 305. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, J.; Zhuang, Z.; Zhang, W.; Wang, Z.; Liu, Y.; Li, J.; Liang, T.; He, R.; Wang, K. Comprehensive Analysis of a ceRNA Network Identifies lncR-C3orf35 Associated with Poor Prognosis in Osteosarcoma. Biomed. Res. Int. 2020, 2020, 3178037. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Li, Q.; Zhou, D.; Liu, P. Expression of high mobility group box 1 protein predicts a poorer prognosis for patients with osteosarcoma. Oncol. Lett. 2016, 11, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhao, C.L.; Zhang, J.; Cheng, J.W.; Hu, J.P.; Yu, P.; Yang, M.H.; Xia, Y.Z.; Yin, Y.; Zhang, Z.Z.; et al. Enhancing immunogenicity and release of in situ-generated tumor vesicles for autologous vaccines. J. Control. Release 2025, 381, 113614. [Google Scholar] [CrossRef]
- Yi, Z.; Gao, J. Effect of Gynosaponin on the malignant biological behaviors of osteosarcoma cells by regulating the HMGB1-RAGE signaling pathway. Hebei Med. J. 2024, 46, 1451–1457. [Google Scholar]
- Zhang, H.; Yang, G.; Liang, W.; Xie, B.; Wang, Y. MiRNA-129-5p targeting HMGB1 inhibits proliferation and migration of osteosarcoma cells. Cancer Res. Prev. Treat. 2022, 49, 5–11. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Miao, J.; Hu, Y. miR-505 inhibits proliferation of osteosarcoma via HMGB1. FEBS Open Bio 2020, 10, 1251–1260. [Google Scholar] [CrossRef]
- Lv, S.; Guan, M. miRNA-1284, a regulator of HMGB1, inhibits cell proliferation and migration in osteosarcoma. Biosci. Rep. 2018, 38, BSR20171675. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Zhao, X.; Cui, B.; Zhang, L.; Wang, Q. MicroRNA-935 Inhibits Proliferation and Invasion of Osteosarcoma Cells by Directly Targeting High Mobility Group Box 1. Oncol. Res. 2018, 26, 1439–1446. [Google Scholar] [CrossRef]
- Guo, S.; Bai, R.; Liu, W.; Zhao, A.; Zhao, Z.; Wang, Y.; Wang, Y.; Zhao, W.; Wang, W. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol. 2014, 35, 7025–7034. [Google Scholar] [CrossRef]
- Huang, J.; Liu, K.; Song, D.; Ding, M.; Wang, J.; Jin, Q.; Ni, J. Kruppel-like factor 4 promotes high-mobility group box 1-induced chemotherapy resistance in osteosarcoma cells. Cancer Sci. 2016, 107, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, L.; Ju, Y.; Li, W.; Zhang, M.; Jiao, Y.; Zhang, J.; Wang, S.; Wang, Y.; Zhao, M.; et al. A novel androstenedione derivative induces ROS-mediated autophagy and attenuates drug resistance in osteosarcoma by inhibiting macrophage migration inhibitory factor (MIF). Cell Death Dis. 2014, 5, e1361. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Wang, H.; Cai, Z.; Zeng, Z. Mechanism of Corylin Inhibiting the Development of Osteosarcoma: Regulating HMGB1/p38 MAPK Signaling. Discov. Med. 2025, 37, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wei, X.; Gao, S.; Chen, W.; Geng, Y.; Liu, J.; Guan, H. Circ_LRP6 facilitates osteosarcoma progression via the miR-122-5p/miR-204-5p/HMGB1 axis. Environ. Toxicol. 2023, 38, 2462–2475. [Google Scholar] [CrossRef]
- Lou, P.; Ding, T.; Zhan, X. Long Noncoding RNA HNF1A-AS1 Regulates Osteosarcoma Advancement Through Modulating the miR-32-5p/HMGB1 Axis. Cancer Biother. Radiopharm. 2021, 36, 371–381. [Google Scholar] [CrossRef]
- Liu, K.; Huang, J.; Ni, J.; Song, D.; Ding, M.; Wang, J.; Huang, X.; Li, W. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle 2017, 16, 578–587. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.J.; Zhou, J.H.; Chen, R.; Cen, C.Q. LncRNA HULC induces the progression of osteosarcoma by regulating the miR-372-3p/HMGB1 signalling axis. Mol. Med. 2020, 26, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, G.; Wang, W.; Qiu, E.; Pei, Y.; Zhang, X. Magnoflorine inhibits the malignant phenotypes and increases cisplatin sensitivity of osteosarcoma cells via regulating miR-410-3p/HMGB1/NF-kappaB pathway. Life Sci. 2020, 256, 117967. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Xing, Y.; Zhang, X.; Wang, J.; Chen, B. CircBBS9 accelerates the malignant progression of osteosarcoma through sponging miR-485-3p/HMGB1 axis. J. Orthop. Sci. 2024, 29, 1130–1139. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.J.; Wang, C.Y.; Shen, M.Z.; Jiang, S.; Li, T.; Di, W.C.; Chen, Y.; et al. Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury. Acta Pharmacol. Sin. 2022, 43, 520–528. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, L.; Sun, Y.; Wang, X.; Shi, X. HMGB1 and Toll-like receptors: Potential therapeutic targets in autoimmune diseases. Mol. Med. 2023, 29, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Hindi, N.; Carrillo-Garcia, J.; Blanco-Alcaina, E.; Renshaw, M.; Luna, P.; Duran, J.; Jimenez, N.; Sancho, P.; Ramos, R.; Moura, D.S.; et al. Platinum-Based Regimens Are Active in Advanced Pediatric-Type Rhabdomyosarcoma in Adults and Depending on HMGB1 Expression. Int. J. Mol. Sci. 2023, 24, 856. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, D.; Jiang, X.; He, G. HMGB1: From Molecular Functions to Clinical Applications in Cancer and Inflammatory Diseases. Med. Res. Rev. 2025. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, F.; Zhou, Z.; Wu, M.; Huang, Q.; Jiang, X.; Wen, X.; Ye, L. HMGB1 and Its Signaling Pathway in Osteosarcoma: Current Advances in Targeted Therapy. Curr. Issues Mol. Biol. 2025, 47, 887. https://doi.org/10.3390/cimb47110887
Liu Z, Wang F, Zhou Z, Wu M, Huang Q, Jiang X, Wen X, Ye L. HMGB1 and Its Signaling Pathway in Osteosarcoma: Current Advances in Targeted Therapy. Current Issues in Molecular Biology. 2025; 47(11):887. https://doi.org/10.3390/cimb47110887
Chicago/Turabian StyleLiu, Zhuosheng, Fucai Wang, Zhihan Zhou, Mei Wu, Qinghua Huang, Xinpeng Jiang, Xuan Wen, and Liuting Ye. 2025. "HMGB1 and Its Signaling Pathway in Osteosarcoma: Current Advances in Targeted Therapy" Current Issues in Molecular Biology 47, no. 11: 887. https://doi.org/10.3390/cimb47110887
APA StyleLiu, Z., Wang, F., Zhou, Z., Wu, M., Huang, Q., Jiang, X., Wen, X., & Ye, L. (2025). HMGB1 and Its Signaling Pathway in Osteosarcoma: Current Advances in Targeted Therapy. Current Issues in Molecular Biology, 47(11), 887. https://doi.org/10.3390/cimb47110887






