Cellular and Molecular Pathways in Diabetes-Associated Heart Failure: Emerging Mechanistic Insights and Therapeutic Opportunities
Abstract
1. Introduction
2. Molecular and Cellular Mechanisms of Heart Failure in Diabetes Mellitus
2.1. Overview of Cardiac Energy Metabolism
2.2. Impaired Energy Metabolism in the Diabetic Heart
2.3. Mitochondrial Dysfunction in Diabetes-Related Heart Failure
2.4. Cardiac Endoplasmic Reticulum Stress (ERS) in Diabetes-Related Heart Failure
2.5. Role of Inflammation in Diabetes-Related Heart Failure
2.6. NF-κB–Mediated Inflammatory Cascade
2.7. RAAS Activation in the Diabetic Heart
- Direct hyperglycemia-induced Angiotensin II (Ang-II) synthesis—Elevated glucose levels stimulate cardiomyocyte production of Ang-II, largely through chymase-dependent pathways. Chymases, a family of serine proteases expressed in mast cells, fibroblasts, and vascular endothelial cells, facilitate intracellular Ang-II formation, which has been linked to apoptosis, oxidative stress, and myocardial fibrosis in diabetic models [65,66]. Chymase inhibition has shown notable therapeutic potential in halting cardiac and vascular injury in DM [67,68].
- Increased myocardial sensitivity to Ang-II—Hyperglycemia enhances tissue responsiveness to Ang-II, promoting more pronounced vasoconstriction and contractile responses. In experimental models, elevated glucose augmented Ang-II–mediated aortic contraction through activation of the angiotensin type-1 receptor (AT1R) [69].
- AGE-mediated RAAS stimulation—AGEs, formed under conditions of sustained hyperglycemia, oxidative stress, and dyslipidemia, can activate intracellular signaling cascades that promote pro-inflammatory mediator release, oxidative stress, and RAAS activation. Importantly, AGEs also potentiate chymase-dependent Ang-II production [70,71,72].
- Ang-II exerts multiple effects that initially serve as compensatory mechanisms, such as vasoconstriction, stimulation of growth factors, and promotion of vascular smooth muscle cell and fibroblast proliferation, but over time, these changes contribute to maladaptive remodeling, hypertrophy, and progression to HF [73].
2.8. Role of Autophagy in Diabetes-Related Heart Failure
- •
- Microautophagy involves direct invagination of the lysosomal or vacuolar membrane to engulf cytoplasmic material.
- •
- CMA selectively targets soluble cytosolic proteins for lysosomal degradation via chaperone proteins.
- •
- •
- •
- AMPK, an intracellular energy sensor, stimulates autophagy both directly, by phosphorylating ULK1 and other ATG proteins, and indirectly, by regulating autophagy-related gene expression via transcription factors [82].
2.8.1. Autophagy in Type 1 Diabetes Mellitus
2.8.2. Autophagy in Type 2 Diabetes Mellitus
3. Role of Epigenetics in Diabetes-Related Heart Failure
3.1. DNA Methylation
3.2. Histone Modifications
3.3. Role of MicroRNAs in Diabetes-Related Heart Failure
4. Pathomechanisms in Diabetes-Associated Heart Attacks
5. Pharmacological Management of Heart Failure
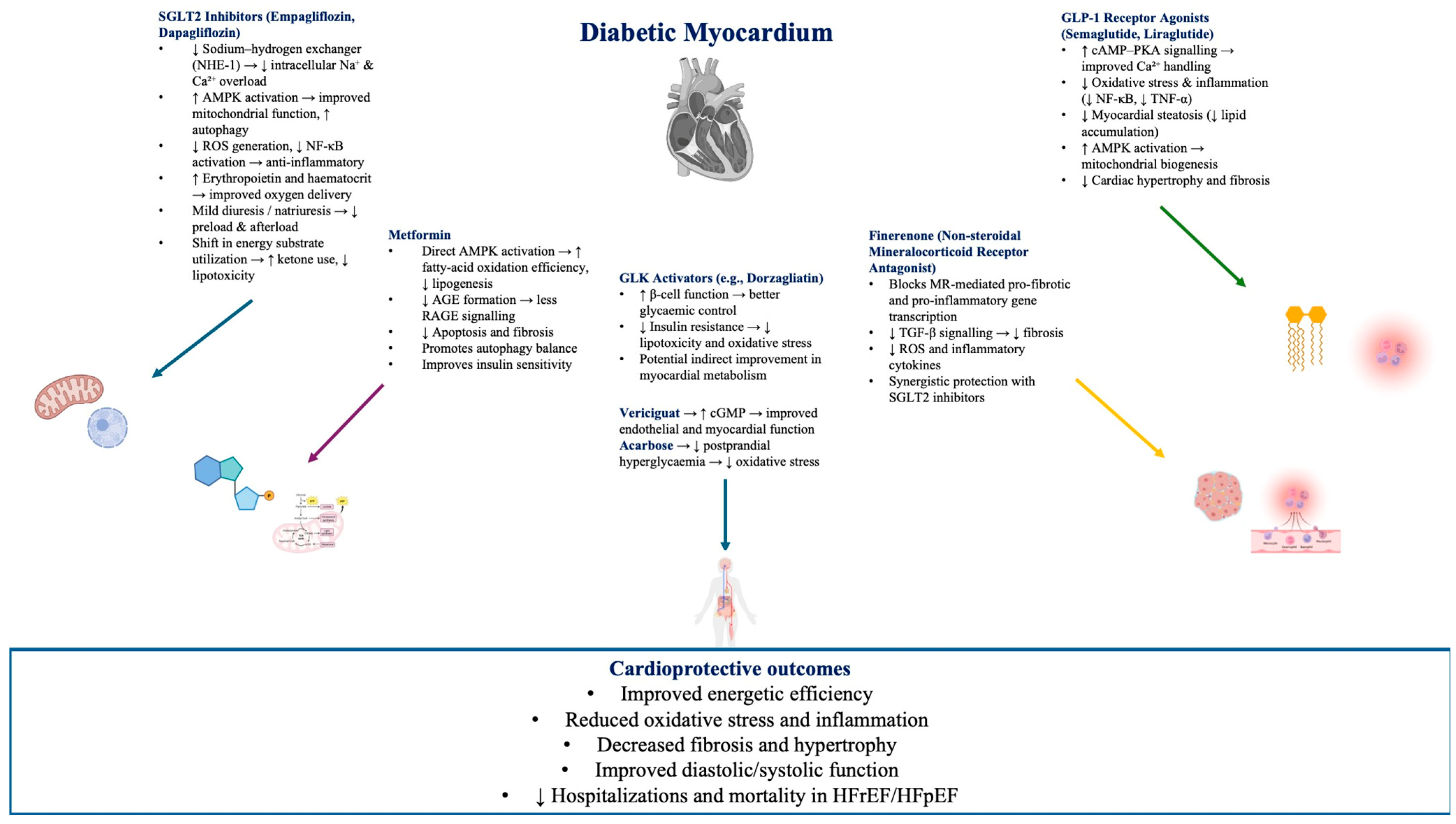
5.1. Antidiabetic Agents and Heart Failure
5.2. SGLT2 Inhibitors and Heart Failure
Potential Mechanisms of Action for SGLT2 in HF
5.3. Finerenone—Non-Steroidal Mineralocorticoid Receptor Antagonists in Heart Failure
5.4. Vericiguat—An Emerging Therapy in Heart Failure Management
- NO-dependent pathway—it enhances the sensitivity of sGC receptors to NO by stabilizing the NO-sGC binding site, leading to increased cGMP synthesis in cardiomyocytes and vascular smooth muscle cells.
- NO-independent pathway—it binds directly to an alternative site on the sGC receptor, further boosting cGMP production [117].
5.5. Considerations in Selecting Research Endpoints for SGLT2 Inhibitor Trials in HFpEF
6. Comprehensive Discussion and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Lawati, J.A. Diabetes Mellitus: A Local and Global Public Health Emergency! Oman Med. J. 2017, 32, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Mengstie, M.A.; Abebe, E.C.; Teklemariam, A.B.; Mulu, A.T.; Teshome, A.A.; Zewde, E.A.; Muche, Z.T.; Azezew, M.T. Molecular and cellular mechanisms in diabetic heart failure: Potential therapeutic targets. Front. Endocrinol. 2022, 13, 947294. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Giugliano, D.; Meier, J.J.; Esposito, K. Heart failure and type 2 diabetes: From cardiovascular outcome trials, with hope. Diabetes Obes. Metab. 2019, 21, 1081–1087. [Google Scholar] [CrossRef]
- Thomas, M.C. Type 2 Diabetes and Heart Failure: Challenges and Solutions. Curr. Cardiol. Rev. 2016, 12, 249–255. [Google Scholar] [CrossRef]
- Schütt, K.A. Herzinsuffizienz bei Menschen mit Diabetes mellitus. Der Diabetol. 2021, 17, 607–617. [Google Scholar] [CrossRef]
- Ceriello, A.; Catrinoiu, D.; Chandramouli, C.; Cosentino, F.; Dombrowsky, A.C.; Itzhak, B.; Lalic, N.M.; Prattichizzo, F.; Schnell, O.; Seferović, P.M.; et al. Heart failure in type 2 diabetes: Current perspectives on screening, diagnosis and management. Cardiovasc. Diabetol. 2021, 20, 218. [Google Scholar] [CrossRef]
- Hausner, E.A.; Elmore, S.A.; Yang, X. Overview of the Components of Cardiac Metabolism. Drug Metab. Dispos. 2019, 47, 673–688. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Nabben, M. CD36 (SR-B2) as a Target to Treat Lipid Overload-Induced Cardiac Dysfunction. J. Lipid Atheroscler. 2020, 9, 66. [Google Scholar] [CrossRef]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef]
- Angelini, A.; Pi, X.; Xie, L. Dioxygen and Metabolism; Dangerous Liaisons in Cardiac Function and Disease. Front. Physiol. 2017, 8, 1044. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Pascual, F.; Coleman, R.A. Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Varma, U.; Koutsifeli, P.; Benson, V.L.; Mellor, K.M.; Delbridge, L.M.D. Molecular mechanisms of cardiac pathology in diabetes—Experimental insights. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.; Chen, G.X. Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, Z.V. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in healthy heart and in cardiac disease. Int. J. Cardiol. 2017, 230, 70–75. [Google Scholar] [CrossRef]
- Maria, Z.; Campolo, A.R.; Lacombe, V.A. Diabetes Alters the Expression and Translocation of the Insulin-Sensitive Glucose Transporters 4 and 8 in the Atria. PLoS ONE 2015, 10, e0146033. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Sun, Q.; Lopaschuk, G.D. The Contribution of Cardiac Fatty Acid Oxidation to Diabetic Cardiomyopathy Severity. Cells 2021, 10, 3259. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, C.; de Souza Junior, A.L.; Carbonaro, M.; Bolsoni-Lopes, A.; Figueroa, D.; de Souza, L.E.; Silva, K.A.S.; Consolim-Colombo, F.; Curi, R.; Irigoyen, M.C. Glucose and fatty acid metabolism in infarcted heart from streptozotocin-induced diabetic rats after 2 weeks of tissue remodeling. Cardiovasc. Diabetol. 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Peterson, L.R.; McGill, J.B.; Matthew, S.; Lesniak, D.; Dence, C.; Gropler, R.J. Increased Myocardial Fatty Acid Metabolism in Patients with Type 1 Diabetes Mellitus. J. Am. Coll. Cardiol. 2006, 47, 598–604. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef]
- Zheng, L.; Li, B.; Lin, S.; Chen, L.; Li, H. Role and mechanism of cardiac insulin resistance in occurrence of heart failure caused by myocardial hypertrophy. Aging 2019, 11, 6584–6590. [Google Scholar] [CrossRef]
- Aroor, A.R.; Mandavia, C.H.; Sowers, J.R. Insulin Resistance and Heart Failure. Heart Fail. Clin. 2012, 8, 609–617. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Jiang, W.; Qin, Y.; Gong, G. Mitochondrial Dynamics in Adult Cardiomyocytes and Heart Diseases. Front. Cell Dev. Biol. 2020, 8, 584800. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial Dysfunction in Diabetic Cardiomyopathy: The Possible Therapeutic Roles of Phenolic Acids. Int. J. Mol. Sci. 2020, 21, 6043. [Google Scholar] [CrossRef]
- Dorn, G.W.; Vega, R.B.; Kelly, D.P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015, 29, 1981–1991. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Fatty Acid Oxidation and Its Relation with Insulin Resistance and Associated Disorders. Ann. Nutr. Metab. 2016, 68 (Suppl. S3), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, J.; Zirlik, A.; Bugger, H. Mitochondrial Mechanisms in Diabetic Cardiomyopathy. Diabetes Metab. J. 2020, 44, 33. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Hoek, J.; Sheu, S.S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef]
- Davidson, S.M.; Adameová, A.; Barile, L.; Cabrera-Fuentes, H.A.; Lazou, A.; Pagliaro, P.; Stensløkken, K.; Garcia-Dorado, D.; Action, E.-C.C. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J. Cell Mol. Med. 2020, 24, 3795–3806. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Ramaccini, D.; Morciano, G.; Pedriali, G.; Kahsay, A.E.; Bouhamida, E.; Giorgi, C.; Wieckowski, M.R.; Pinton, P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules 2020, 10, 998. [Google Scholar] [CrossRef]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS–STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef]
- Ou, L.; Zhang, A.; Cheng, Y.; Chen, Y. The cGAS-STING Pathway: A Promising Immunotherapy Target. Front. Immunol. 2021, 12, 795048. [Google Scholar] [CrossRef]
- Cai, Z.; Yuan, S.; Luan, X.; Feng, J.; Deng, L.; Zuo, Y.; Li, J. Pyroptosis-Related Inflammasome Pathway: A New Therapeutic Target for Diabetic Cardiomyopathy. Front Pharmacol. 2022, 13, 842313. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Liang, H.; Du, W.; Wang, L. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm. Sin. B 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Q.; Chen, Z.; Chen, L.X. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Sicari, D.; Delaunay-Moisan, A.; Combettes, L.; Chevet, E.; Igbaria, A. A guide to assessing endoplasmic reticulum homeostasis and stress in mammalian systems. FEBS J. 2020, 287, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic Reticulum Stress Sensing in the Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Wang, S.; Binder, P.; Fang, Q.; Wang, Z.; Xiao, W.; Liu, W.; Wang, X. Endoplasmic reticulum stress in the heart: Insights into mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1293–1304. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Chaudhary, M.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum (ER) Stress Response Failure in Diseases. Trends Cell Biol. 2020, 30, 672–675. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. Metabolic Stress and Cardiovascular Disease in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1911–1924. [Google Scholar] [CrossRef]
- Ruan, Y.; Zeng, J.; Jin, Q.; Chu, M.; Ji, K.; Wang, Z.; Li, L. Endoplasmic reticulum stress serves an important role in cardiac ischemia/reperfusion injury (Review). Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, H.; Dong, R.; Wu, Y.Q. Sequential changes of endoplasmic reticulum stress and apoptosis in myocardial fibrosis of diabetes mellitus-induced rats. Mol. Med. Rep. 2016, 13, 5037–5044. [Google Scholar] [CrossRef]
- Horáková, L.; Strosova, M.K.; Spickett, C.M.; Blaskovic, D. Impairment of calcium ATPases by high glucose and potential pharmacological protection. Free Radic. Res. 2013, 47 (Suppl. S1), 81–92. [Google Scholar] [CrossRef]
- Takada, A.; Miki, T.; Kuno, A.; Kouzu, H.; Sunaga, D.; Itoh, T.; Tanno, M.; Yano, T.; Sato, T.; Ishikawa, S.; et al. Role of ER Stress in Ventricular Contractile Dysfunction in Type 2 Diabetes. PLoS ONE 2012, 7, e39893. [Google Scholar] [CrossRef]
- Zhihao, L.; Jingyu, N.; Lan, L.; Michael, S.; Rui, G.; Xiyun, B.; Xiaozhi, L.; Guanwei, F. SERCA2a: A key protein in the Ca2+ cycle of the heart failure. Heart Fail. Rev. 2020, 25, 523–535. [Google Scholar] [CrossRef]
- Wang, L.; Myles, R.C.; Lee, I.J.; Bers, D.M.; Ripplinger, C.M. Role of Reduced Sarco-Endoplasmic Reticulum Ca2+-ATPase Function on Sarcoplasmic Reticulum Ca2+ Alternans in the Intact Rabbit Heart. Front. Physiol. 2021, 12, 656516. [Google Scholar] [CrossRef] [PubMed]
- Prola, A.; Nichtova, Z.; Pires Da Silva, J.; Piquereau, J.; Monceaux, K.; Guilbert, A.; Gressette, M.; Ventura-Clapier, R.; Garnier, A.; Zahradnik, I.; et al. Endoplasmic reticulum stress induces cardiac dysfunction through architectural modifications and alteration of mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2019, 115, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Vázquez-Carrera, M. Emerging Actors in Diabetic Cardiomyopathy: Heartbreaker Biomarkers or Therapeutic Targets? Trends Pharmacol. Sci. 2018, 39, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Kaur, N.; Guan, Y.; Raja, R.; Ruiz-Velasco, A.; Liu, W. Mechanisms and Therapeutic Prospects of Diabetic Cardiomyopathy Through the Inflammatory Response. Front. Physiol. 2021, 12, 694864. [Google Scholar] [CrossRef]
- Senatus, L.; MacLean, M.; Arivazhagan, L.; Egana-Gorrono, L.; Lopez-Diez, R.; Manigrasso, M.B.; Ruiz, H.H.; Vasquez, C.; Wilson, R.; Shekhtman, A.; et al. Inflammation Meets Metabolism Roles: For the Receptor for Advanced Glycation End Products Axis in Cardiovascular Disease. Immunometabolism 2021, 3, e210024. [Google Scholar] [CrossRef]
- Ramesh, P.; Yeo, J.L.; Brady, E.M.; McCann, G.P. Role of inflammation in diabetic cardiomyopathy. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221083530. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, I.; Hajduch, E.; Luchiari, B.; Nadruz, W.; Le Goff, W.; Sposito, A.C. The Reciprocal Relationship between LDL Metabolism and Type 2 Diabetes Mellitus. Metabolites 2021, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Bonelli, A.; Paccone, A.; Buccolo, S.; Iovine, M.; Botti, G.; Maurea, N. Oxidized Low-Density Lipoproteins increases nivolumab-induced cardiotoxicity through TLR4/NF-KB and NLRP3 pathways. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.2837. [Google Scholar] [CrossRef]
- Verboom, L.; Hoste, E.; van Loo, G. OTULIN in NF-κB signaling, cell death, and disease. Trends Immunol. 2021, 42, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Froogh, G.; Kandhi, S.; Duvvi, R.; Le, Y.; Weng, Z.; Alruwaili, N.; Ashe, J.O.; Sun, D.; Huang, A. The contribution of chymase-dependent formation of ANG II to cardiac dysfunction in metabolic syndrome of young rats: Roles of fructose and EETs. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H985–H993. [Google Scholar] [CrossRef]
- Singh, V.P.; Le, B.; Khode, R.; Baker, K.M.; Kumar, R. Intracellular Angiotensin II Production in Diabetic Rats Is Correlated with Cardiomyocyte Apoptosis, Oxidative Stress, and Cardiac Fibrosis. Diabetes 2008, 57, 3297–3306. [Google Scholar] [CrossRef]
- Ahmad, S.; Ferrario, C.M. Chymase inhibitors for the treatment of cardiac diseases: A patent review (2010–2018). Expert. Opin. Ther. Pat. 2018, 28, 755–764. [Google Scholar] [CrossRef]
- Maeda, Y.; Inoguchi, T.; Takei, R.; Sawada, F.; Sasaki, S.; Fujii, M.; Kobayashi, K.; Urata, H.; Nishiyama, A.; Takayanagi, R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am. J. Physiol. Ren. Physiol. 2010, 299, F1328–F1338. [Google Scholar] [CrossRef]
- Arun, K.; Kaul, C.; Ramarao, P. High glucose concentration augments angiotensin II mediated contraction via AT1 receptors in rat thoracic aorta. Pharmacol. Res. 2004, 50, 561–568. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Bi, Y.-G.; Liu, X.-D.; Zhao, Y.; Han, J.-F.; Wei, M.; Zhang, Q.-Y. Autophagy was involved in the protective effect of metformin on hyperglycemia-induced cardiomyocyte apoptosis and Connexin43 downregulation in H9c2 cells. Int. J. Med. Sci. 2017, 14, 698–704. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T. Advanced Glycation end Products, Oxidative Stress and Diabetic Nephropathy. Oxid. Med. Cell Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Koka, V.; Wang, W.; Huang, X.R.; Kim-Mitsuyama, S.; Truong, L.D.; Lan, H.Y. Advanced Glycation End Products Activate a Chymase-Dependent Angiotensin II–Generating Pathway in Diabetic Complications. Circulation 2006, 113, 1353–1360. [Google Scholar] [CrossRef]
- Vukelic, S.; Griendling, K.K. Angiotensin II, From Vasoconstrictor to Growth Factor. Circ. Res. 2014, 114, 754–757. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and Autophagy-Related Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.W.Y.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Tooze, S.A. Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov. 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.; Williamson, L.; Chan, E. Advances in Autophagy Regulatory Mechanisms. Cells 2016, 5, 24. [Google Scholar] [CrossRef]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Tamargo-Gómez, I.; Mariño, G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018, 19, 3812. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160. [Google Scholar] [CrossRef]
- Munasinghe, P.; Katare, R. Maladaptive autophagy in diabetic heart disease. Int. J. Clin. Exp. Physiol. 2016, 3, 155. [Google Scholar] [CrossRef]
- Kobayashi, S.; Liang, Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 252–261. [Google Scholar] [CrossRef]
- Li, X.; Ke, X.; Li, Z.; Li, B. Vaspin prevents myocardial injury in rats model of diabetic cardiomyopathy by enhancing autophagy and inhibiting inflammation. Biochem. Biophys. Res. Commun. 2019, 514, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Kobayashi, S.; Chen, K.; Timm, D.; Volden, P.; Huang, Y.; Gulick, J.; Yue, Z.; Robbins, J.; Epstein, P.N.; et al. Diminished Autophagy Limits Cardiac Injury in Mouse Models of Type 1 Diabetes. J. Biol. Chem. 2013, 288, 18077–18092. [Google Scholar] [CrossRef]
- Kanamori, H.; Naruse, G.; Yoshida, A.; Watanabe, T.; Kawaguchi, T.; Tanaka, T.; Yamada, Y.; Takasugi, H.; Mikami, A.; Minatoguchi, S.; et al. Morphological characteristics in diabetic cardiomyopathy associated with autophagy. J. Cardiol. 2021, 77, 30–40. [Google Scholar] [CrossRef]
- Kobayashi, S.; Xu, X.; Chen, K.; Liang, Q. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 2012, 8, 577–592. [Google Scholar] [CrossRef]
- Ouyang, C.; You, J.; Xie, Z. The interplay between autophagy and apoptosis in the diabetic heart. J. Mol. Cell Cardiol. 2014, 71, 71–80. [Google Scholar] [CrossRef]
- Venkatesh, I.; Makky, K. Teaching Epigenetic Regulation of Gene Expression Is Critical in 21st-Century Science Education: Key Concepts & Teaching Strategies. Am. Biol. Teach. 2020, 82, 372–380. [Google Scholar] [CrossRef]
- Russell-Hallinan, A.; Karuna, N.; Lezoualc’H, F.; Matullo, G.; Baker, H.; Bernard, M.; Devaux, Y.; Badimon, L.; Vilahur, G.; Rieusset, J.; et al. Established and Emerging Roles of Epigenetic Regulation in Diabetic Cardiomyopathy. Diabetes Metab. Res. Rev. 2025, 41, e70081. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Garg, R.; Bahl, A.; Khullar, M. Molecular Mechanisms and Epigenetic Regulation in Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 725532. [Google Scholar] [CrossRef]
- Aristizabal, M.J.; Anreiter, I.; Halldorsdottir, T.; Odgers, C.L.; McDade, T.W.; Goldenberg, A.; Mostafavi, S.; Kobor, M.S.; Binder, E.B.; Sokolowski, M.B.; et al. Biological embedding of experience: A primer on epigenetics. Proc. Natl. Acad. Sci. USA 2020, 117, 23261–23269. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, J.; Zhang, Q.; Xiao, X. Novel insights into DNA methylation and its critical implications in diabetic vascular complications. Biosci. Rep. 2017, 37, BSR20160611. [Google Scholar] [CrossRef]
- Pepin, M.E.; Wende, A.R. Epigenetics in the Development of Diabetic Cardiomyopathy. Epigenomics 2019, 11, 469–472. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Qiu, C.; Zhu, H.; Pan, S.; Jia, H.; Kang, H.; Guan, G.; Hui, R.; Zhu, L.; et al. Diabetes mellitus exacerbates post-myocardial infarction heart failure by reducing sarcolipin promoter methylation. ESC Heart Fail. 2020, 7, 1935–1948. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Wu, C.-K.; Lee, J.-K.; Chang, S.-N.; Kuo, Y.-M.; Wang, Y.-C.; Lai, L.-P.; Chiang, F.-T.; Hwang, J.-J.; Lin, J.-L. TNF- down-regulates sarcoplasmic reticulum Ca2+ ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF- B to promoter response element. Cardiovasc. Res. 2015, 105, 318–329. [Google Scholar] [CrossRef]
- Yerra, V.G.; Advani, A. Histones and heart failure in diabetes. Cell. Mol. Life Sci. 2018, 75, 3193–3213. [Google Scholar] [CrossRef]
- Qi, Y.K.; Ai, H.S.; Li, Y.M.; Yan, B. Total Chemical Synthesis of Modified Histones. Front. Chem. 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Demetriadou, C.; Koufaris, C.; Kirmizis, A. Histone N-alpha terminal modifications: Genome regulation at the tip of the tail. Epigenetics Chromatin 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, A.W.; Akhmedov, A.; Suades, R.; Costantino, S.; Paneni, F.; Caidahl, K.; Mohammed, S.A.; Hage, C.; Gkolfos, C.; et al. Hyperglycemia Induces Myocardial Dysfunction via Epigenetic Regulation of JunD. Circ. Res. 2020, 127, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, L.M.; Natarajan, R. The role of epigenetics in the pathology of diabetic complications. Am. J. Physiol. Ren. Physiol. 2010, 299, F14–F25. [Google Scholar] [CrossRef]
- Ke, X.; Lin, Z.; Ye, Z.; Leng, M.; Chen, B.; Jiang, C.; Jiang, X.; Li, G. Histone Deacetylases in the Pathogenesis of Diabetic Cardiomyopathy. Front. Endocrinol. 2021, 12, 679655. [Google Scholar] [CrossRef]
- Deng, J.; Liao, Y.; Liu, J.; Liu, W.; Yan, D. Research Progress on Epigenetics of Diabetic Cardiomyopathy in Type 2 Diabetes. Front. Cell Dev. Biol. 2021, 9, 777258. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Rawal, S.; Ram, T.P.; Coffey, S.; Williams, M.J.; Saxena, P.; Bunton, R.W.; Galvin, I.F.; Katare, R. Differential expression pattern of cardiovascular microRNAs in the human type-2 diabetic heart with normal ejection fraction. Int. J. Cardiol. 2016, 202, 40–43. [Google Scholar] [CrossRef]
- Divakaran, V.; Mann, D.L. The Emerging Role of MicroRNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2008, 103, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S. Role of microRNAs in the pathogenesis of diabetic cardiomyopathy. Biomed. Rep. 2017, 6, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.-D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef]
- Moorthy, S.; Koshy, T.; Srinivasan, V.; Silambanan, S. Circulating Biomarkers and MicroRNAs in the Diagnosis, Prognosis and Treatment of Diabetic Cardiomyopathy-A Review. Cardiol. Cardiovasc. Med. 2020, 4, 18. [Google Scholar] [CrossRef]
- Dai, B.; Li, H.; Fan, J.; Zhao, Y.; Yin, Z.; Nie, X.; Wang, D.W.; Chen, C. MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc. Diabetol. 2018, 17, 123. [Google Scholar] [CrossRef]
- Asrih, M.; Steffens, S. Emerging role of epigenetics and miRNA in diabetic cardiomyopathy. Cardiovasc. Pathol. 2013, 22, 117–125. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium–glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Dukat, A.; Böhm, M.; Lacourcière, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Hulot, J.-S.; Trochu, J.-N.; Donal, E.; Galinier, M.; Logeart, D.; De Groote, P.; Juillière, Y. Vericiguat for the treatment of heart failure: Mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin. Pharmacother. 2021, 22, 1847–1855. [Google Scholar] [CrossRef]
- Butler, J.; Zannad, F.; Filippatos, G.; Anker, S.D.; Packer, M. Totality of evidence in trials of sodium–glucose co-transporter-2 inhibitors in the patients with heart failure with reduced ejection fraction: Implications for clinical practice. Eur. Heart J. 2020, 41, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.T.; McAlister, F.A.; Blackburn, D.F.; Majumdar, S.R.; Tsuyuki, R.T.; Varney, J.; Johnson, J.A. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: Systematic review. BMJ 2007, 335, 497. [Google Scholar] [CrossRef] [PubMed]
- He, B.K.; Ning, Z.Q.; Li, Z.B.; Shan, S.; Pan, D.S.; Ko, B.C.B.; Li, P.P.; Shen, Z.F.; Dou, G.F.; Zhang, B.L.; et al. In Vitro and In Vivo Characterizations of Chiglitazar, a Newly Identified PPAR Pan-Agonist. PPAR Res. 2012, 2012, 546548. [Google Scholar] [CrossRef]
- Rohatgi, A.; McGuire, D.K. Effects of the Thiazolidinedione Medications on Micro- and Macrovascular Complications in Patients with Diabetes—Update 2008. Cardiovasc. Drugs Ther. 2008, 22, 233–240. [Google Scholar] [CrossRef]
- Norris, S.; Carson, S.; Roberts, C. Comparative Effectiveness of Pioglitazone and Rosiglitazone in Type 2 Diabetes, Prediabetes, and the Metabolic Syndrome: A Meta-Analysis. Curr. Diabetes Rev. 2007, 3, 127–140. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Wolski, K.; Nicholls, S.J.; Nissen, S.E. Pioglitazone and Risk of Cardiovascular Events in Patients with Type 2 Diabetes Mellitus. JAMA 2007, 298, 1180. [Google Scholar] [CrossRef]
- Avogaro, A.; Fadini, G.P. Insulin treatment in patients with diabetes and heart failure: Defendant on the stand. Eur. J. Heart Fail. 2018, 20, 896–897. [Google Scholar] [CrossRef]
- Clawson, R.; Weidman-Evans, E.; Fort, A. Which drug is best for a patient with type 2 diabetes and heart failure? JAAPA 2021, 34, 49–52. [Google Scholar] [CrossRef]
- Hanefeld, M.; Schaper, F. Acarbose: Oral antidiabetes drug with additional cardiovascular benefits. Expert Rev. Cardiovasc. Ther. 2008, 6, 153–163. [Google Scholar] [CrossRef]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Mehta, C.; Sharma, A.; Nissen, S.E.; Rossignol, P.; Zannad, F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes: A renal function stratified analysis of the EXAMINE trial. BMC Med. 2020, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Solomon, N.; DeVore, A.D.; Sharma, A.; Felker, G.M.; Hernandez, A.F.; Heidenreich, P.A.; Matsouaka, R.A.; Green, J.B.; Butler, J.; et al. Clinical Outcomes with Metformin and Sulfonylurea Therapies Among Patients with Heart Failure and Diabetes. JACC Heart Fail. 2022, 10, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 Inhibitors in Type 2 Diabetes Mellitus and Heart Failure—A Concise Review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef]
- Anabtawi, A.; Miles, J.M. Metformin: Nonglycemic Effects and Potential Novel Indications. Endocr. Pract. 2016, 22, 999–1007. [Google Scholar] [CrossRef]
- Aguilar, D.; Chan, W.; Bozkurt, B.; Ramasubbu, K.; Deswal, A. Metformin Use and Mortality in Ambulatory Patients With Diabetes and Heart Failure. Circ. Heart Fail. 2011, 4, 53–58. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S46–S60. [Google Scholar] [CrossRef]
- Lenzen, S. A Fresh View of Glycolysis and Glucokinase Regulation: History and Current Status. J. Biol. Chem. 2014, 289, 12189–12194. [Google Scholar] [CrossRef]
- Zeng, J.; Gan, S.; Mi, N.; Liu, Y.; Su, X.; Zhang, W.; Zhang, J.; Yu, F.; Dong, X.; Han, M.; et al. Diabetes remission in drug-naïve patients with type 2 diabetes after dorzagliatin treatment: A prospective cohort study. Diabetes Obes. Metab. 2023, 25, 2878–2887. [Google Scholar] [CrossRef]
- Ilieșiu, A.M.; Hodorogea, A.S. Treatment of Heart Failure with Preserved Ejection Fraction. Adv. Exp. Med. Biol. 2018, 1067, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Shah, S.J.; Petrie, M.C.; Borlaug, B.A.; Abildstrøm, S.Z.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Verma, S.; et al. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: A pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials. Lancet 2024, 403, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Bidulka, P.; Lugo-Palacios, D.G.; Carroll, O.; O’nEill, S.; Adler, A.I.; Basu, A.; Silverwood, R.J.; Bartlett, J.W.; Nitsch, D.; Charlton, P.; et al. Comparative effectiveness of second line oral antidiabetic treatments among people with type 2 diabetes mellitus: Emulation of a target trial using routinely collected health data. BMJ 2024, 385, e077097. [Google Scholar] [CrossRef]
- Kittipibul, V.; Cox, Z.L.; Chesdachai, S.; Fiuzat, M.; Lindenfeld, J.; Mentz, R.J. Genitourinary Tract Infections in Patients Taking SGLT2 Inhibitors. J. Am. Coll. Cardiol. 2024, 83, 1568–1578. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Caparrotta, T.M.; Blackbourn, L.A.K.; McGurnaghan, S.J.; Chalmers, J.; Lindsay, R.; McCrimmon, R.; McKnight, J.; Wild, S.; Petrie, J.R.; Philip, S.; et al. Prescribing Paradigm Shift? Applying the 2019 European Society of Cardiology–Led Guidelines on Diabetes, Prediabetes, and Cardiovascular Disease to Assess Eligibility for Sodium–Glucose Cotransporter 2 Inhibitors or Glucagon-Like Peptide 1 Receptor Agonists as First-Line Monotherapy (or Add-on to Metformin Monotherapy) in Type 2 Diabetes in Scotland. Diabetes Care 2020, 43, 2034–2041. [Google Scholar] [CrossRef]
- Kosiborod, M.; Lam, C.S.; Kohsaka, S.; Kim, D.J.; Karasik, A.; Shaw, J.; Tangri, N.; Goh, S.-Y.; Thuresson, M.; Chen, H.; et al. Cardiovascular Events Associated with SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs. J. Am. Coll. Cardiol. 2018, 71, 2628–2639. [Google Scholar] [CrossRef]
- Pasternak, B.; Ueda, P.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Wintzell, V.; Melbye, M.; et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ 2019, 366, l4772. [Google Scholar] [CrossRef]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Armaly, Z.; Hamo-Giladi, D.B.; Abassi, Z. Novel perspectives regarding the physiologic mechanisms by which gliflozins induce reticulocytosis and erythrocytosis. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E621–E623. [Google Scholar] [CrossRef]
- Zhang, N.; Feng, B.; Ma, X.; Sun, K.; Xu, G.; Zhou, Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2019, 18, 107. [Google Scholar] [CrossRef]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc. Diabetol. 2018, 17, 6. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef]
- Pabel, S.; Hamdani, N.; Singh, J.; Sossalla, S. Potential Mechanisms of SGLT2 Inhibitors for the Treatment of Heart Failure with Preserved Ejection Fraction. Front. Physiol. 2021, 12, 752370. [Google Scholar] [CrossRef]
- Bonora, B.M.; Avogaro, A.; Fadini, G.P. Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence. Diabetes Metab. Syndr. Obes. 2020, 13, 161–174. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- González-Juanatey, J.R.; Górriz, J.L.; Ortiz, A.; Valle, A.; Soler, M.J.; Facila, L. Cardiorenal benefits of finerenone: Protecting kidney and heart. Ann. Med. 2023, 55, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium–Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Marzolla, V.; Infante, M.; Armani, A.; Rizzo, M.; Caprio, M. Efficacy and safety of finerenone for treatment of diabetic kidney disease: Current knowledge and future perspective. Expert. Opin. Drug Saf. 2022, 21, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Bauersachs, J.; Soltani, S. Herzinsuffizienz: Leitlinien-Update der ESC 2023. Herz 2024, 49, 19–21. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Blanton, R.M. cGMP Signaling and Modulation in Heart Failure. J. Cardiovasc. Pharmacol. 2020, 75, 385–398. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.J.S.; Tolppanen, H. Drug Treatment of Heart Failure with Reduced Ejection Fraction: Defining the Role of Vericiguat. Drugs 2021, 81, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Follmann, M.; Ackerstaff, J.; Redlich, G.; Wunder, F.; Lang, D.; Kern, A.; Fey, P.; Griebenow, N.; Kroh, W.; Becker-Pelster, E.-M.; et al. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J. Med. Chem. 2017, 60, 5146–5161. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Mulder, H.; Reyes, E.; Cowie, M.R.; Lassus, J.; Hernandez, A.F.; Ezekowitz, J.A.; Butler, J.; O’Connor, C.M.; Koglin, J.; et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur. J. Heart Fail. 2021, 23, 1313–1321. [Google Scholar] [CrossRef]
- Butler, J.; Packer, M.; Greene, S.J.; Fiuzat, M.; Anker, S.D.; Anstrom, K.J.; Carson, P.E.; Cooper, L.B.; Fonarow, G.C.; Hernandez, A.F.; et al. Heart Failure End Points in Cardiovascular Outcome Trials of Sodium Glucose Cotransporter 2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation 2019, 140, 2108–2118. [Google Scholar] [CrossRef]
- Fu, S.; Litwin, S.E.; Tedford, R.J. Lessons from SGLT-2 inhibitors: Rethinking endpoints for heart failure studies. Nat. Med. 2021, 27, 1872–1873. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Butler, J.; Abboud, F.M.; Armstrong, P.W.; Adamopoulos, S.; Atherton, J.J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.O.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: Population based cohort study. BMJ 2024, 385, e078242. [Google Scholar] [CrossRef]
- Green, J.B.; Mottl, A.K.; Bakris, G.; Heerspink, H.J.L.; Mann, J.F.E.; McGill, J.B.; Nangaku, M.; Rossing, P.; Scott, C.; Gay, A.; et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. 2023, 38, 894–903. [Google Scholar] [CrossRef]

| Name | Method | Result | Conclusion |
|---|---|---|---|
| The DAPA-HF study | N = 4744; Dapagliflozin 10 mg vs. placebo; Follow-up: 18.2 years | Reduced cardiovascular death or HHF by 26% and all-cause mortality by 17% in HFrEF. | Reduces risk of ventricular arrhythmia, cardiac arrest, or sudden death in HFrEF. |
| The EMPEROR-Reduced study | N = 3730; Empagliflozin 10 mg vs. placebo; Follow-up: 1.5 years | Reduced first cardiovascular death or HF (±CKD) and recurrent HF hospitalizations; slowed eGFR decline. | Empagliflozin use is recommended in HFrEF. |
| The DELIVER study | N = 6263; Dapagliflozin 10 mg vs. placebo; Follow-up: 2.3 years | Reduced cardiovascular death and HHF by 18%, regardless of LV function. | Reduces risk of primary composite endpoint. |
| The PRESERVED-HF study | N = 324; Dapagliflozin 10 mg vs. placebo; Follow-up: 12 weeks | Improved symptoms, physical limitations, and 6-min walk test. | Improves symptoms and activity limitations in HFpEF. |
| The CAMEO-DAPA study | N = 38; NYHA II/III, LVEF > 50%, PCWP; Follow-up: 24 weeks | Resting PCWP ↓ 3.5 mmHg; Exercise PCWP ↓ 6.1 mmHg. | Offers potential benefits for HFpEF. |
| The EMPEROR-Preserved study | N = 5988; Empagliflozin 10 mg vs. placebo; Follow-up: 26.2 months | Improved cardiovascular death and HHF in HFpEF; unaffected by HR. | Benefit independent of comorbid diabetes. |
| The EMPA-RESPONSE-AHF study | N = 80; Empagliflozin 10 mg vs. placebo; Follow-up: 60 days | Reduced composite endpoint: worsening HF, HF rehospitalization, death. | Safe and well tolerated in acute decompensated HF. |
| The EMPA-REG OUTCOME study | N = 7020; Empagliflozin 10 or 25 mg vs. placebo | Reduced 3P-MACE by 14% and CV death by 38%. | Prolonged survival in patients of all ages. |
| The EMBRACE-HF study | N = 65; Empagliflozin 10 mg vs. placebo; Follow-up: 12 weeks | Reduced mean pulmonary artery diastolic pressure. | Reduced pulmonary artery diastolic pressure. |
| The EMPULSE study | N = 530; Empagliflozin 10 mg vs. placebo; Follow-up: 90 days | Patients were 36% more likely to have clinical benefit; no heterogeneity. | Clinical benefit within 90 days in acute HF. |
| The EMPAG-HF study | Empagliflozin 25 mg vs. placebo; Follow-up at discharge & 30 days | No additional renal injury in acute decompensated HF. | Safe and well tolerated. |
| The VERTIS CV study | N = 8246; Ertugliflozin 5/15 mg vs. placebo; Follow-up: 3.5 years | Non-inferior for 3P-MACE; reduced first and all HF events. | Effect not influenced by baseline HF or LVEF. |
| The CHIENT-HF study | N = 476; Canagliflozin 100 mg vs. placebo | Improved symptoms regardless of EF or T2DM. | Improves prognosis, symptoms, and quality of life. |
| CVD-REAL study | Patients from 6 countries; SGLT2i vs. other antidiabetics | Lower HHF and mortality; no heterogeneity between countries. | Associated with reduced HHF and mortality. |
| Scandinavian registry cohort | Registry data from Denmark, Norway, Sweden; 19% CVD history, 6% HF history | 83% dapagliflozin, 16% empagliflozin, 1% canagliflozin; 34% lower HF risk vs. DPP-4i. | Reduced primary HF risk by 34%. |
| Real-world Taiwan study | N = 12,681 T2DM; dapagliflozin (n = 5812) vs. empagliflozin (n = 6869) | Similar CV event risk, but dapagliflozin reduced HF more than empagliflozin. | Dapagliflozin superior in reducing HF risk. |
| Class | Common Medications | Effects in Heart Failure | Clinical Practice Recommendations |
|---|---|---|---|
| SGLT2 inhibitors | Dapagliflozin, Empagliflozin | Beneficial—reduce HHF, cardiovascular death, and CKD progression in HFrEF and HFpEF | Recommended for symptomatic HFrEF and HFpEF patients regardless of DM status; high-quality evidence (Grade A) |
| GLP-1 RA | Semaglutide | Beneficial—improve symptoms in HFpEF, reduce weight, attenuate remodeling and inflammation | Consider when SGLT2 inhibitors are contraindicated or not tolerated; especially for HFpEF with obesity |
| DPP-4 inhibitors | Saxagliptin (harmful), Alogliptin (neutral) | Mixed—saxagliptin increases HHF risk; others neutral | Saxagliptin not recommended in T2DM with HF; others with caution |
| GKA | Dorzagliatin | Potential cardiovascular benefits—improve β-cell function, glycemic control | Promising for CHD and HF, but more RCT data needed before recommendation |
| Metformin | Metformin | Beneficial—reduces mortality, HF readmissions, improves myocardial metabolism | Recommended for stable T2DM with HF and normal renal function; avoid in acute/decompensated HF |
| Glycosidase inhibitor | Acarbose | Beneficial—reduces HF risk | Second- or third-line; avoid or discontinue if digoxin is used |
| Glinides | Repaglinide | Neutral—no increased CV risk | Second- or third-line; monitor closely in HF |
| Sulfonylureas | Glimepiride | Potentially harmful—increased mortality risk | Not recommended for T2DM with HF |
| Insulin | Insulin | Potentially harmful—may worsen HF outcomes | Use rapid-/short-acting forms with close monitoring in HF |
| TZDs | Pioglitazone | Harmful—increase HF and mortality risk | Not recommended for T2DM with HF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ktenopoulos, N.; Anagnostopoulou, L.; Apostolos, A.; Iliakis, P.; Karakasis, P.; Milaras, N.; Theofilis, P.; Fragoulis, C.; Drakopoulou, M.; Synetos, A.; et al. Cellular and Molecular Pathways in Diabetes-Associated Heart Failure: Emerging Mechanistic Insights and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2025, 47, 886. https://doi.org/10.3390/cimb47110886
Ktenopoulos N, Anagnostopoulou L, Apostolos A, Iliakis P, Karakasis P, Milaras N, Theofilis P, Fragoulis C, Drakopoulou M, Synetos A, et al. Cellular and Molecular Pathways in Diabetes-Associated Heart Failure: Emerging Mechanistic Insights and Therapeutic Opportunities. Current Issues in Molecular Biology. 2025; 47(11):886. https://doi.org/10.3390/cimb47110886
Chicago/Turabian StyleKtenopoulos, Nikolaos, Lilian Anagnostopoulou, Anastasios Apostolos, Panagiotis Iliakis, Paschalis Karakasis, Nikias Milaras, Panagiotis Theofilis, Christos Fragoulis, Maria Drakopoulou, Andreas Synetos, and et al. 2025. "Cellular and Molecular Pathways in Diabetes-Associated Heart Failure: Emerging Mechanistic Insights and Therapeutic Opportunities" Current Issues in Molecular Biology 47, no. 11: 886. https://doi.org/10.3390/cimb47110886
APA StyleKtenopoulos, N., Anagnostopoulou, L., Apostolos, A., Iliakis, P., Karakasis, P., Milaras, N., Theofilis, P., Fragoulis, C., Drakopoulou, M., Synetos, A., Latsios, G., Tsioufis, K., & Toutouzas, K. (2025). Cellular and Molecular Pathways in Diabetes-Associated Heart Failure: Emerging Mechanistic Insights and Therapeutic Opportunities. Current Issues in Molecular Biology, 47(11), 886. https://doi.org/10.3390/cimb47110886










