Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications
Abstract
1. Introduction
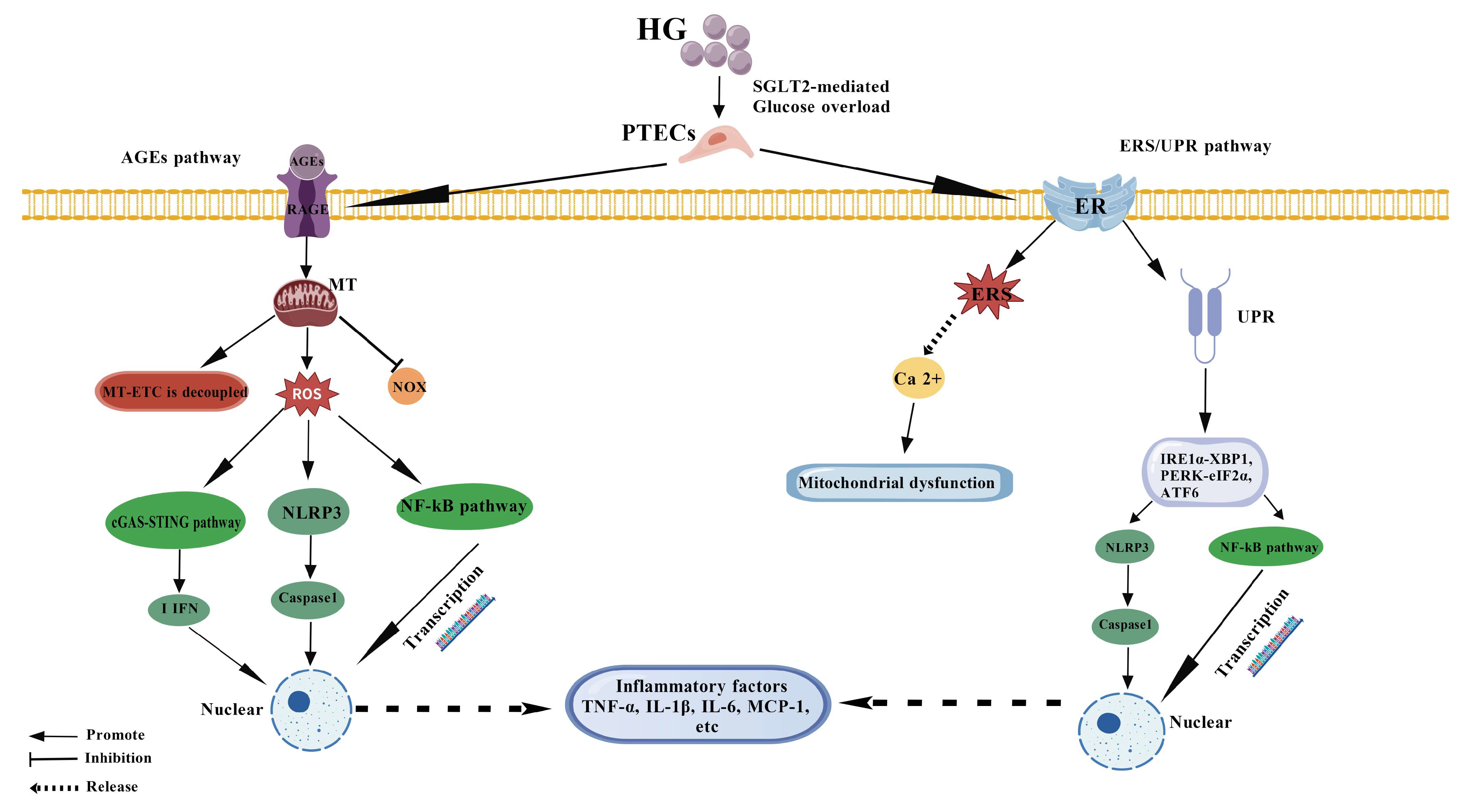
2. Molecular Mechanism and Network Regulation of Inflammatory Response of PTECs
2.1. Activation of ROS-Dependent Inflammatory Pathways
2.2. Amplifier Effect of AGEs-RAGE Axis
2.3. Pro-Inflammatory Effects of ER Stress and Unfolded Protein Response
3. The Triggering Pathway and Downstream Effects of PTECs Apoptosis
3.1. Exogenous Apoptotic Pathway: Signaling Cascade Mediated by Death Receptor
3.2. Endogenous Apoptotic Pathway: The Central Role of Mitochondria and Endoplasmic Reticulum Stress
3.3. Apoptotic PTECs as Active Participants in Microenvironment Remodeling
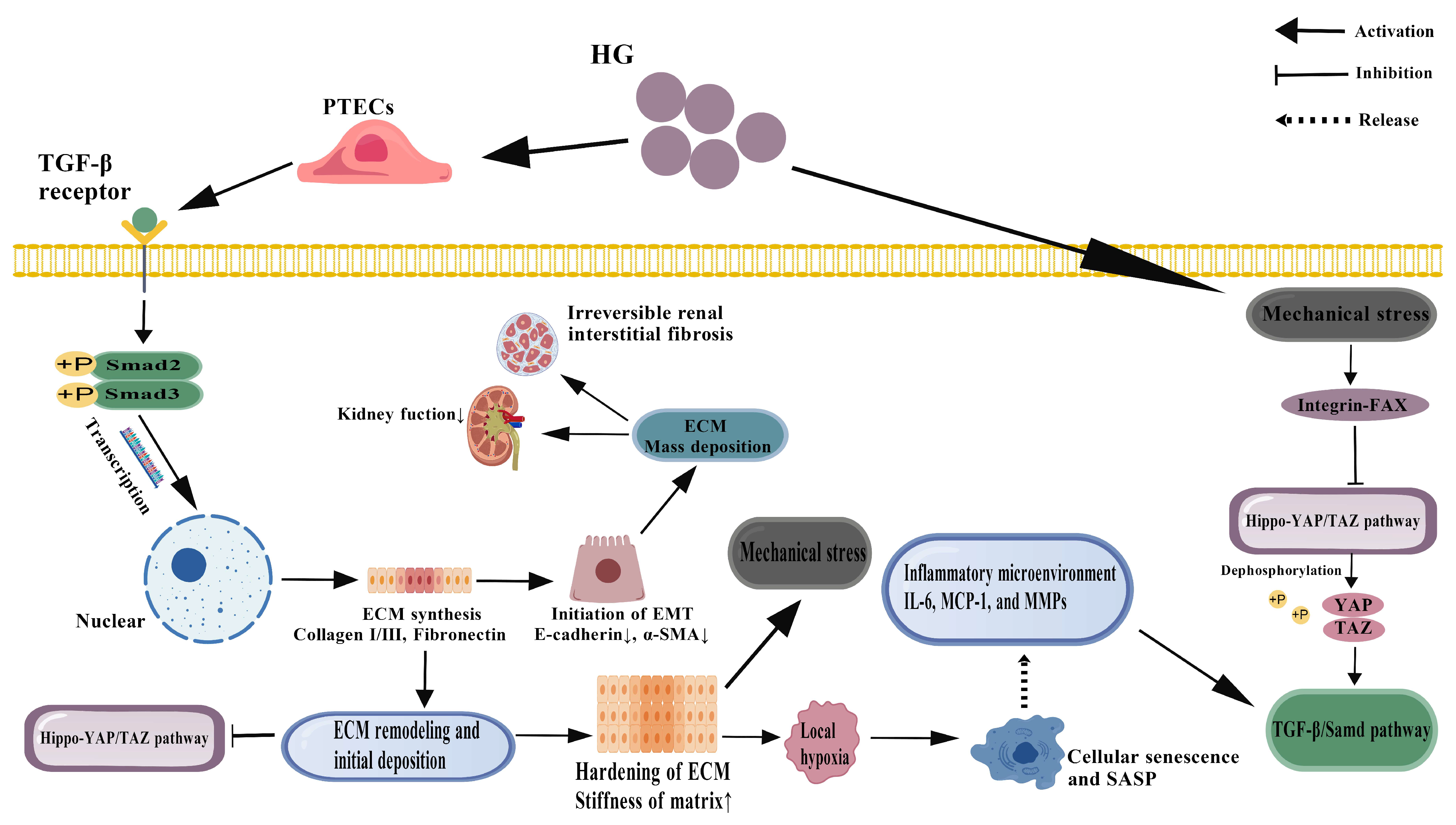
4. Dynamic Evolution of PTECs Fibrosis and Remodeling of Microenvironment
4.1. Double-Edged Sword Effect of TGF-β1 Signaling Pathway
4.2. Mechanobiological Regulation of Hippo-YAP/TAZ Pathway
4.3. Temporal and Spatial Heterogeneity of Fibrosis Progression and Window of Reversibility
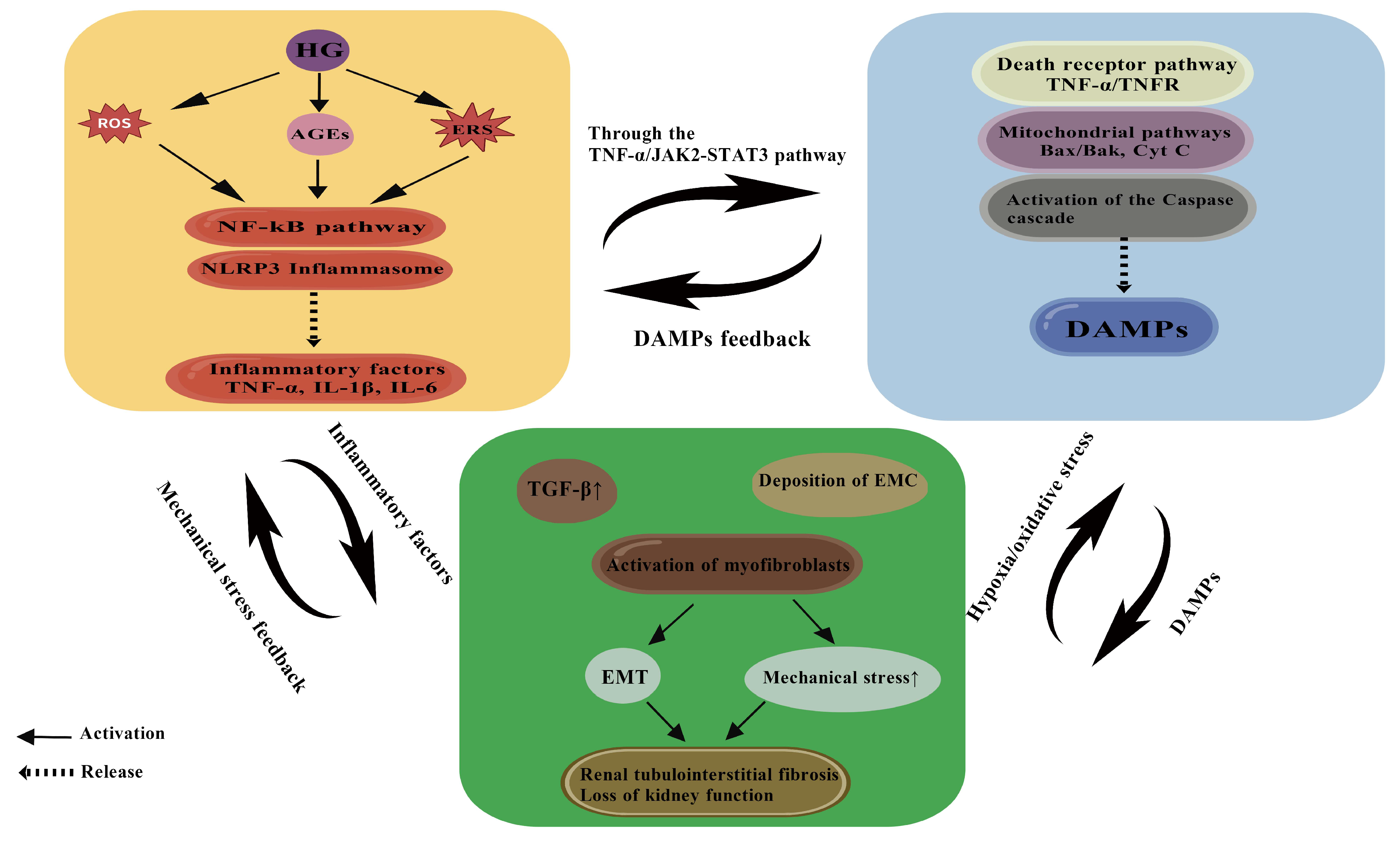
5. Interaction of Inflammation–Apoptosis–Fibrosis Network
6. Treatment Strategy: From Molecular Mechanism to Clinical Translation
6.1. Monotargeted Agents: Precision and Specificity
6.2. Multi-Targeted Formulations: Synergistic Network Modulation
6.3. Future Directions: Integrated Traditional Chinese and Western Medicine and Individualized Treatment
| Types of Drugs | Name of Drug | Remarks/Mechanism Description | The Pathological Process of Action | Level of Evidence | Key Quantitative Outcomes |
|---|---|---|---|---|---|
| Western medicine | Finerenone [163,164,165] | Mineralocorticoid receptor (MR) → inhibits NF-κB/NLRP3 FIDELIO-DKD, FIGARO-DKD | Inflammation, Fibrosis | Preclinical/Clinical |
|
| Western medicine | Empagliflozin [165,166] | SGLT2 → AMPK activation, mitochondrial ROS reduction EMPA-REG OUTCOME | Inflammation, Apoptosis, Fibrosis | Preclinical/Clinical |
|
| Western medicine | Dapagliflozin [167,169] | SGLT2 → inhibits HMGB1/TLR4/NF-κB YAP/TAZ DAPA-CKD | Inflammation, Fibrosis | Preclinical/Clinical |
|
| Western medicine | Canagliflozin [168,170] | Inhibits Hhip-Hedgehog pathway CREDENCE | Apoptosis, Fibrosis | Preclinical/Clinical |
|
| Western medicine | Gemigliptin [171] | NLRP3 inflammasome inhibitor | Inflammation | Preclinical/Early clinical |
|
| Western medicine | Baricitinib [172] | JAK1/2 → inhibits JAK2/STAT3 | Apoptosis, Inflammation | Preclinical |
|
| Western medicine | Simvastatin [173] | Nrf2 activation; inhibits NADPH oxidase and NF-κB | Apoptosis | Preclinical |
|
| Western medicine | Calcitriol [174] | Inhibits p38-MAPK phosphorylation | Apoptosis | Preclinical |
|
| Western medicine | 4-PBA [175] | ER stress inhibitor → ATF4/CHOP pathway | Apoptosis, ERS | Preclinical |
|
| Western medicine | TUDCA [176] | ER stress modulator | Apoptosis, ERS | Preclinical |
|
| Western medicine | Liraglutide [177,178] | Inhibits JAK2/STAT3 Enhance uric acid excretion → improve renal function | Inflammation, Fibrosis | Preclinical/Clinical |
|
| Traditional Chinese Medicine | Quercetin [17,179] | Inhibits PI3K/Akt and JAK2/STAT3 | Inflammation, Apoptosis, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Myricetin [180] | Inhibits PI3K/Akt and TGF-β1/Smad3 | Inflammation, Apoptosis, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Emodin [181] | Inhibits mTOR/p70S6K → suppresses EMT | Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Astragaloside IV [182] | Upregulates Bcl-2/Bax, promotes PINK1/Parkin mitophagy | Apoptosis, Oxidative Stress | Preclinical/Clinical |
|
| Traditional Chinese Medicine | Tanshinone IIA [183] | Inhibits TGF-β1/Smad → suppresses EMT | EMT, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Tripterygium glycosides [184] | Inhibits TLR4/NF-κB | Inflammation | Clinical (hepatotoxicity risk) |
|
| Traditional Chinese Medicine | Cordycepin [185] | Inhibits Drp1-mediated mitochondrial fission | Fibrosis, Apoptosis | Preclinical |
|
| Traditional Chinese Medicine | Puerarin [186] | Inhibits ferroptosis and TGF-β1/Smad3 | Fibrosis, Oxidative Stress | Preclinical |
|
| Traditional Chinese Medicine | Baicalin [187] | miR-124/TLR4/NF-κB axis | Inflammation, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Ginsenoside Rg3 [188] | Inhibits MAPK/NF-κB | Inflammation, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Icariin [189] | Activates Nrf2 via GPER/p62/Keap1 | Apoptosis | Preclinical |
|
| Traditional Chinese Medicine | Spinach extract [190] | Inhibits AGEs/RAGE axis | Inflammation, Apoptosis, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Huangkui capsule [191,192] | Multi-target: PI3K/Akt, JAK2/STAT3, TGF-β1/Smad3 | Inflammation, Apoptosis, Fibrosis | Clinical/Preclinical |
|
| Traditional Chinese Medicine | Liuwei Dihuang pill [193] | Inhibits TGF-β/SMADs, MAPK, NF-κB; upregulates cytoglobin | Inflammation, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Yi-Shen-Hua-Shi granule [194] | Modulates gut microbiota → “gut-kidney axis” | Inflammation | Preclinical |
|
| Traditional Chinese Medicine | Shenyan Kangfu Tablets [195] | Modulates PI3K-Akt and HIF-1 pathways | Tubular Injury, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Dang-Gui-Bu-Xue decoction [196] | Inhibits AGEs/RAGE pathway | Inflammation, Fibrosis | Preclinical |
|
| Traditional Chinese Medicine | Tangshen Formula [197] | Modulates gut microbiota and amino acid metabolism | Inflammation | Preclinical |
|
| Traditional Chinese Medicine | Tangshenkang Granule [198] | Improves lipid metabolism, suppresses oxidative stress and inflammation | Fibrosis | Preclinical |
|
7. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godon, J.P. Evidence of increased proximal sodium and water reabsorption in experimental glomerulonephritis. Role of a natriuretic factor of renal origin. Nephron 1978, 21, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E. Proximal Tubulopathy: Prime Mover and Key Therapeutic Target in Diabetic Kidney Disease. Diabetes 2017, 66, 791–800. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. 2020, 472, 1345–1370. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- ElKhooly, I.A.; El-Bassossy, H.M.; Mohammed, H.O.; Atwa, A.M.; Hassan, N.A. Vitamin B1 and calcitriol enhance glibenclamide suppression of diabetic nephropathy: Role of HMGB1/TLR4/NF-κB/TNF-α/Nrf2/α-SMA trajectories. Life Sci. 2024, 357, 123046. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Wang, W.; Liu, Y.; Zhao, C.; Yang, K.; Dai, W.; Ou, Y.; Yin, X.; Long, Y.; et al. GSTK1 and RETREG1/FAM134B-mediated reticulophagy attenuates tubular injury in diabetic nephropathy through endoplasmic reticulum stress and apoptosis. Autophagy 2025, 8, 1–16. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Chuan, L.; Wei, S.; Wang, J.; Yang, X.; Ru, J. miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int. Immunopharmacol. 2020, 89 Pt A, 107016. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Yin, P.; Gao, J.; Na, L.; Sun, Y.; Wang, Z.; Zhang, Z.; Zhao, C. Ruxolitinib Alleviates Renal Interstitial Fibrosis in UUO Mice. Int. J. Biol. Sci. 2020, 16, 194–203. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.-L.; Xu, K.-X.; Zheng, Z.-H.; Cheng, G.-Z.; Wu, H.-J.; Liu, J. The Hippo pathway and its correlation with acute kidney injury. Zool. Res. 2022, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, H.; Liu, Y. The fibrogenic niche in kidney fibrosis: Components and mechanisms. Nat. Rev. Nephrol. 2022, 18, 545–557. [Google Scholar] [CrossRef]
- Wilson, P.C.; Wu, H.; Kirita, Y.; Uchimura, K.; Ledru, N.; Rennke, H.G.; Welling, P.A.; Waikar, S.S.; Humphreys, B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2019, 116, 19619–19625. [Google Scholar] [CrossRef]
- Filippatos, G.; Pitt, B.; Agarwal, R.; Farmakis, D.; Ruilope, L.M.; Rossing, P.; Bauersachs, J.; Mentz, R.J.; Kolkhof, P.; Scott, C.; et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO-DKD trial. Eur. J. Heart Fail. 2022, 24, 996–1005. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.; Bonaca, M.P.; Mosenzon, O.; Kato, E.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39, Correction in Lancet 2019, 393, 30. [Google Scholar] [CrossRef]
- Xu, W.-L.; Zhou, P.-P.; Yu, X.; Tian, T.; Bao, J.-J.; Ni, C.-R.; Zha, M.; Wu, X.; Yu, J.-Y. Myricetin induces M2 macrophage polarization to alleviate renal tubulointerstitial fibrosis in diabetic nephropathy via PI3K/Akt pathway. World J. Diabetes 2024, 15, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Fu, Y.; Dai, J.; Lu, J.; Liu, G.; Yang, X. Huangkui capsule combined with finerenone attenuates diabetic nephropathy by regulating the JAK2/STAT3 signaling pathway based on network pharmacology, molecular docking, and experimental verification. Front. Pharmacol. 2025, 16, 1625286. [Google Scholar] [CrossRef]
- Widowati, W.; Prahastuti, S.; Tjokropranoto, R.; Onggowidjaja, P.; Kusuma, H.S.W.; Afifah, E.; Arumwardana, S.; Maulana, M.A.; Rizal, R. Quercetin prevents chronic kidney disease on mesangial cells model by regulating inflammation, oxidative stress, and TGF-β1/SMADs pathway. PeerJ 2022, 10, e13257. [Google Scholar] [CrossRef]
- Zhao, J.; Chan, Y.-C.; He, B.; Duan, T.-T.; Yu, Z.-L. A patent herbal drug Yi-Shen-Hua-Shi granule ameliorates C-BSA-induced chronic glomerulonephritis and inhabits TGFβ signaling in rats. J. Ethnopharmacol. 2019, 236, 258–262. [Google Scholar] [CrossRef]
- Gao, Y.; Su, X.; Xue, T.; Zhang, N. The beneficial effects of astragaloside IV on ameliorating diabetic kidney disease. Biomed. Pharmacother. 2023, 163, 114598. [Google Scholar] [CrossRef]
- Shen, X.; Dong, X.; Han, Y.; Li, Y.; Ding, S.; Zhang, H.; Sun, Z.; Yin, Y.; Li, W.; Li, W. Ginsenoside Rg1 ameliorates glomerular fibrosis during kidney aging by inhibiting NOX4 and NLRP3 inflammasome activation in SAMP8 mice. Int. Immunopharmacol. 2020, 82, 106339. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Harumi, T.; Watanabe, J.; Fujita, Y.; Honjo, J.; Shimizu, N.; Makino, Y.; Haneda, M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: A possible role of HIF-1α expression and oxygen metabolism. Diabetes 2011, 60, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Zhou, H.; Shenoi, R.; Morris, M.; Lainez-Mas, B.; Goedeke, L.; Rajendran, B.K.; Setia, O.; Aryal, B.; Kanasaki, K.; et al. Renal Angptl4 is a key fibrogenic molecule in progressive diabetic kidney disease. Sci. Adv. 2024, 10, eadn6068. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, M.; Zheng, X.; Li, S.; Fan, Y.; Wang, Y.; Peng, H.; Chen, S.; Yang, J.; Tan, L.; et al. YAP1 preserves tubular MT quality control to mitigate diabetic kidney disease. Redox Biol. 2024, 78, 103435. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Wen, W.; Zheng, H.; Li, W.; Huang, G.; Chen, P.; Zhu, X.; Cao, Y.; Li, J.; Huang, X.; Huang, Y. Transcription factor EB: A potential integrated network regulator in metabolic-associated cardiac injury. Metabolism 2023, 147, 155662. [Google Scholar] [CrossRef]
- Mohammadi, S.; Khorasani, M. Implications of the cGAS-STING pathway in diabetes: Risk factors and therapeutic strategies. Int. J. Biol. Macromol. 2024, 278 Pt 1, 134210. [Google Scholar] [CrossRef]
- Zarin, P.; Shwartz, Y.; Ortiz-Lopez, A.; Hanna, B.S.; Sassone-Corsi, M.; Hsu, Y.-C.; Mathis, D.; Benoist, C. Treg cells require Izumo1R to regulate γδT cell-driven inflammation in the skin. Proc. Natl. Acad. Sci. USA 2023, 120, e2221255120. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, D.; Dai, S.; Liu, F.; Zhang, W.; Shen, T. Desflurane attenuates renal ischemia-reperfusion injury by modulating ITGB1/CD9 and reducing oxidative stress in tubular epithelial cells. Redox Biol. 2025, 80, 103490. [Google Scholar] [CrossRef]
- Cimmino, T.P.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Habashy, N.H.; Kodous, A.S.; Abu-Serie, M.M. Targeting ROS/NF-κB sigaling pathway by the seedless black Vitis vinifera polyphenols in CCl4-intoxicated kidney, lung, brain, and spleen in rats. Sci. Rep. 2021, 11, 16575. [Google Scholar] [CrossRef]
- Chang, L.; Yeh, E.; Chuang, Y.; Wu, C.; Kuo, C.; Lii, C.; Yang, Y.; Chen, H.; Li, C. Luteolin Inhibits Indoxyl Sulfate-Induced ICAM-1 and MCP-1 Expression by Inducing HO-1 Expression in EA.hy926 Human Endothelial Cells. Environ. Toxicol. 2024, 39, 5112–5123. [Google Scholar] [CrossRef]
- Han, J.; Tong, X.-Y.; Zheng, Y.-Y.; Cheng, J.-H.; Ouyang, J.-M.; Li, K. Corn Silk Polysaccharides Before and After Selenization Reduced Calcium Oxalate Crystal-Induced HK-2 Cells Pyroptosis by Inhibiting the NLRP3-GSDMD Signaling Pathway. J. Inflamm. Res. 2025, 18, 3623–3638. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, F. The cGAS-cGAMP-STING Pathway: A Molecular Link Between Immunity and Metabolism. Diabetes 2019, 68, 1099–1108. [Google Scholar] [CrossRef]
- Jha, J.C.; Dai, A.; Garzarella, J.; Charlton, A.; Urner, S.; Østergaard, J.A.; Okabe, J.; Holterman, C.E.; Skene, A.; Power, D.A.; et al. Independent of Renox, NOX5 Promotes Renal Inflammation and Fibrosis in Diabetes by Activating ROS-Sensitive Pathways. Diabetes 2022, 71, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Y.; Wang, S.; He, X.; Liu, M.; Bai, B.; Tian, C.; Sun, R.; Yu, T.; Chu, X. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol. 2021, 46, 102089. [Google Scholar] [CrossRef]
- Kushwaha, K.; Garg, S.S.; Gupta, J. Targeting epigenetic regulators for treating diabetic nephropathy. Biochimie 2022, 202, 146–158. [Google Scholar] [CrossRef]
- Takahashi, A.; Takabatake, Y.; Kimura, T.; Maejima, I.; Namba, T.; Yamamoto, T.; Matsuda, J.; Minami, S.; Kaimori, J.-Y.; Matsui, I.; et al. Autophagy Inhibits the Accumulation of Advanced Glycation End Products by Promoting Lysosomal Biogenesis and Function in the Kidney Proximal Tubules. Diabetes 2017, 66, 1359–1372. [Google Scholar] [CrossRef]
- Qin, J.; Peng, Z.; Yuan, Q.; Li, Q.; Peng, Y.; Wen, R.; Hu, Z.; Liu, J.; Xia, X.; Deng, H.; et al. AKF-PD alleviates diabetic nephropathy via blocking the RAGE/AGEs/NOX and PKC/NOX Pathways. Sci. Rep. 2019, 9, 4407. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Chang, X.; Xue, Y.; Liu, G.; Zhang, T.; Chen, W.; Fan, W.; Tian, J.; Ren, X. Isoliquiritigenin Alleviates Diabetic Kidney Disease via Oxidative Stress and the TLR4/NF-κB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2024, 68, e2400215. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zheng, S. Inhibition of NADPH Oxidase 5 (NOX5) Suppresses High Glucose-Induced Oxidative Stress, Inflammation and Extracellular Matrix Accumulation in Human Glomerular Mesangial Cells. Med. Sci. Monit. 2020, 26, e919399. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Hwang, I.; Han, S.H.; Shin, J.-S.; Shin, O.S.; Yu, J.-W. Advanced glycation end products impair NLRP3 inflammasome-mediated innate immune responses in macrophages. J. Biol. Chem. 2017, 292, 20437–20448. [Google Scholar] [CrossRef]
- Zhu, D.; Ni, Y.; Chen, C.; Dong, Z.; Wang, L.; Zhang, W. Geniposide ameliorates diabetic nephropathy in type 2 diabetic mice by targeting AGEs-RAGE-dependent inflammatory pathway. Phytomedicine 2024, 135, 156046. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Delguste, F.; Durand, A.; Guilbaud, A.; Rousselin, C.; Schmidt, A.M.; Tessier, F.; Boulanger, E.; Neviere, R. Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining MT dynamics and autophagy-lysosome pathway. Free Radic. Biol. Med. 2017, 112, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Steenbeke, M.; De Bruyne, S.; De Buyzere, M.; Lapauw, B.; Speeckaert, R.; Petrovic, M.; Delanghe, J.R.; Speeckaert, M.M. The role of soluble receptor for advanced glycation end-products (sRAGE) in the general population and patients with diabetes mellitus with a focus on renal function and overall outcome. Crit. Rev. Clin. Lab. Sci. 2021, 58, 113–130. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Koide, H.; Ueda, Y.; Takeuchi, M.; Yamagishi, S. Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin. Cardiol. 2011, 34, 372–377. [Google Scholar] [CrossRef]
- Jang, M.; Oh, S.W.; Lee, Y.; Kim, J.Y.; Ji, E.S.; Kim, P. Targeting extracellular matrix glycation to attenuate fibroblast activation. Acta Biomater. 2022, 141, 255–263. [Google Scholar] [CrossRef]
- Fan, W.; Adebowale, K.; Váncza, L.; Li, Y.; Rabbi, F.; Kunimoto, K.; Chen, D.; Mozes, G.; Chiu, D.K.-C.; Li, Y.; et al. Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver. Nature 2024, 626, 635–642. [Google Scholar] [CrossRef]
- Ozerov, A.; Merezhkina, D.; Zubkov, F.I.; Litvinov, R.; Ibragimova, U.; Valuisky, N.; Borisov, A.; Spasov, A. Synthesis and antiglycation activity of 3-phenacyl substituted thiazolium salts, new analogs of Alagebrium. Chem. Biol. Drug Des. 2024, 103, e14391. [Google Scholar] [CrossRef]
- Wang, F.; Peng, Y.; Liang, L.; Ruan, Y.; Yu, S.; Mo, X.; Tan, W.; Xu, X.; Jia, J.; Peng, J.; et al. HNF4A Regulated APEH Deficiency Promotes UPR Activation in Diabetic Kidney Disease. FASEB J. 2025, 39, e70649. [Google Scholar] [CrossRef]
- Zhang, P.; Su, C.; Jiang, Z.; Zheng, C. Herpes Simplex Virus 1 UL41 Protein Suppresses the IRE1/XBP1 Signal Pathway of the Unfolded Protein Response via Its RNase Activity. J. Virol. 2017, 91, e02056-16. [Google Scholar] [CrossRef] [PubMed]
- Luhr, M.; Torgersen, M.L.; Szalai, P.; Hashim, A.; Brech, A.; Staerk, J.; Engedal, N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019, 294, 8197–8217. [Google Scholar] [CrossRef]
- Vosbigian, K.A.; Wright, S.J.; Steiert, B.P.; Rosche, K.L.; Fisk, E.A.; Ramirez-Zepp, E.; Park, J.M.; Shelden, E.A.; Shaw, D.K. ATF6 enables pathogen infection in ticks by inducing stomatin and altering cholesterol dynamics. Proc. Natl. Acad. Sci. USA 2025, 122, e2501045122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Zhu, W.; Shi, X.; Xu, S. The endoplasmic reticulum-MT crosstalk is involved in the mitigation mechanism of eucalyptol on imidacloprid toxicity in Ctenopharyngodon idellus kidney cells. Fish Shellfish Immunol. 2022, 127, 99–108. [Google Scholar] [CrossRef]
- Masuda, M.; Miyazaki-Anzai, S.; Levi, M.; Ting, T.C.; Miyazaki, M. PERK-eIF2α-ATF4-CHOP signaling contributes to TNFα-induced vascular calcification. J. Am. Heart Assoc. 2013, 2, e000238. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, H.; Xiang, H.; Ke, H.; Dong, C.; Song, Q.; Zhou, J.; Jiang, Q.; Wang, Y.; Chen, L.; et al. Selenium participates in the formation of kidney stones by alleviating endoplasmic reticulum stress and apoptosis of renal tubular epithelial cells. Redox Rep. 2024, 29, 2416825. [Google Scholar] [CrossRef]
- Wang, W.-W.; Liu, Y.-L.; Wang, M.-Z.; Li, H.; Liu, B.-H.; Tu, Y.; Yuan, C.-C.; Fang, Q.-J.; Chen, J.-X.; Wang, J.; et al. Inhibition of Renal Tubular Epithelial Mesenchymal Transition and Endoplasmic Reticulum Stress-Induced Apoptosis with Shenkang Injection Attenuates Diabetic Tubulopathy. Front. Pharmacol. 2021, 12, 662706. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Su, Y.; Liu, H.; Su, Q.; Shen, T.; Zhang, M.; Mi, X.; Zhang, Y.; Yue, S.; et al. PDIA4 targets IRE1α/sXBP1 to alleviate NLRP3 inflammasome activation and renal tubular injury in diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167645. [Google Scholar] [CrossRef]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Zhou, Z.; Yu, H.; Zhang, S.; Huang, S.; Wei, Z.; Ren, K.; Jin, Y. Unraveling the complex interplay between Mitochondria-Associated Membranes (MAMs) and cardiovascular Inflammation: Molecular mechanisms and therapeutic implications. Int. Immunopharmacol. 2024, 141, 112930. [Google Scholar] [CrossRef]
- Li, D.; Zhang, K.; Xu, C.; Jiang, Y.; Shan, J.; Zhang, Z.; Cai, J. Cypermethrin induces apoptosis, autophagy and inflammation via ERS-ROS-NF-κB axis in hepatocytes of carp (Cyprinus carpio). Pestic. Biochem. Physiol. 2023, 196, 105625. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, H.; Li, M.; Zhuang, J.; Peng, Y.; Zhou, H.; Xu, C.; Yu, Q.; Fu, X.; Cao, S.; et al. Autophagy protein NRBF2 attenuates endoplasmic reticulum stress-associated neuroinflammation and oxidative stress via promoting autophagosome maturation by interacting with Rab7 after SAH. J. Neuroinflamm. 2021, 18, 210. [Google Scholar] [CrossRef]
- Patel, S.; Sathyanathan, V.; Salaman, S.D. Molecular mechanisms underlying cisplatin-induced nephrotoxicity and the potential ameliorative effects of essential oils: A comprehensive review. Tissue Cell 2024, 88, 102377. [Google Scholar] [CrossRef] [PubMed]
- Ruby, M.; Gifford, C.C.; Pandey, R.; Raj, V.S.; Sabbisetti, V.S.; Ajay, A.K. Autophagy as a Therapeutic Target for Chronic Kidney Disease and the Roles of TGF-β1 in Autophagy and Kidney Fibrosis. Cells 2023, 12, 412. [Google Scholar] [CrossRef]
- Shen, S.; Ji, C.; Wei, K. Cellular Senescence and Regulated Cell Death of Tubular Epithelial Cells in Diabetic Kidney Disease. Front. Endocrinol. 2022, 13, 924299. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N. Endothelial Toxicity of High Glucose and its by-Products in Diabetic Kidney Disease. Toxins 2019, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Green, D.R.; Droin, N.; Pinkoski, M. Activation-induced cell death in T cells. Immunol. Rev. 2003, 193, 70–81. [Google Scholar] [CrossRef]
- Pimentel, J.M.; Zhou, J.Y.; Wu, G.S. The Role of TRAIL in Apoptosis and Immunosurveillance in Cancer. Cancers 2023, 15, 2752. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Meng, Y.; Yan, B.; Zhou, Q.; Wang, X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol. Cell 2024, 84, 170–179. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 2011, 1813, 558–563. [Google Scholar] [CrossRef]
- Kelly, D.J.; Stein-Oakley, A.; Zhang, Y.; Wassef, L.; Maguire, J.; Koji, T.; Thomson, N.; Wilkinson-Berka, J.L.; Gilbert, R.E. Fas-induced apoptosis is a feature of progressive diabetic nephropathy in transgenic (mRen-2)27 rats: Attenuation with renin-angiotensin blockade. Nephrology 2004, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Yi, B.; Yao, B.-Q.; Xia, T.; Yang, Y.-F.; Zhang, Z.-H.; Chen, C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol. Res. 2020, 156, 104778. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Nakano, H. Necroptosis and Its Involvement in Various Diseases. Adv. Exp. Med. Biol. 2024, 1444, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, Y.; Zhao, Y.; Huang, S.; Xin, X.; Jiang, L.; Wang, H.; Wu, W.; Qu, L.; Xiang, C.; et al. Nephropathy Is Aggravated by Fatty Acids in Diabetic Kidney Disease through Tubular Epithelial Cell Necroptosis and Is Alleviated by an RIPK-1 Inhibitor. Kidney Dis. 2023, 9, 408–423. [Google Scholar] [CrossRef]
- Kang, J.S.; Cho, N.-J.; Lee, S.W.; Lee, J.G.; Lee, J.-H.; Yi, J.; Choi, M.S.; Park, S.; Gil, H.-W.; Oh, J.C.; et al. RIPK3 causes mitochondrial dysfunction and albuminuria in diabetic podocytopathy through PGAM5-Drp1 signaling. Metabolism 2024, 159, 155982. [Google Scholar] [CrossRef]
- Siegmund, D.; Wagner, J.; Wajant, H. TNF Receptor Associated Factor 2 (TRAF2) Signaling in Cancer. Cancers 2022, 14, 4055. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.T.J.; Wu, H.; Kirita, Y.; Chen, C.; Malvin, N.P.; Yoshimura, Y.; Muto, Y.; Humphreys, B.D. TNIK depletion induces inflammation and apoptosis in injured renal proximal tubule epithelial cells. Am. J. Physiol. Ren. Physiol. 2024, 326, F827–F838. [Google Scholar] [CrossRef]
- Lytrivi, M.; Castell, A.-L.; Poitout, V.; Cnop, M. Recent Insights into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Bai, Y.; Chuffa, L.G.d.A.; Loh, D.; Fan, L.; Cardinali, D.P. Function of intramitochondrial melatonin and its association with Warburg metabolism. Cell Signal 2025, 131, 111754. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Low, I.C.; Kang, J.; Pervaiz, S. Bcl-2: A prime regulator of mitochondrial redox metabolism in cancer cells. Antioxid. Redox Signal 2011, 15, 2975–2987. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Ferri, K.F.; Kroemer, G. Mitochondria--the suicide organelles. Bioessays 2001, 23, 111–115. [Google Scholar] [CrossRef]
- Mignotte, B.; Vayssiere, J.L. Mitochondria and apoptosis. Eur. J. Biochem. 1998, 252, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; Jiang, L.; Shan, B.; Xiao, P.; Ai, J.; Li, N.; Qi, F.; Niu, S. Mechanism of Heshouwuyin inhibiting the Cyt c/Apaf-1/Caspase-9/Caspase-3 pathway in spermatogenic cell apoptosis. BMC Complement. Med. Ther. 2020, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Obitsu, S.; Sakata, K.; Teshima, R.; Kondo, K. Eleostearic acid induces RIP1-mediated atypical apoptosis in a kinase-independent manner via ERK phosphorylation, ROS generation and mitochondrial dysfunction. Cell Death Dis. 2013, 4, e674. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, X.; Guo, J.; Wang, Y.; Jiang, Y.; Li, S.; Liu, Y.N.; Liu, W.J. JinChan YiShen TongLuo Formula ameliorate mitochondrial dysfunction and apoptosis in diabetic nephropathy through the HIF-1α-PINK1-Parkin pathway. J. Ethnopharmacol. 2024, 328, 117863. [Google Scholar] [CrossRef]
- Duan, T.; Sun, L.; Xi, Y.; Li, C.; Zhao, Q.; Xu, L.; Yang, M.; Liu, C.; Xiao, L.; Deng, T.; et al. Mito-tempo ameliorates tubular injury of diabetic nephropathy via inhibiting mt-dsRNA release and PKR/eIF2α pathway activation. Free Radic. Biol. Med. 2025, 237, 147–159. [Google Scholar] [CrossRef]
- Lewko, B.; Wodzińska, M.; Daca, A.; Płoska, A.; Obremska, K.; Kalinowski, L. Urolithin A Ameliorates the TGF Beta-Dependent Impairment of Podocytes Exposed to High Glucose. J. Pers. Med. 2024, 14, 914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, B.; Cheng, S.; Fan, H.; Liu, S.; Zhou, B.; Liu, W.; Liang, R.; Tang, Y.; Zhang, Y. KDELR2 knockdown synergizes with temozolomide to induce glioma cell apoptosis through the CHOP and JNK/p38 pathways. Transl. Cancer Res. 2021, 10, 3491–3506. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.-H.; Gao, F.F.; Lee, M.; Yuk, J.-M.; Cha, G.-H.; Chu, J.-Q.; Wang, H.; Lee, Y.-H. Involvement of endoplasmic reticulum stress response and IRE1-mediated ASK1/JNK/Mcl-1 pathways in silver nanoparticle-induced apoptosis of human retinal pigment epithelial cells. Toxicology 2020, 442, 152540. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Calì, T. Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role. Cell Calcium 2021, 94, 102343. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Yang, M.; Yang, J.; Zhao, C.; Han, Y.; Zhao, H.; Jiang, N.; Wei, L.; Xiao, Y.; et al. PACS-2 Ameliorates Tubular Injury by Facilitating Endoplasmic Reticulum-Mitochondria Contact and Mitophagy in Diabetic Nephropathy. Diabetes 2022, 71, 1034–1050. [Google Scholar] [CrossRef]
- Kumar, V. Toll-Like Receptors in Adaptive Immunity. Handb. Exp. Pharmacol. 2022, 276, 95–131. [Google Scholar] [CrossRef]
- Pandey, A.; Li, Z.; Gautam, M.; Ghosh, A.; Man, S.M. Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis. Immunol. Rev. 2025, 329, e13406. [Google Scholar] [CrossRef]
- Liang, K.; Xu, L.; Ta, Y.; Wang, R.; Ma, S.; Xiao, C.; Zhang, X.; Gao, Y.; Li, M. Exploring the mechanism of TLR4/NF-κB signaling pathway in hypoxic myocardial injury: Implications for traditional Chinese medicine therapy. Fitoterapia 2025, 185, 106721. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, M.; Jin, Z.; Zheng, C.; Ye, H.; Weng, H. Dapagliflozin ameliorates diabetic renal injury through suppressing the self-perpetuating cycle of inflammation mediated by HMGB1 feedback signaling in the kidney. Eur. J. Pharmacol. 2023, 943, 175560. [Google Scholar] [CrossRef]
- Wang, J.S.; Chiu, Y.T. Systemic hypoxia enhances exercise-mediated bactericidal and subsequent apoptotic responses in human neutrophils. J. Appl. Physiol. 2009, 107, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, X.; Liu, N.; He, J.C.; Zhong, Y. Relationship between Macrophages and Tissue Microenvironments in Diabetic Kidneys. Biomedicines 2023, 11, 1889. [Google Scholar] [CrossRef]
- Verçosa, B.L.A.; Muniz-Junqueira, M.I.; Menezes-Souza, D.; Magalhães, L.M.D.; Fujiwara, R.T.; Melo, M.N.; Vasconcelos, A.C. Enhanced apoptotic index, chemokines and inflammatory recruitment in renal tissues shows relationship with the clinical signs in Leishmania-infected dogs. Vet. Parasitol. 2021, 300, 109611. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Tontanahal, A. Extracellular vesicles in renal inflammatory and infectious diseases. Free Radic. Biol. Med. 2021, 171, 42–54. [Google Scholar] [CrossRef]
- Scalzone, A.; Sanjurjo-Rodríguez, C.; Berlinguer-Palmini, R.; Dickinson, A.M.; Jones, E.; Wang, X.-N.; Crossland, R.E. Functional and Molecular Analysis of Human Osteoarthritic Chondrocytes Treated with Bone Marrow-Derived MSC-EVs. Bioengineering 2024, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zheng, Z.; Guan, M.; Zhang, Q.; Li, Y.; Wang, L.; Xue, Y. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Phillips, P.C.A.; de Sousa Loreto Aresta Branco, M.; Cliff, C.L.; Ward, J.K.; Squires, P.E.; Hills, C.E. Targeting senescence to prevent diabetic kidney disease: Exploring molecular mechanisms and potential therapeutic targets for disease management. Diabet. Med. 2025, 42, e15408. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Du, Y.; Liu, Y.; Zhang, Z.; Zhang, X.; Li, J.; Huang, G.; Liu, F.; Li, B.; et al. p16INK4a Deletion Alleviated Obesity-Associated Kidney Fibrosis by Regulating Metabolic Reprogramming and the Inflammasome Pathway. J. Cell Mol. Med. 2025, 29, e70444. [Google Scholar] [CrossRef]
- Yang, M.; Wu, S.; Dai, Q.; Qin, W.; Zhang, Y.; Lei, Y.; Song, H.; Zheng, T.; Guan, M.; Huang, G.; et al. Andrographolide prevents renal fibrosis via decelerating lipotoxicity-mediated premature senescence of tubular epithelial cells. Biochem. Pharmacol. 2024, 230 Pt 3, 116615. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Hu, S.; Qin, W.; Shi, J.; Hou, Q.; Wang, X.; Xu, X.; Zhang, M.; Zeng, C.; Liu, Z.; et al. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight. 2019, 4, e122912, Correction in JCI Insight. 2019, 4, e130986. [Google Scholar] [CrossRef]
- Huang, J.; Meng, P.; Liang, Y.; Li, X.; Zhou, S.; Li, J.; Wang, X.; Miao, J.; Shen, W.; Zhou, L. Tubular CD44 plays a key role in aggravating AKI through NF-κB p65-mediated mitochondrial dysfunction. Cell Death Dis. 2025, 16, 119. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Eddy, A.A. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 1996, 7, 2495–2508. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, H.; Li, X.; Pan, J.; Li, H.; Wang, L.; Zhang, P.; Chen, J.; Qiu, S.; Xie, Y.; et al. Discovery of PRDM16-Mediated TRPA1 Induction as the Mechanism for Low Tubulo-Interstitial Fibrosis in Diabetic Kidney Disease. Adv. Sci. 2024, 11, e2306704. [Google Scholar] [CrossRef]
- Tang, R.; Xiao, X.; Lu, Y.; Li, H.; Zhou, Q.; Nuro-Gyina, P.K.; Li, X. Interleukin-22 attenuates renal tubular cells inflammation and fibrosis induced by TGF-β1 through Notch1 signaling pathway. Ren. Fail. 2020, 42, 381–390. [Google Scholar] [CrossRef]
- Miyajima, A.; Chen, J.; Kirman, I.; Poppas, D.P.; Vaughan, E.D.; Felsen, D. Interaction of nitric oxide and transforming growth factor-beta1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J. Urol. 2000, 164, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Militi, S.; Nibhani, R.; Pook, M.; Pauklin, S. SMAD2/3-SMYD2 and developmental transcription factors cooperate with cell-cycle inhibitors to guide tissue formation. Protein Cell 2025, 16, 260–285. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Zhai, Y.; Xiong, R.-P.; Chen, X.; Liu, P.; Peng, Y.; Zhao, Y.; Ning, Y.-L.; Yang, N.; Zhou, Y.-G. Ski mediates TGF-β1-induced fibrosarcoma cell proliferation and promotes tumor growth. J. Cancer 2020, 11, 5929–5940. [Google Scholar] [CrossRef]
- Samuvel, D.J.; Lemasters, J.J.; Chou, C.J.; Zhong, Z. LP340, a novel histone deacetylase inhibitor, decreases liver injury and fibrosis in mice: Role of oxidative stress and microRNA-23a. Front. Pharmacol. 2024, 15, 1386238. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Song, H.; Yang, C. Lapatinib ameliorates skin fibrosis by inhibiting TGF-β1/Smad and non-Smad signaling pathway. Sci. Rep. 2025, 15, 8444. [Google Scholar] [CrossRef]
- Livingston, M.J.; Zhang, M.; Kwon, S.-H.; Chen, J.-K.; Li, H.; Manicassamy, S.; Dong, Z. Autophagy activates EGR1 via MAPK/ERK to induce FGF2 in renal tubular cells for fibroblast activation and fibrosis during maladaptive kidney repair. Autophagy 2024, 20, 1032–1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Deng, Y.; Chen, J.; Huang, M.; Zhu, F.; Gao, Z.; Wu, L.; Hong, Q.; Feng, Z.; et al. The PI3K-Akt-mTOR pathway mediates renal pericyte-myofibroblast transition by enhancing glycolysis through HKII. J. Transl. Med. 2023, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-Y.; Juan, Y.-H.; Hung, T.-W.; Tsai, Y.-P.; Ting, Y.-H.; Lee, C.-C.; Tsai, J.-P.; Hsieh, Y.-H. β-Mangostin Alleviates Renal Tubulointerstitial Fibrosis via the TGF-β1/JNK Signaling Pathway. Cells 2024, 13, 1701. [Google Scholar] [CrossRef]
- Wang, J.; Ge, S.; Wang, Y.; Liu, Y.; Qiu, L.; Li, J.; Huang, X.; Sun, L. Puerarin Alleviates UUO-Induced Inflammation and Fibrosis by Regulating the NF-κB P65/STAT3 and TGFβ1/Smads Signaling Pathways. Drug Des. Dev. Ther. 2021, 15, 3697–3708. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, J.; Yang, R.; Bai, J.; Deng, B.; Cheng, L.; Gao, F.; Xie, J.; Zhang, B. The CaMKII Inhibitory Peptide AIP Alleviates Renal Fibrosis Through the TGF-β/Smad and RAF/ERK Pathways. J. Pharmacol. Exp. Ther. 2023, 386, 310–322. [Google Scholar] [CrossRef]
- Gagnani, R.; Srivastava, M.; Suri, M.; Singh, H.; Navik, U.S.; Bali, A. A focus on c-Jun-N-terminal kinase signaling in sepsis-associated multiple organ dysfunction: Mechanisms and therapeutic strategies. Int. Immunopharmacol. 2024, 143 Pt 3, 113552. [Google Scholar] [CrossRef]
- Zhao, S.; Li, W.; Yu, W.; Rao, T.; Li, H.; Ruan, Y.; Yuan, R.; Li, C.; Ning, J.; Li, S.; et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics 2021, 11, 8660–8673. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Ma, X.; An, L.; Liu, Y.; Wang, Q. Interaction of circulating TGFβ regulatory miRNAs in different severity of diabetic kidney disease. Arch. Physiol. Biochem. 2024, 130, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Gifford, C.C.; Tang, J.; Costello, A.; Khakoo, N.S.; Nguyen, T.Q.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin. Sci. 2021, 135, 275–303. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Chang, C.W.; Shen, Y.C.; Yan, S.J. HP1a-mediated heterochromatin formation inhibits high dietary sugar-induced tumor progression. Cell Death Dis. 2021, 12, 1130. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Shen, H.; Huang, X.; Zhao, Y.; Wu, D.; Xue, K.; Yao, J.; Wang, Y.; Tang, N.; Qiu, Y. The Hippo pathway links adipocyte plasticity to adipose tissue fibrosis. Nat. Commun. 2022, 13, 6030. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; He, Q.; Bulus, N.; Fogo, A.B.; Zhang, M.-Z.; Harris, R.C. YAP Activation in Renal Proximal Tubule Cells Drives Diabetic Renal Interstitial Fibrogenesis. Diabetes 2020, 69, 2446–2457. [Google Scholar] [CrossRef]
- Wolf, M.T.F.; Bonsib, S.M.; Larsen, C.P.; Hildebrandt, F. Nephronophthisis: A pathological and genetic perspective. Pediatr. Nephrol. 2024, 39, 1977–2000. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Wang, L.; Tao, Q.; Tu, C. Hippo/YAP signaling pathway: A new therapeutic target for diabetes mellitus and vascular complications. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231220134. [Google Scholar] [CrossRef]
- Zhu, B.; Li, F.; Yu, J.; Liang, Z.; Ke, X.; Wang, Y.; Song, Z.; Li, Z.; Li, G.; Guo, Y. PIEZO1 mediates matrix stiffness-induced tumor progression in kidney renal clear cell carcinoma by activating the Ca2+/Calpain/YAP pathway. Biochim. Biophys. Acta Mol. Cell Res. 2025, 1872, 119871. [Google Scholar] [CrossRef]
- Tilston-Lunel, A.; Mazzilli, S.; Kingston, N.M.; Szymaniak, A.D.; Hicks-Berthet, J.; Kern, J.G.; Abo, K.; Reid, M.E.; Perdomo, C.; Wilson, A.A.; et al. Aberrant epithelial polarity cues drive the development of precancerous airway lesions. Proc. Natl. Acad. Sci. USA 2021, 118, e2019282118. [Google Scholar] [CrossRef]
- Wang, L.; Choi, K.; Su, T.; Li, B.; Wu, X.; Zhang, R.; Driskill, J.H.; Li, H.; Lei, H.; Guo, P.; et al. Multiphase coalescence mediates Hippo pathway activation. Cell 2022, 185, 4376–4393.e18. [Google Scholar] [CrossRef]
- Wang, T.-T.; Wu, L.-L.; Wu, J.; Zhang, L.-S.; Shen, W.-J.; Zhao, Y.-H.; Liu, J.-N.; Fu, B.; Wang, X.; Li, Q.-G.; et al. 14-3-3ζ inhibits maladaptive repair in renal tubules by regulating YAP and reduces renal interstitial fibrosis. Acta Pharmacol. Sin. 2023, 44, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chrysopoulou, M.; Rinschen, M.M. Metabolic Rewiring and Communication: An Integrative View of Kidney Proximal Tubule Function. Annu. Rev. Physiol. 2024, 86, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Wong, D.W.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell Signal 2019, 57, 76–88. [Google Scholar] [CrossRef]
- Xia, W.; Chen, X.; Zhu, Z.; Chen, H.; Li, B.; Wang, K.; Huang, L.; Liu, Z.; Chen, Z. Knockdown of lncRNA MALAT1 attenuates renal interstitial fibrosis through miR-124-3p/ITGB1 axis. Sci. Rep. 2023, 13, 18076. [Google Scholar] [CrossRef]
- Fu, Z.; Geng, X.; Liu, C.; Shen, W.; Dong, Z.; Sun, G.; Cai, G.; Chen, X.; Hong, Q. Identification of common and specific fibrosis-related genes in three common chronic kidney diseases. Ren. Fail. 2024, 46, 2295431. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef]
- Yamashita, N.; Kramann, R. Mechanisms of kidney fibrosis and routes towards therapy. Trends Endocrinol. Metab. 2024, 35, 31–48. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Docherty, M.-H.; O’sullivan, E.D.; Bonventre, J.V.; Ferenbach, D.A. Cellular Senescence in the Kidney. J. Am. Soc. Nephrol. 2019, 30, 726–736. [Google Scholar] [CrossRef]
- Tsukui, T.; Wolters, P.J.; Sheppard, D. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature 2024, 631, 627–634. [Google Scholar] [CrossRef]
- Baues, M.; Klinkhammer, B.M.; Ehling, J.; Gremse, F.; van Zandvoort, M.A.; Reutelingsperger, C.P.; Daniel, C.; Amann, K.; Bábíčková, J.; Kiessling, F.; et al. A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo. Kidney Int. 2020, 97, 609–614. [Google Scholar] [CrossRef]
- Ma, X.; Ma, J.; Leng, T.; Yuan, Z.; Hu, T.; Liu, Q.; Shen, T. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, C. Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease. Antioxidants 2024, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Pinoșanu, E.A.; Pîrșcoveanu, D.; Albu, C.V.; Burada, E.; Pîrvu, A.; Surugiu, R.; Sandu, R.E.; Serb, A.F. Rhoa/ROCK, mTOR and Secretome-Based Treatments for Ischemic Stroke: New Perspectives. Curr. Issues Mol. Biol. 2024, 46, 3484–3501. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Girerd, S.; Jaisser, F. Mineralocorticoid receptor antagonists and kidney diseases: Pathophysiological basis. Kidney Int. 2019, 96, 302–319. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diab Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Yao, L.; Liang, X.; Liu, Y.; Li, B.; Hong, M.; Wang, X.; Chen, B.; Liu, Z.; Wang, P. Non-steroidal mineralocorticoid receptor antagonist finerenone ameliorates mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in diabetic tubulopathy. Redox Biol. 2023, 68, 102946. [Google Scholar] [CrossRef]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients With Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef]
- Wanner, C.; Nangaku, M.; Kraus, B.J.; Zinman, B.; Mattheus, M.; Hantel, S.; Schumacher, M.; Ohneberg, K.; Schmoor, C.; Inzucchi, S.E. How do SGLT2 inhibitors protect the kidney? A mediation analysis of the EMPA-REG OUTCOME trial. Nephrol. Dial. Transplant. 2024, 39, 1504–1513, Correction in Nephrol. Dial. Transplant. 2025, 60, 1261. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Jardine, M.J.; Zhou, Z.; Mahaffey, K.W.; Oshima, M.; Agarwal, R.; Bakris, G.; Bajaj, H.S.; Bull, S.; Cannon, C.P.; Charytan, D.M.; et al. Renal, Cardiovascular, and Safety Outcomes of Canagliflozin by Baseline Kidney Function: A Secondary Analysis of the CREDENCE Randomized Trial. J. Am. Soc. Nephrol. 2020, 31, 1128–1139. [Google Scholar] [CrossRef]
- Liu, X.; Xu, C.; Xu, L.; Li, X.; Sun, H.; Xue, M.; Li, T.; Yu, X.; Sun, B.; Chen, L. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism 2020, 111, 154334. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Li, N.; Yang, X.; Zhou, L.; Li, H.; Wang, T.; Xie, M.; Liu, H. Dapagliflozin delays renal fibrosis in diabetic kidney disease by inhibiting YAP/TAZ activation. Life Sci. 2023, 322, 121671. [Google Scholar] [CrossRef]
- Chang, S.Y.; Liao, M.C.; Miyata, K.N.; Pang, Y.; Zhao, X.P.; Peng, J.; Rivard, A.; Ingelfinger, J.R.; Chan, J.S.D.; Zhang, S.L. Canagliflozin inhibits hedgehog interacting protein (Hhip) induction of tubulopathy in diabetic Akita mice. Transl. Res. 2025, 277, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Choi, Y.-K.; Woo, H.-I.; Jung, Y.-A.; Lee, S.; Lee, S.; Park, M.; Lee, I.-K.; Jung, G.-S.; Park, K.-G. Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome. Diabetes Metab. J. 2019, 43, 830–839. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Brosius, F.C.; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, X.; Zhao, Y.; Zhou, M.; Yang, Y.; Zhang, X. Calcitriol attenuates renal tubular epithelial cells apoptosis via inhibiting p38MAPK signaling in diabetic nephropathy. Acta Diabetol. 2020, 57, 1327–1335. [Google Scholar] [CrossRef]
- l-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocin-induced type 1 diabetes. Biomed. Pharmacother. 2018, 105, 290–298. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, K.; Hou, L.; Liao, J.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; Hu, L.; Pan, J.; et al. Endoplasmic reticulum stress contributes to pyroptosis through NF-κB/NLRP3 pathway in diabetic nephropathy. Life Sci. 2023, 322, 121656. [Google Scholar] [CrossRef]
- Marquardt, A.; Al-Dabet, M.M.; Ghosh, S.; Kohli, S.; Manoharan, J.; ElWakiel, A.; Gadi, I.; Bock, F.; Nazir, S.; Wang, H.; et al. Farnesoid X Receptor Agonism Protects against Diabetic Tubulopathy: Potential Add-On Therapy for Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3182–3189. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Bjornstad, P.; Kramer, M.H.H.; Diamant, M.; Hoorn, E.J.; Joles, J.A.; van Raalte, D.H. Effect of immediate and prolonged GLP-1 receptor agonist administration on uric acid and kidney clearance: Post-hoc analyses of four clinical trials. Diabetes Obes. Metab. 2018, 20, 1235–1245. [Google Scholar] [CrossRef]
- Zitman-Gal, T.; Einbinder, Y.; Ohana, M.; Katzav, A.; Kartawy, A.; Benchetrit, S. Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J. Diabetes 2019, 11, 656–664. [Google Scholar] [CrossRef]
- Liu, F.; Feng, Q.; Yang, M.; Yang, Y.; Nie, J.; Wang, S. Quercetin prevented diabetic nephropathy by inhibiting renal tubular epithelial cell apoptosis via the PI3K/AKT pathway. Phytother. Res. 2024, 38, 3594–3606. [Google Scholar] [CrossRef]
- Mao, Z.-M.; Shen, S.-M.; Wan, Y.-G.; Sun, W.; Chen, H.-L.; Huang, M.-M.; Yang, J.-J.; Wu, W.; Tang, H.-T.; Tang, R.-M. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J. Ethnopharmacol. 2015, 173, 256–265. [Google Scholar] [CrossRef]
- Huilian, H.; Ya, L.; Jin-Dong, L. The mechanism of emodin on the transdifferentiation of renal tubular epithelial cells based on mTOR pathway. Chin. J. Hosp. Pharm. 2019, 33, 1729–1733. [Google Scholar] [CrossRef]
- Liu, X.H. Effect of Astragaloside IV on high glucose-induced apoptosis of renal tubular epithelial cells and expression of mitophagy-related proteins. J. Guangzhou Univ. Chin. Med. 2019, 4, 251–255. [Google Scholar] [CrossRef]
- Cao, L.; Huang, B.; Fu, X.; Yang, J.; Lin, Y.; Lin, F. Effects of tanshinone IIA on the regulation of renal proximal tubular fibrosis. Mol. Med. Rep. 2017, 15, 4247–4252. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Zhang, X.-N.; Li, L.; Yang, W.; Wang, S.-S.; Guo, X.; Sun, P.; Chen, L.-M. Tripterygium Glycosides Tablet Ameliorates Renal Tubulointerstitial Fibrosis via the Toll-Like Receptor 4/Nuclear Factor Kappa B Signaling Pathway in High-Fat Diet Fed and Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2015, 2015, 390428. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, S.; Chen, J.; Zhang, J.; Lu, Y.; Gu, Q.; Yan, Z.; Chen, W.; Chen, A.; Fang, Y.; et al. Cordycepin Ameliorates Renal Interstitial Fibrosis by Inhibiting Drp1-Mediated Mitochondrial Fission. Drug Des. Dev. Ther. 2025, 19, 1271–1287. [Google Scholar] [CrossRef]
- Hou, B.; Ma, P.; Yang, X.; Zhao, X.; Zhang, L.; Zhao, Y.; He, P.; Du, G.; Qiang, G. In silico prediction and experimental validation to reveal the protective mechanism of Puerarin against excessive extracellular matrix accumulation through inhibiting ferroptosis in diabetic nephropathy. J. Ethnopharmacol. 2024, 319 Pt 2, 117281. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, L.; Liang, R.; Yang, C.; Wang, P. Baicalin suppresses renal fibrosis through microRNA-124/TLR4/NF-κB axis in streptozotocin-induced diabetic nephropathy mice and high glucose-treated human proximal tubule epithelial cells. J. Physiol. Biochem. 2020, 76, 407–416. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.-G.; Liu, Z.; Gong, X.-J.; Hu, J.-N.; Wang, Y.-P.; Liu, W.-C.; Lin, X.-H.; Wang, Z.; Li, W. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 2021, 267, 113500. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Pan, Z.; Yao, W.; Gao, X.; Wang, X.; Ding, X. Icariin Prevents Extracellular Matrix Accumulation and Ameliorates Experimental Diabetic Kidney Disease by Inhibiting Oxidative Stress via GPER Mediated p62-Dependent Keap1 Degradation and Nrf2 Activation. Front. Cell Dev. Biol. 2020, 8, 559. [Google Scholar] [CrossRef]
- Flores-Estrada, J.; Cano-Martínez, A.; Ibarra-Lara, L.; Jiménez, A.; Palacios-Reyes, C.; García, L.J.P.; Ortiz-López, M.G.; Rodríguez-Peña, O.N.; Hernández-Portilla, L.B. Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis. Int. J. Mol. Sci. 2025, 26, 4730. [Google Scholar] [CrossRef]
- Xu, Z.J.; Shu, S.; Li, Z.J.; Liu, Y.M.; Zhang, R.Y.; Zhang, Y. Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-β/SMADS, MAPK, and NF-kB and upregulating expression of cytoglobin in renal tissues. Medicine 2017, 96, e5879. [Google Scholar] [CrossRef]
- Han, C.; Shen, Z.; Cui, T.; Ai, S.-S.; Gao, R.-R.; Liu, Y.; Sui, G.-Y.; Hu, H.-Z.; Li, W. Yi-Shen-Hua-Shi granule ameliorates diabetic kidney disease by the “gut-kidney axis”. J. Ethnopharmacol. 2023, 307, 116257. [Google Scholar] [CrossRef]
- Liu, J.; Gao, L.-D.; Fu, B.; Yang, H.-T.; Zhang, L.; Che, S.-Q.; Xu, Y.; Du, X.; Liu, Z.-C.; Xue, Y.; et al. Efficacy and safety of Zicuiyin decoction on diabetic kidney disease: A multicenter, randomized controlled trial. Phytomedicine 2022, 100, 154079. [Google Scholar] [CrossRef]
- Zhu, Z.; Luan, G.; Peng, S.; Fang, Y.; Fang, Q.; Shen, S.; Wu, K.; Qian, S.; Jia, W.; Ye, J.; et al. Huangkui capsule attenuates diabetic kidney disease through the induction of mitophagy mediated by STING1/PINK1 signaling in tubular cells. Phytomedicine 2023, 119, 154975. [Google Scholar] [CrossRef]
- Wang, X.; He, Q.; Chen, Q.; Xue, B.; Wang, J.; Wang, T.; Liu, H.; Chen, X. Network pharmacology combined with metabolomics to study the mechanism of Shenyan Kangfu Tablets in the treatment of diabetic nephropathy. J. Ethnopharmacol. 2021, 270, 113817. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Qi, Y.; Chen, P.; Chen, L.; Jiang, Y.; Fan, Z.; Guan, H.; Bai, L.; Liu, J.; Zhao, D.; et al. Dang-Gui-Bu-Xue decoction against diabetic nephropathy via modulating the carbonyl compounds metabolic profile and AGEs/RAGE pathway. Phytomedicine 2024, 135, 156104. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Zhang, H.-J.; Zhang, W.; Feng, K.; Liu, H.; Zhao, H.-L.; Li, P. Tangshen Formula alleviates inflammatory injury against aged diabetic kidney disease through modulating gut microbiota composition and related amino acid metabolism. Exp. Gerontol. 2024, 188, 112393. [Google Scholar] [CrossRef]
- Hu, S.-J.; Shu, B.; Jin, H.; Li, X.-F.; Mao, J.-R.; Ren, K.-J.; Gao, L.; Yang, L.; Wu, Y.-W.; Wang, Y.-J. Therapeutic Role of Tangshenkang Granule () in Rat Model with Diabetic Nephropathy. Chin. J. Integr. Med. 2018, 24, 600–605. [Google Scholar] [CrossRef]
- Gu, L.-Y.; Sun, Y.; Tang, H.-T.; Xu, Z.-X. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway. J. Ethnopharmacol. 2021, 281, 113548. [Google Scholar] [CrossRef]
- Satirapoj, B. Tubulointerstitial Biomarkers for Diabetic Nephropathy. J. Diabetes Res. 2018, 2018, 2852398. [Google Scholar] [CrossRef]
- Mou, X.; Zhou, D.-Y.; Zhou, D.-Y.; Ma, J.-R.; Liu, Y.-H.; Chen, H.-P.; Hu, Y.-B.; Shou, C.-M.; Chen, J.-W.; Liu, W.-H.; et al. Serum TGF-β1 as a Biomarker for Type 2 Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0149513. [Google Scholar] [CrossRef]
- Xu, W.; Yi, F.; Liao, H.; Zhu, C.; Zou, X.; Dong, Y.; Zhou, W.; Sun, Z.; Yin, J. The Potential and Challenges of Human Pluripotent Stem Cells in the Treatment of Diabetic Nephropathy. Front. Biosci. (Landmark Ed.) 2025, 30, 28283. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-L.; Zhang, Y.; Pang, L.; Dong, Y.-F.; Li, M.-Y.; Liao, H.; Li, R.-S. Integrated Analysis of Single-Cell RNA-Seq and Bulk RNA-Seq Combined with Multiple Machine Learning Identified a Novel Immune Signature in Diabetic Nephropathy. Diabetes Metab. Syndr. Obes. 2023, 16, 1669–1684, Correction in Diabetes Metab. Syndr. Obes. 2025, 18, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Hopp, K. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin. J. Am. Soc. Nephrol. 2020, 15, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakashima, M.; Ishikiriyama, T.; Nakashima, H.; Yamagata, A.; Imakiire, T.; Kinoshita, M.; Seki, S.; Kumagai, H.; Oshima, N. Effects of L-Carnitine Treatment on Kidney Mitochondria and Macrophages in Mice with Diabetic Nephropathy. Kidney Blood Press. Res. 2022, 47, 277–290. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 29. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-chips technologies—A guide from disease models to opportunities for drug development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, C.; Fu, Y.; Xie, L.; Kong, Y.; Yang, X. Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications. Curr. Issues Mol. Biol. 2025, 47, 885. https://doi.org/10.3390/cimb47110885
Liu X, Zhang C, Fu Y, Xie L, Kong Y, Yang X. Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications. Current Issues in Molecular Biology. 2025; 47(11):885. https://doi.org/10.3390/cimb47110885
Chicago/Turabian StyleLiu, Xuanke, Chunjiang Zhang, Yanjie Fu, Linlin Xie, Yijing Kong, and Xiaoping Yang. 2025. "Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications" Current Issues in Molecular Biology 47, no. 11: 885. https://doi.org/10.3390/cimb47110885
APA StyleLiu, X., Zhang, C., Fu, Y., Xie, L., Kong, Y., & Yang, X. (2025). Inflammation, Apoptosis, and Fibrosis in Diabetic Nephropathy: Molecular Crosstalk in Proximal Tubular Epithelial Cells and Therapeutic Implications. Current Issues in Molecular Biology, 47(11), 885. https://doi.org/10.3390/cimb47110885





