CNPY3 Promotes Human Breast Cancer Progression and Metastasis via Modulation of the Tumor Microenvironment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cell Lines
2.2. Reagents
2.3. Oncomine Database
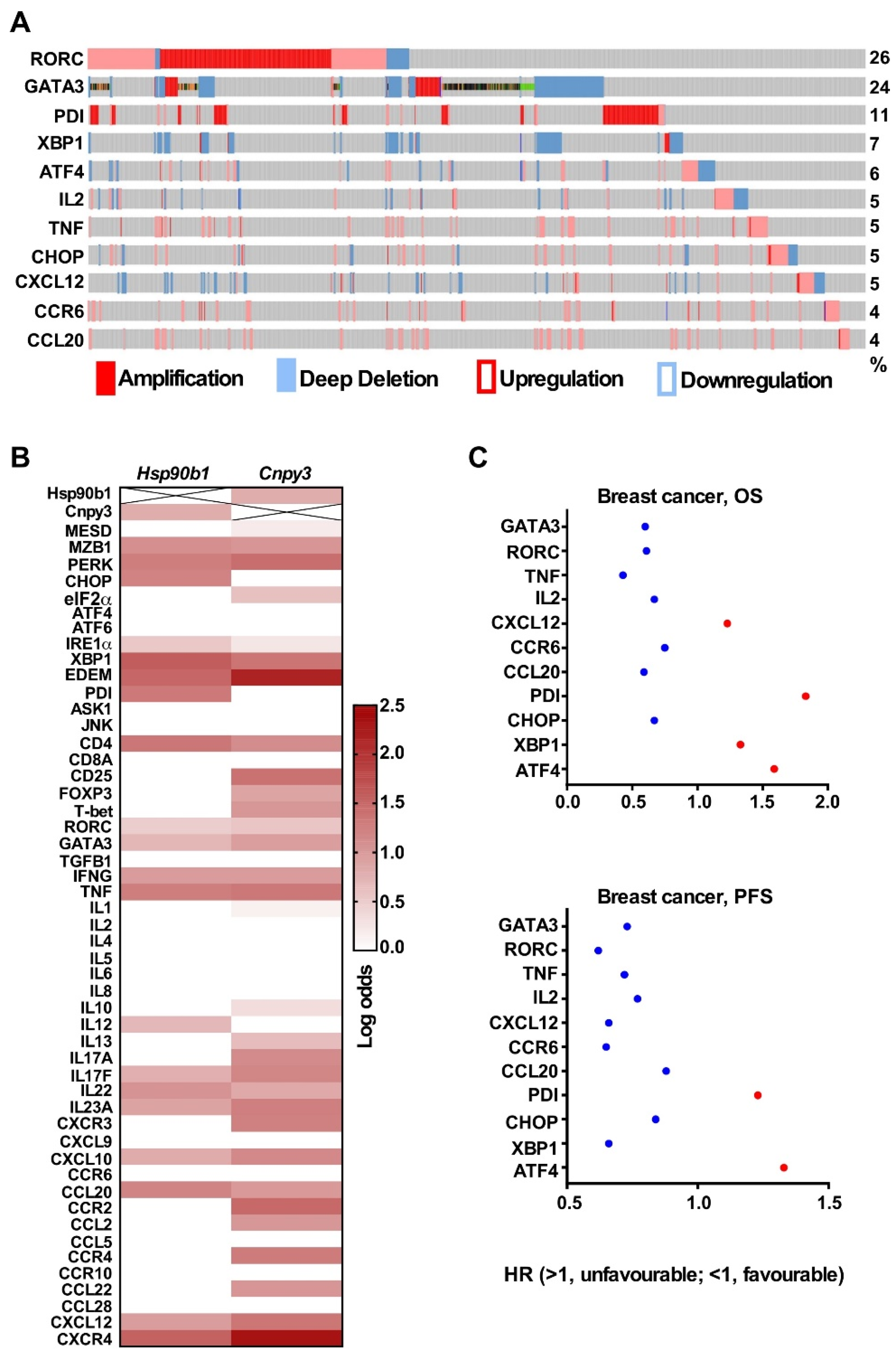
2.4. The cBioPortal for Cancer Genomics
2.5. Kaplan–Meier Plotter
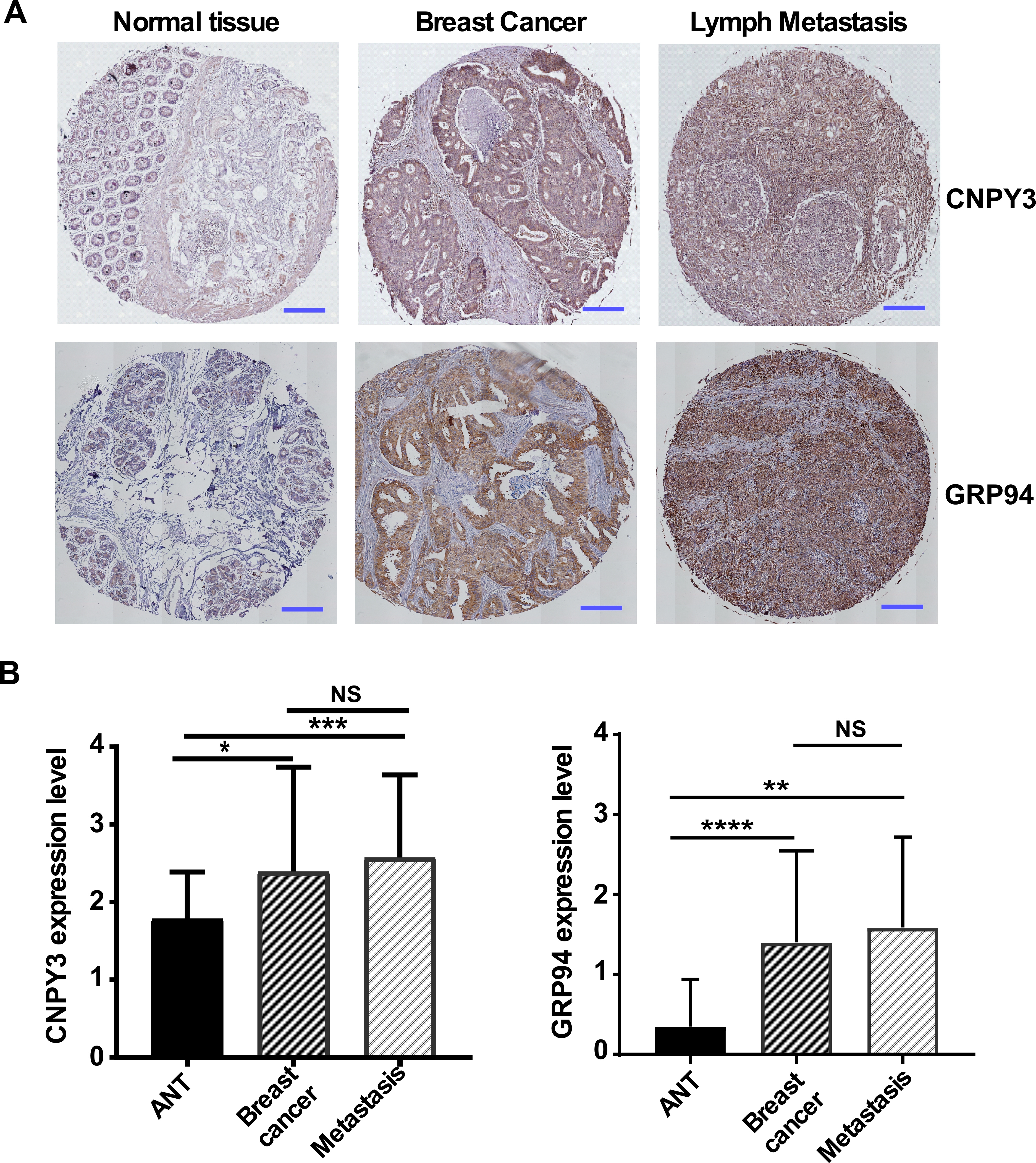
2.6. Tumor Tissue Array and Patient Data
2.7. Immunohistochemistry (IHC)
2.8. CNPY3 CRIPR/Cas9 Viral Vectors and Transduction
2.9. Protein Extraction and Western Blot
2.10. Colony Formation Assay
2.11. Human Breast Cancer Xenograft Model
2.12. Statistical Analysis
3. Results
3.1. The Expression of CNPY3 and GRP94 Is Upregulated in Human Cancers
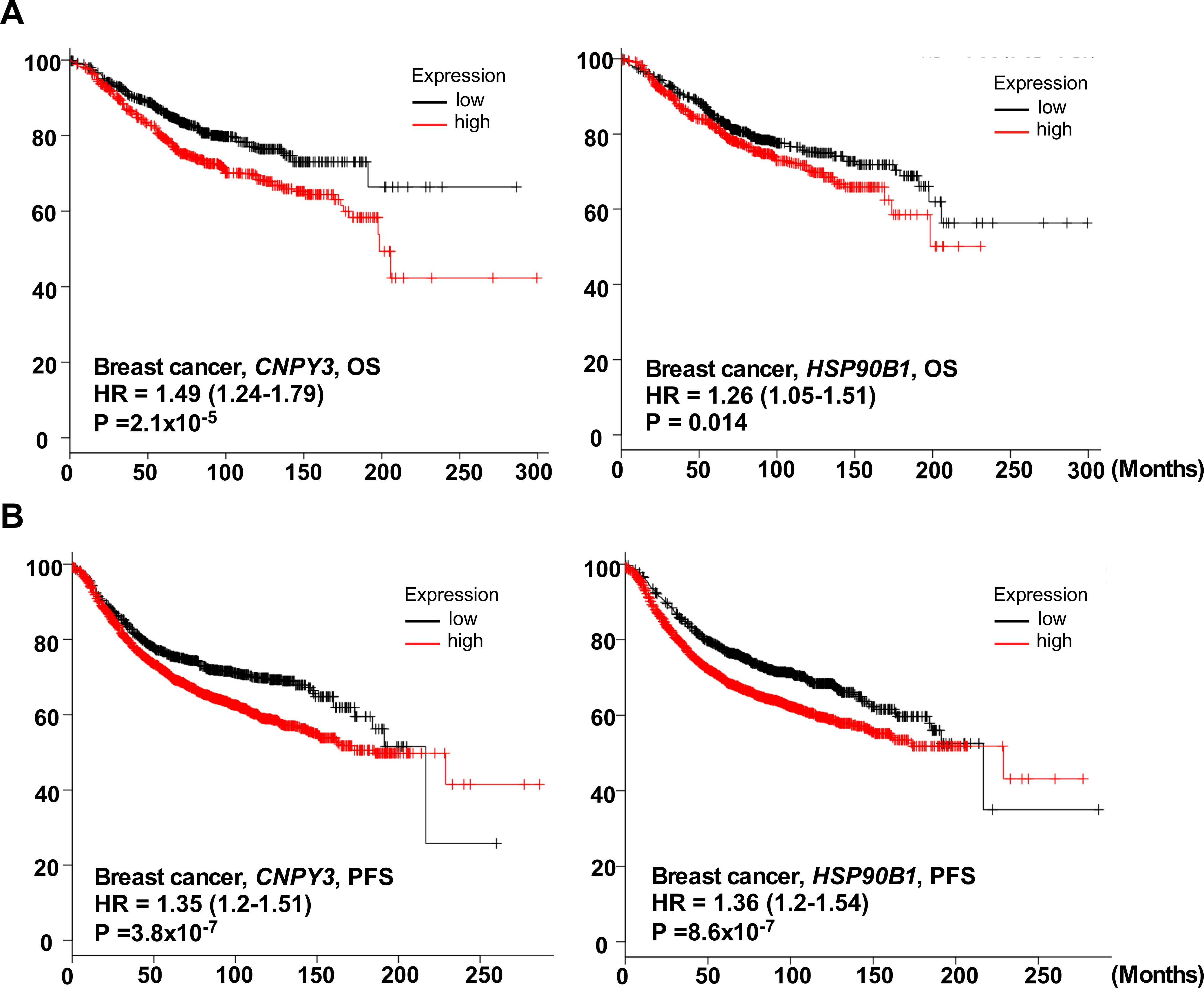
3.2. CNPY3 and GRP94 Are Hallmarks of Human Breast Cancer Development and Progression
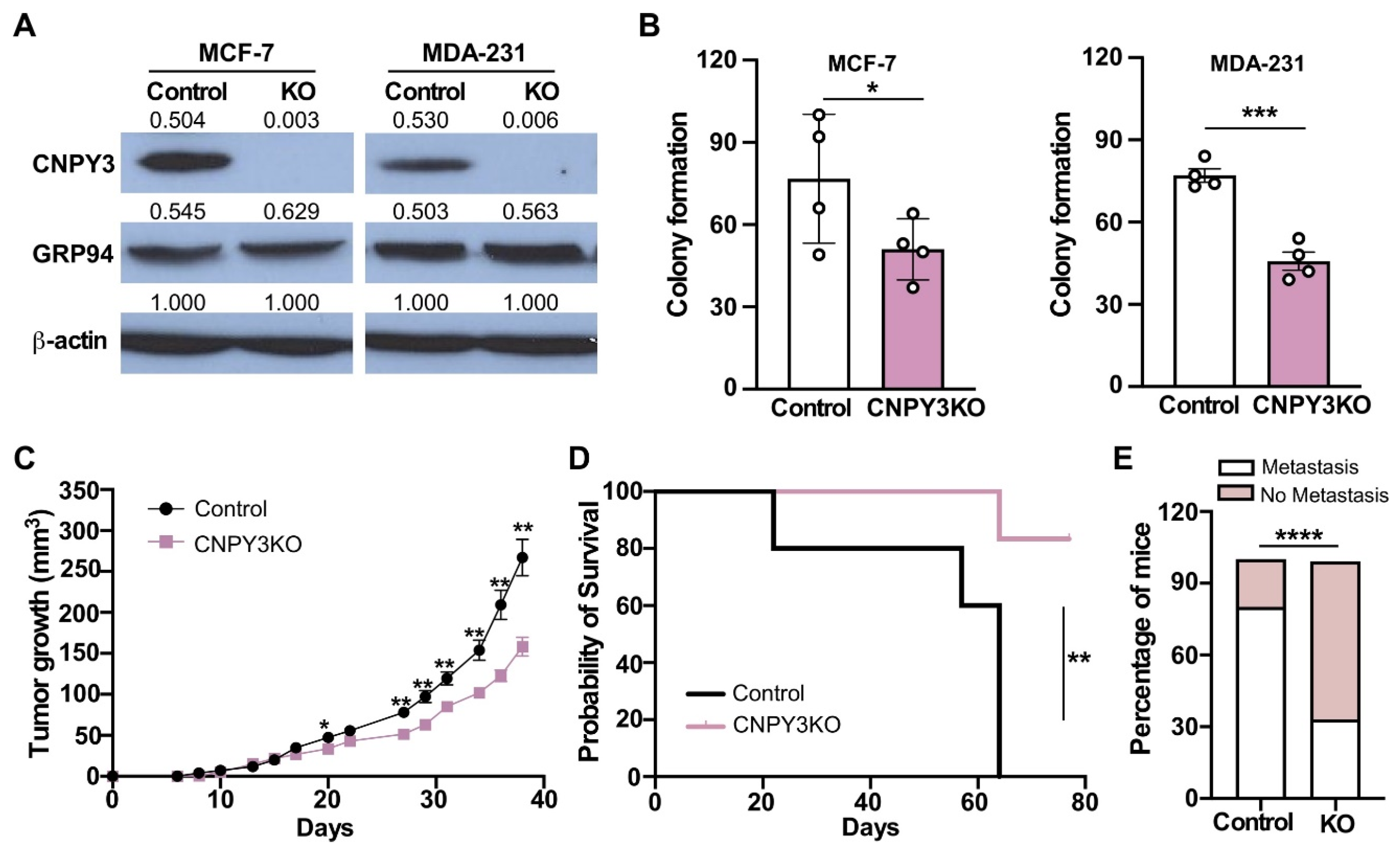
3.3. Deletion of CNPY3 Suppresses Human Breast Tumor Growth and METASTASIS In Vitro and In Vivo
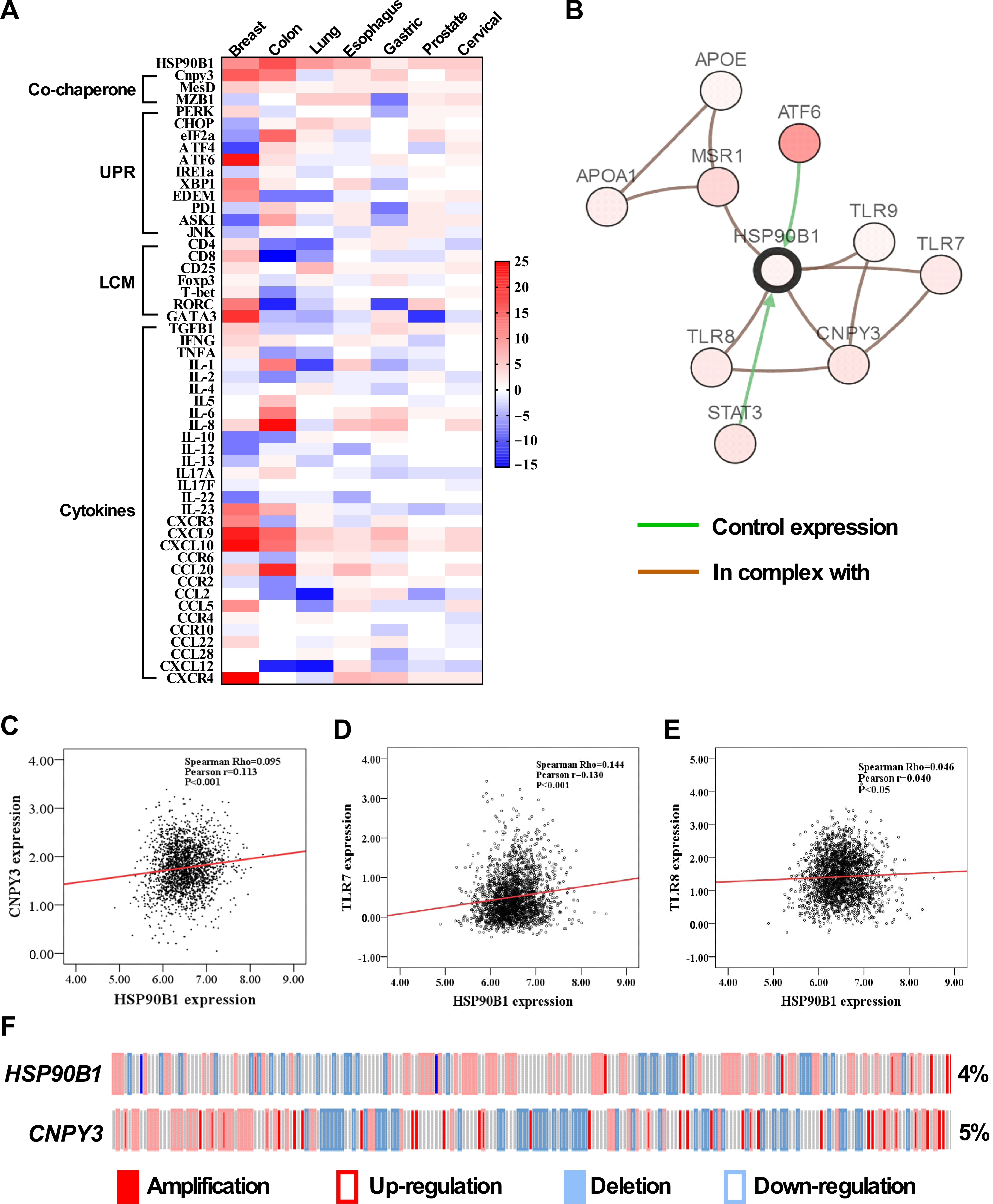
3.4. CNPY3 and GRP94 Alterations Regulate UPR Pathway Genes and Immune-Related Genes in the Tumor Microenvironment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Heritz, J.A.; Backe, S.J.; Mollapour, M. Molecular chaperones: Guardians of tumor suppressor stability and function. Oncotarget 2024, 15, 679–696. [Google Scholar] [CrossRef]
- Hwang, S.M.; Chang, S.; Rodriguez, P.C.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress responses in anticancer immunity. Nat. Rev. Cancer 2025, 25, 684–702. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Singh, M.K.; Han, S.; Ju, S.; Ranbhise, J.S.; Ha, J.; Yeo, S.G.; Kim, S.S.; Kang, I. Hsp70: A Multifunctional Chaperone in Maintaining Proteostasis and Its Implications in Human Disease. Cells 2025, 14, 509. [Google Scholar] [CrossRef] [PubMed]
- Ansa-Addo, E.A.; Thaxton, J.; Hong, F.; Wu, B.X.; Zhang, Y.; Fugle, C.W.; Metelli, A.; Riesenberg, B.; Williams, K.; Gewirth, D.T.; et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr. Top. Med. Chem. 2016, 16, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Staron, M.; Yang, Y.; Liu, B.; Li, J.; Shen, Y.; Zuniga-Pflucker, J.C.; Aguila, H.L.; Goldschneider, I.; Li, Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood 2010, 115, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Weekes, M.P.; Antrobus, R.; Talbot, S.; Hor, S.; Simecek, N.; Smith, D.L.; Bloor, S.; Randow, F.; Lehner, P.J. Proteomic plasma membrane profiling reveals an essential role for gp96 in the cell surface expression of LDLR family members, including the LDL receptor and LRP6. J. Proteome Res. 2012, 11, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular Chaperone GRP94/GP96 in Cancers: Oncogenesis and Therapeutic Target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef]
- Hua, Y.; White-Gilbertson, S.; Kellner, J.; Rachidi, S.; Usmani, S.Z.; Chiosis, G.; Depinho, R.; Li, Z.; Liu, B. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin. Cancer Res. 2013, 19, 6242–6251. [Google Scholar] [CrossRef]
- Metelli, A.; Wu, B.X.; Fugle, C.W.; Rachidi, S.; Sun, S.; Zhang, Y.; Wu, J.; Tomlinson, S.; Howe, P.H.; Yang, Y.; et al. Surface Expression of TGFbeta Docking Receptor GARP Promotes Oncogenesis and Immune Tolerance in Breast Cancer. Cancer Res. 2016, 76, 7106–7117. [Google Scholar] [CrossRef]
- Cho, Y.B.; Kim, J.W.; Heo, K.; Kim, H.J.; Yun, S.; Lee, H.S.; Shin, H.G.; Shim, H.; Yu, H.; Kim, Y.H.; et al. An internalizing antibody targeting of cell surface GRP94 effectively suppresses tumor angiogenesis of colorectal cancer. Biomed. Pharmacother. 2022, 150, 113051. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Li, C.; Wang, J.; Zhang, J.; Zhou, Y.; Sun, F.; Wang, H.; Ma, F.; Qian, H. Comprehensive analysis reveals GRP94 is associated with worse prognosis of breast cancer. Transl. Cancer Res. 2021, 10, 298–309. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Long, L.; Zhang, P.; Zhang, Y.; Ji, N. gp96 Expression in Gliomas and Its Association with Tumor Malignancy and T Cell Infiltrating Level. J. Oncol. 2022, 2022, 9575867. [Google Scholar] [CrossRef]
- Klarica Gembic, T.; Grebic, D.; Gulic, T.; Golemac, M.; Avirovic, M. Predictive and Prognostic Values of Glycoprotein 96, Androgen Receptors, and Extranodal Extension in Sentinel Lymph Node-Positive Breast Cancer: An Immunohistochemical Retrospective Study. J. Clin. Med. 2024, 13, 7665. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Han, J.; Huang, J.; Li, S.; Pang, H. Hypoxia-Induced Intracellular and Extracellular Heat Shock Protein gp96 Increases Paclitaxel-Resistance and Facilitates Immune Evasion in Breast Cancer. Front. Oncol. 2021, 11, 784777. [Google Scholar] [CrossRef]
- Takahashi, K.; Shibata, T.; Akashi-Takamura, S.; Kiyokawa, T.; Wakabayashi, Y.; Tanimura, N.; Kobayashi, T.; Matsumoto, F.; Fukui, R.; Kouro, T.; et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 2007, 204, 2963–2976. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Saitoh, S.I.; Fukui, R.; Shibata, T.; Sato, R.; Murakami, Y. Dynamic control of nucleic-acid-sensing Toll-like receptors by the endosomal compartment. Int. Immunol. 2021, 33, 835–840. [Google Scholar] [CrossRef]
- Saitoh, S.; Miyake, K. Mechanism regulating cell surface expression and activation of Toll-like receptor 4. Chem. Rec. 2006, 6, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Kobayashi, M.; Akashi-Takamura, S.; Tanimura, N.; Konno, K.; Takahashi, K.; Ishii, T.; Mizutani, T.; Iba, H.; Kouro, T.; et al. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J. Immunol. 2006, 177, 1772–1779. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A. Localisation and trafficking of Toll-like receptors: An important mode of regulation. Curr. Opin. Immunol. 2010, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.E.; Tapping, R.I. Cell surface trafficking of TLR1 is differentially regulated by the chaperones PRAT4A and PRAT4B. J. Biol. Chem. 2012, 287, 16550–16562. [Google Scholar] [CrossRef]
- Ghait, M.; Husain, R.A.; Duduskar, S.N.; Haack, T.B.; Rooney, M.; Gohrig, B.; Bauer, M.; Rubio, I.; Deshmukh, S.D. The TLR-chaperone CNPY3 is a critical regulator of NLRP3-inflammasome activation. Eur. J. Immunol. 2022, 52, 907–923. [Google Scholar] [CrossRef]
- Gao, X.C.; Zhou, B.H.; Ji, Z.X.; Li, Q.; Liu, H.N. Canopy FGF signaling regulator 3 affects prognosis, immune infiltration, and PI3K/AKT pathway in colon adenocarcinoma. World J. Gastrointest. Oncol. 2024, 16, 3284–3298. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.X.; Liu, P.; Yang, J.; Wen, S. Mining expression and prognosis of topoisomerase isoforms in non-small-cell lung cancer by using Oncomine and Kaplan-Meier plotter. PLoS ONE 2017, 12, e0174515. [Google Scholar] [CrossRef]
- Yan, H.; Hu, K.; Wu, W.; Li, Y.; Tian, H.; Chu, Z.; Koeffler, H.P.; Yin, D. Low Expression of DYRK2 (Dual Specificity Tyrosine Phosphorylation Regulated Kinase 2) Correlates with Poor Prognosis in Colorectal Cancer. PLoS ONE 2016, 11, e0159954. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, A.S. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell. Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D.; et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets subvert T cell immunity against cancer via GARP-TGFbeta axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475. [Google Scholar] [CrossRef]
- Chavany, C.; Mimnaugh, E.; Miller, P.; Bitton, R.; Nguyen, P.; Trepel, J.; Whitesell, L.; Schnur, R.; Moyer, J.; Neckers, L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 1996, 271, 4974–4977. [Google Scholar] [CrossRef]
- Patel, P.D.; Yan, P.; Seidler, P.M.; Patel, H.J.; Sun, W.; Yang, C.; Que, N.S.; Taldone, T.; Finotti, P.; Stephani, R.A.; et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013, 9, 677–684. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef]
- Yu, F.; Sharma, S.; Edwards, J.; Feigenbaum, L.; Zhu, J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat. Immunol. 2015, 16, 197–206. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Sundrud, M.S.; Trivigno, C. Identity crisis of Th17 cells: Many forms, many functions, many questions. Semin. Immunol. 2013, 25, 263–272. [Google Scholar] [CrossRef]
- Yoshida, N.; Kinugasa, T.; Miyoshi, H.; Sato, K.; Yuge, K.; Ohchi, T.; Fujino, S.; Shiraiwa, S.; Katagiri, M.; Akagi, Y.; et al. A High RORgammaT/CD3 Ratio is a Strong Prognostic Factor for Postoperative Survival in Advanced Colorectal Cancer: Analysis of Helper T Cell Lymphocytes (Th1, Th2, Th17 and Regulatory T Cells). Ann. Surg. Oncol. 2016, 23, 919–927. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Zhao, R.; Sun, S.; Guo, B.; Li, Z.; Liu, B. CNPY3 Promotes Human Breast Cancer Progression and Metastasis via Modulation of the Tumor Microenvironment. Curr. Issues Mol. Biol. 2025, 47, 883. https://doi.org/10.3390/cimb47110883
Duan X, Zhao R, Sun S, Guo B, Li Z, Liu B. CNPY3 Promotes Human Breast Cancer Progression and Metastasis via Modulation of the Tumor Microenvironment. Current Issues in Molecular Biology. 2025; 47(11):883. https://doi.org/10.3390/cimb47110883
Chicago/Turabian StyleDuan, Xiaofeng, Ran Zhao, Shaoli Sun, Beichu Guo, Zihai Li, and Bei Liu. 2025. "CNPY3 Promotes Human Breast Cancer Progression and Metastasis via Modulation of the Tumor Microenvironment" Current Issues in Molecular Biology 47, no. 11: 883. https://doi.org/10.3390/cimb47110883
APA StyleDuan, X., Zhao, R., Sun, S., Guo, B., Li, Z., & Liu, B. (2025). CNPY3 Promotes Human Breast Cancer Progression and Metastasis via Modulation of the Tumor Microenvironment. Current Issues in Molecular Biology, 47(11), 883. https://doi.org/10.3390/cimb47110883




