Expression of TIM-3 and Gal-9 Immune Checkpoints in Chronic Lymphocytic Leukemia: The Potential Role of Interleukin-27
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Samples
2.3. PBMCs Isolation
2.4. Cell Cultures
2.5. Flow Cytometry Staining and Analysis
2.6. Statistical Analysis
3. Results
3.1. Experimental Design and Approach
3.2. IL-27 Effects on Lymphocyte Population Distribution
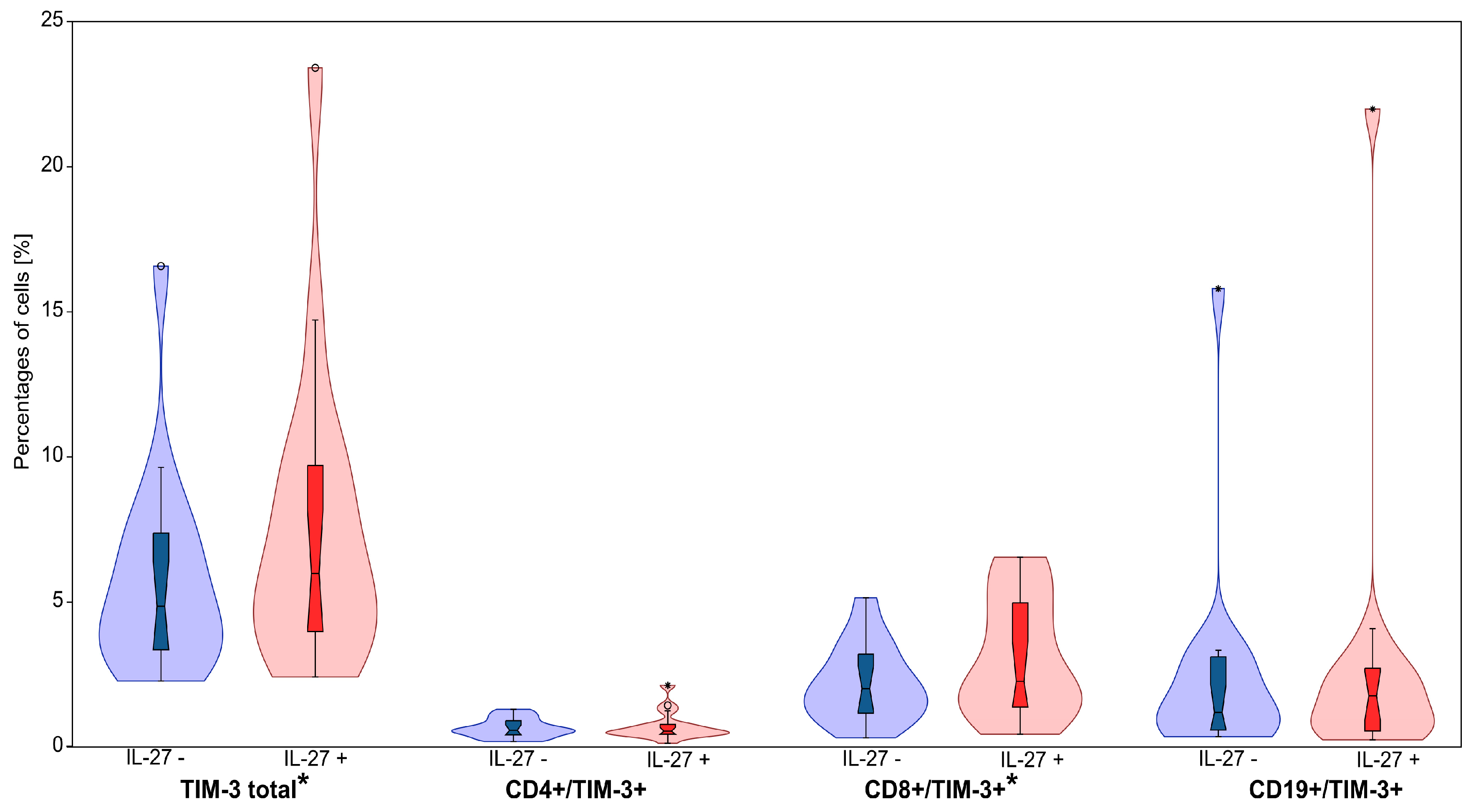
3.3. IL-27 Upregulates TIM-3 on CD8+ T Cells
3.4. IL-27 Modestly Increases Intracellular Gal-9 Expression
4. Discussion
4.1. Study Limitations and Future Directions
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| APC | Allophycocyanin |
| CLL | Chronic Lymphocytic Leukemia |
| FITC | Fluorescein Isothiocyanate |
| Gal-9 | Galectin-9 |
| IFN | Interferon |
| IL | Interleukin |
| NFIL3 | Nuclear Factor, Interleukin 3 regulated |
| NK | Natural Killer cells |
| PBMC | Peripheral Blood Mononuclear Cells |
| PBS | Phosphate-Buffered Saline |
| PD-L1 | Programmed Death-Ligand 1 |
| PE | Phycoerythrin |
| PerCP | Peridinin-Chlorophyll-Protein |
| RT | Room Temperature |
| SD | Standard Deviation |
| SEM | Standard Error of Mean |
| SSC | Side Scatter |
| T-bet | T-box transcription factor |
| Tc | T cytotoxic lymphocyte |
| Th | T helper lymphocyte |
| TIM-3 | T cell Immunoglobulin and Mucin domain-3 |
| Treg | T regulatory lymphocyte |
References
- Taghiloo, S.; Allahmoradi, E.; Ebadi, R.; Tehrani, M.; Hosseini-Khah, Z.; Janbabaei, G.; Shekarriz, R.; Asgarian-Omran, H. Upregulation of Galectin-9 and PD-L1 Immune Checkpoints Molecules in Patients with Chronic Lymphocytic Leukemia. Asian Pac. J. Cancer Prev. 2017, 18, 2269–2274. [Google Scholar] [CrossRef]
- Chronic Lymphocytic Leukemia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470433/ (accessed on 15 January 2025).
- Billard, C. Apoptosis inducers in chronic lymphocytic leukemia. Oncotarget 2014, 5, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri-Doulabi, E.; Abbaspour, S.; Rostami, S.; Faranoush, M.; Ghahramanfard, F.; Pak, F.; Barati, M.; Kokhaei, P.; Momtazi-Borojeni, A.A. Evaluating the mechanism underlying antitumor effect of interleukin 27 on B cells of chronic lymphocytic leukemia patients. J. Cell. Physiol. 2020, 235, 9424–9431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Thornley, T.B.; Gao, W.; Larocca, R.; Turka, L.A.; Kuchroo, V.K.; Strom, T.B. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J. Clin. Investig. 2012, 122, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef]
- Han, G.; Chen, G.; Shen, B.; Li, Y. Tim-3: An activation marker and activation limiter of innate immune cells. Front. Immunol. 2013, 4, 449. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Brusa, D.; Serra, S.; Coscia, M.; Rossi, D.; D’Arena, G.; Laurenti, L.; Jaksic, O.; Fedele, G.; Inghirami, G.; Gaidano, G.; et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013, 98, 953–963. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediat. Inflamm. 2017, 2017, 3958069. [Google Scholar] [CrossRef]
- Awasthi, A.; Carrier, Y.; Peron, J.P.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Weiner, H.L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Ghaffari, A.A.; Cheng, G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J. Immunol. 2010, 185, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Lewandowski, A.; Markocka-Mączka, K.; Grabowski, K. Concentration of serum interleukin-27 increase in patients with lymph node metastatic gastroesophageal cancer. Adv. Clin. Exp. Med. 2013, 22, 683–691. [Google Scholar] [PubMed]
- Gonin, J.; Carlotti, A.; Dietrich, C.; Audebourg, A.; Radenen-Bussière, B.; Caignard, A.; Avril, M.-F.; Vacher-Lavenu, M.-C.; Larousserie, F.; Devergne, O. Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas. PLoS ONE 2013, 8, e75694, Correction in PLoS ONE 2013, 8. https://doi.org/10.1371/annotation/e1a0e85e-e632-40f2-9925-e0b71eb18b56. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Maklad, A.M.; Khaled, S.A.; Elyamany, A. Interleukin-27 and interleukin-35 in de novo acute myeloid leukemia: Expression and significance as biological markers. J. Blood Med. 2019, 10, 341–349. [Google Scholar] [CrossRef]
- Pagano, G.; Botana, I.F.; Wierz, M.; Roessner, P.M.; Ioannou, N.; Zhou, X.; Al-Hity, G.; Borne, C.; Gargiulo, E.; Gonder, S.; et al. Interleukin-27 potentiates CD8+ T-cell-mediated antitumor immunity in chronic lymphocytic leukemia. Haematologica 2023, 108, 3011–3024. [Google Scholar] [CrossRef]
- Hirahara, K.; Ghoreschi, K.; Yang, X.P.; Takahashi, H.; Laurence, A.; Vahedi, G.; Sciumè, G.; Hall, A.O.; Dupont, C.D.; Francisco, L.M.; et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity 2012, 36, 1017–1030. [Google Scholar] [CrossRef]
- d’Almeida, S.M.; Kauffenstein, G.; Roy, C.; Basset, L.; Papargyris, L.; Henrion, D.; Catros, V.; Ifrah, N.; Descamps, P.; Croue, A.; et al. The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancertumour-associated macrophages: Regulatory role of IL-27. Oncoimmunology 2016, 5, e1178025. [Google Scholar] [CrossRef]
- Horlad, H.; Ma, C.; Yano, H.; Pan, C.; Ohnishi, K.; Fujiwara, Y.; Endo, S.; Kikukawa, Y.; Okuno, Y.; Matsuoka, M.; et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016, 107, 1696–1704. [Google Scholar] [CrossRef]
- Hus, I.; Giannopoulos, K.; Jamroziak, K.; Wołowiec, D.; Roliński, J.M.; Robak, T. Diagnostic and therapeutic recommendations of the Polish Society of Haematologists and Transfusiologists, and Polish Adult Leukemia Group-CLL for chronic lymphocytic leukemia in 2023. Acta Haematol. Pol. 2023, 54, 342–371. [Google Scholar] [CrossRef]
- Puleo, A.; Weber, C.; Rossi, J.; Chiu, J.; Bowman, J.; Davis, K. Isolation of Peripheral Blood Mononuclear Cells Using Vacutainer® Cellular Preparation Tubes (CPT™). Bio-Protocol 2017, 7, e2103. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Ravindran, B. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs). Bio-Protocol 2013, 3, e323. [Google Scholar] [CrossRef]
- Murugaiyan, G.; Mittal, A.; Lopez-Diego, R.; Maier, L.M.; Anderson, D.E.; Weiner, H.L. IL-27 Is a Key Regulator of IL-10 and IL-17 Production by Human CD4+ T Cells. J. Immunol. 2009, 183, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Koutecký, P.; Smith, T.; Loureiro, J.; Kron, P. Best Practices for Instrument Settings and Raw Data Analysis in Flow Cytometry. Cytom. A 2023, 103, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.A.; Holland, M.; McWilliams, E.; Hodi, F.S.; Severgnini, M. Detection of clinically relevant immune checkpoint markers by multicolor flow cytometry. J. Biol. Methods 2019, 6, e114. [Google Scholar] [CrossRef]
- Alanazi, M.A.; Kwa, F.A.A.; Omar, M.M.A.; Antonipillai, J.; Jackson, D.E. Efficacy and challenges involving combination therapies in CLL. Drug Discov. Today 2024, 29, 104243. [Google Scholar] [CrossRef]
- Tausch, E.; Schneider, C.; Stilgenbauer, S. Risk-stratification in frontline CLL therapy: Standard of care. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 457–466. [Google Scholar] [CrossRef]
- Giannoni, P.; Marini, C.; Cutrona, G.; Sambuceti, G.M.; Fais, F.; de Totero, D. Unraveling the Bone Tissue Microenvironment in Chronic Lymphocytic Leukemia. Cancers 2023, 15, 5058. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Chen, S.S.; Rai, K.R. Chronic Lymphocytic Leukemia. Cold Spring Harb. Perspect. Med. 2021, 11, a035220. [Google Scholar] [CrossRef]
- Taghiloo, S.; Asgarian-Omran, H. Cross-talk between leukemic and immune cells at the tumor microenvironment in chronic lymphocytic leukemia: An update review. Eur. J. Haematol. 2024, 113, 4–15. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Liu, H.; He, X.; Huang, F.; Wang, Y. Role of Tim-3 in regulating tumorigenesis, inflammation, and antitumor immunity therapy. Cancer Biomark. 2021, 32, 237–248. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Carson, W.E., 3rd; Bai, X.F. The role of IL-27 in the induction of anti-tumor cytotoxic T lymphocyte response. Am. J. Transl. Res. 2013, 5, 470–480. [Google Scholar]
- Morishima, N.; Owaki, T.; Asakawa, M.; Kamiya, S.; Mizuguchi, J.; Yoshimoto, T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 2005, 175, 1686–1693. [Google Scholar] [CrossRef]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194, Correction in J. Exp. Med. 2011, 208, 1331. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Zhu, C.; Sakuishi, K.; Xiao, S.; Sun, Z.; Zaghouani, S.; Gu, G.; Wang, C.; Tan, D.J.; Wu, C.; Rangachari, M.; et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat. Commun. 2015, 6, 6072. [Google Scholar] [CrossRef]
- Chihara, N.; Madi, A.; Kondo, T.; Zhang, H.; Acharya, N.; Singer, M.; Nyman, J.; Marjanovic, N.D.; Kowalczyk, M.S.; Wang, C.; et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018, 558, 454–459. [Google Scholar] [CrossRef]
- Anderson, A.C.; Lord, G.M.; Dardalhon, V.; Lee, D.H.; Sabatos-Peyton, C.A.; Glimcher, L.H.; Kuchroo, V.K. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 2010, 40, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Fearon, D.T. T-bet-mediated differentiation of the activated CD8+ T cell. Eur. J. Immunol. 2011, 41, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Fujio, K.; Okamura, T.; Yamamoto, K. Interleukin-27 in T cell immunity. Int. J. Mol. Sci. 2015, 16, 2851–2863. [Google Scholar] [CrossRef]
- Schneider, R.; Yaneva, T.; Beauseigle, D.; El-Khoury, L.; Arbour, N. IL-27 increases the proliferation and effector functions of human naïve CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur. J. Immunol. 2011, 41, 47–59. [Google Scholar] [CrossRef]
- DeLong, J.H.; O’Hara Hall, A.; Rausch, M.; Moodley, D.; Perry, J.; Park, J.; Phan, A.T.; Beiting, D.P.; Kedl, R.M.; Hill, J.A.; et al. IL-27 and TCR Stimulation Promote T Cell Expression of Multiple Inhibitory Receptors. Immunohorizons 2019, 3, 13–25. [Google Scholar] [CrossRef]
- Chiba, Y.; Mizoguchi, I.; Furusawa, J.; Hasegawa, H.; Ohashi, M.; Xu, M.; Owaki, T.; Yoshimoto, T. Interleukin-27 Exerts Its Antitumor Effects by Promoting Differentiation of Hematopoietic Stem Cells to M1 Macrophages. Cancer Res. 2018, 78, 182–194. [Google Scholar] [CrossRef]
- Wdowiak, K.; Gallego-Colon, E.; Francuz, T.; Czajka-Francuz, P.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wybraniec, M.T.; Chudek, J.; Wojnar, J. Increased serum levels of Galectin-9 in patients with chronic lymphocytic leukemia. Oncol. Lett. 2019, 17, 1019–1029. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, X.; Ma, Y.; Du, Y.; Feng, J. A new emerging target in cancer immunotherapy: Galectin-9 (LGALS9). Genes Dis. 2022, 10, 2366–2382. [Google Scholar] [CrossRef]
- Kikushige, Y.; Miyamoto, T.; Yuda, J.; Jabbarzadeh-Tabrizi, S.; Shima, T.; Takayanagi, S.-I.; Niiro, H.; Yurino, A.; Miyawaki, K.; Takenaka, K.; et al. A TIM-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression. Cell Stem Cell 2015, 17, 341–352. [Google Scholar] [CrossRef]
- Gonçalves Silva, I.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Bardelli, M.; Varani, L.; Hussain, R.; Siligardi, G.; Ceccone, G.; et al. The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 2017, 22, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Glycobiology simplified: Diverse roles of glycan recognition in inflammation. J. Leukoc. Biol. 2016, 99, 825–838. [Google Scholar] [CrossRef]
- Golden-Mason, L.; McMahan, R.H.; Strong, M.; Reisdorph, R.; Mahaffey, S.; Palmer, B.E.; Cheng, L.; Kulesza, C.; Hirashima, M.; Niki, T.; et al. Galectin-9 functionally impairs natural killer cells in humans and mice. J. Virol. 2013, 87, 4835–4845. [Google Scholar] [CrossRef]
- Kuchroo, V.K.; Meyers, J.H.; Umetsu, D.T.; DeKruyff, R.H. TIM family of genes in immunity and tolerance. Adv. Immunol. 2006, 91, 227–249. [Google Scholar] [CrossRef]
- Wang, F.; He, W.; Yuan, J.; Wu, K.; Zhou, H.; Zhang, W.; Chen, Z.K. Activation of Tim-3-Galectin-9 pathway improves survival of fully allogeneic skin grafts. Transpl. Immunol. 2008, 19, 12–19. [Google Scholar] [CrossRef]
- Pang, N.; Alimu, X.; Chen, R.; Muhashi, M.; Ma, J.; Chen, G.; Zhao, F.; Wang, L.; Qu, J.; Ding, J. Activated Galectin-9/Tim3 promotes Treg and suppresses Th1 effector function in chronic lymphocytic leukemia. FASEB J. 2021, 35, e21556, Erratum in FASEB J. 2024, 38, e23740. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Funes, S.C.; Manrique de Lara, A.; Altamirano-Lagos, M.J.; Mackern-Oberti, J.P.; Escobar-Vera, J.; Kalergis, A.M. Immune checkpoints and the regulation of tolerogenicity in dendritic cells: Implications for autoimmunity and immunotherapy. Autoimmun. Rev. 2019, 18, 359–368. [Google Scholar] [CrossRef]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Jia, H.; Dilger, P.; Bird, C.; Wadhwa, M. IL-27 Promotes Proliferation of Human Leukemic Cell Lines Through the MAPK/ERK Signaling Pathway and Suppresses Sensitivity to Chemotherapeutic Drugs. J. Interferon Cytokine Res. 2016, 36, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Stumhofer, J.S.; Silver, J.S.; Laurence, A.; Porrett, P.M.; Harris, T.; Turka, L.A.; Ernst, M.; Saris, C.J.M.; O’Shea, J.J.; Hunter, C.A. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007, 8, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Jafarkhani, S.; Hossein-Nataj, H.; Eslami-Jouybari, M.; Ghoreishi, M.; Asgarian-Omran, H. PD-1 and TIM-3 blocking cannot enhance apoptosis of chronic lymphocytic leukemia cells induced by peripheral blood CD8+ T cells. Exp. Oncol. 2022, 44, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, H.; Astaneh, M.; Tehrani, M.; Hossein-Nataj, H.; Zaboli, E.; Shekarriz, R.; Asgarian-Omran, H. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia. Immunol. Res. 2020, 68, 269–279. [Google Scholar] [CrossRef]
- Asayama, T.; Tamura, H.; Ishibashi, M.; Kuribayashi-Hamada, Y.; Onodera-Kondo, A.; Okuyama, N.; Yamada, A.; Shimizu, M.; Moriya, K.; Takahashi, H.; et al. Functional expression of Tim-3 on blasts and clinical impact of its ligand galectin-9 in myelodysplastic syndromes. Oncotarget 2017, 8, 88904–88917. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Q.; Wang, Y.; Wang, L.; Li, Z.; Mor, G.; Liao, A. Newly characterized decidual Tim-3+ Treg cells are abundant during early pregnancy and driven by IL-27 coordinately with Gal-9 from trophoblasts. Hum. Reprod. 2020, 35, 2454–2466. [Google Scholar] [CrossRef]
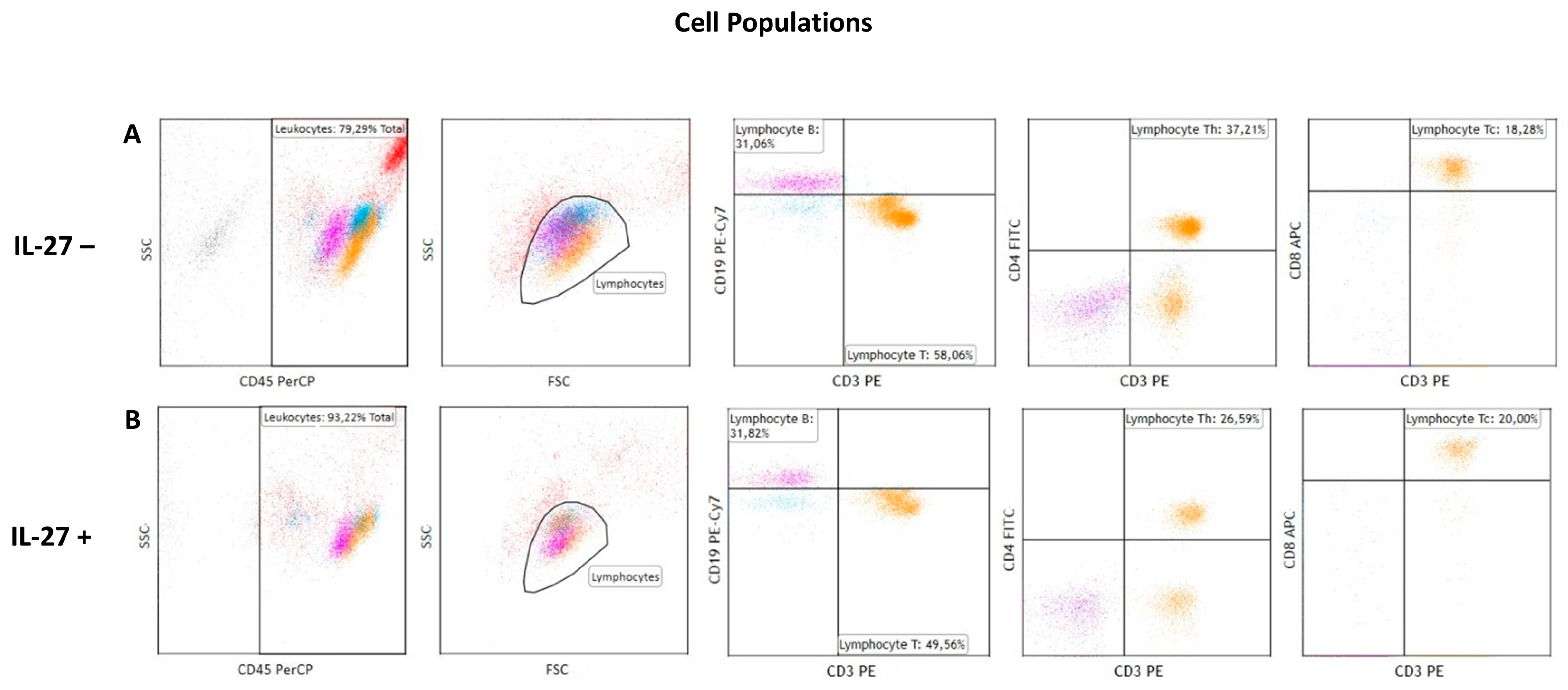

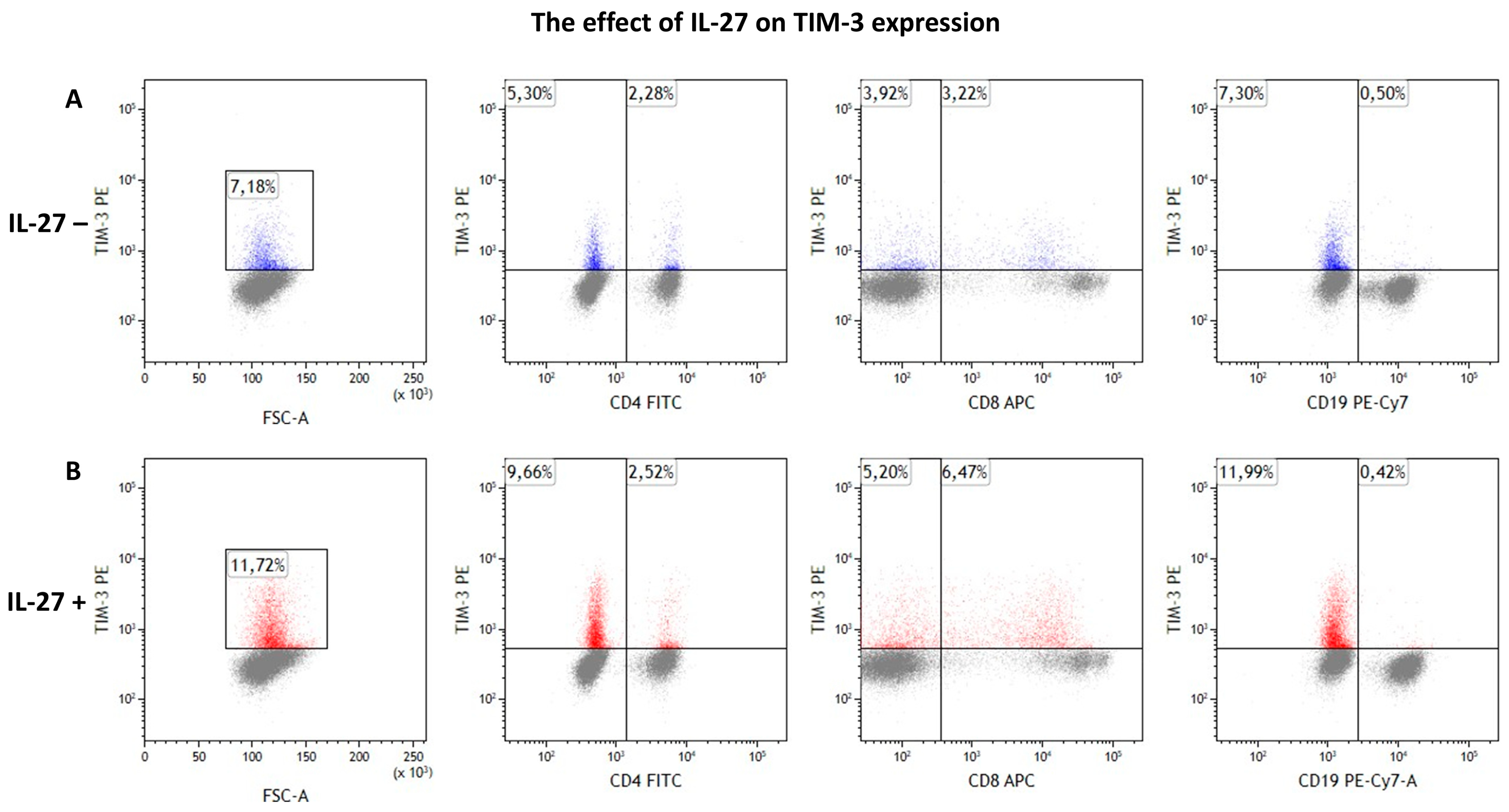
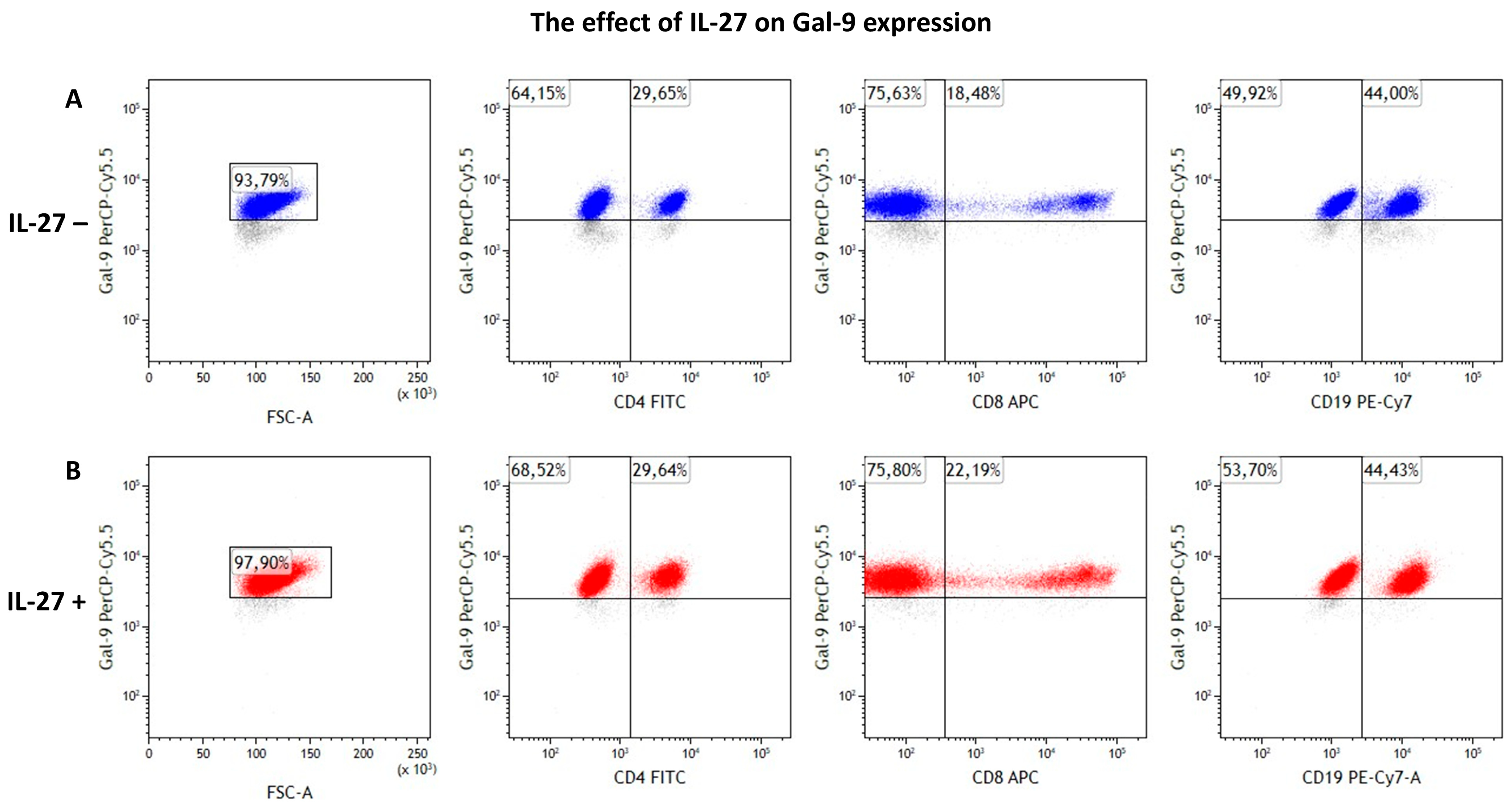
| Cell Population | Cell Culture | Mean | SD | SEM | Median | Min. | Max. | Test Statistic | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| CD3+ (T) | IL-27– | 43.26 | 24.47 | 5.94 | 42.57 | 6.92 | 83.08 | 2.068 W | 0.039 |
| IL-27+ | 36.85 | 21.90 | 5.48 | 40.18 | 0.01 | 69.99 | |||
| CD3+/CD4+ (Th) | IL-27– | 26.71 | 17.26 | 4.19 | 23.59 | 4.60 | 56.01 | 2.585 W | 0.010 |
| IL-27+ | 22.01 | 12.94 | 3.23 | 21.77 | 4.85 | 47.80 | |||
| CD3+/CD8+ (Tc) | IL-27– | 12.69 | 6.74 | 1.63 | 12.70 | 2.09 | 25.33 | 0.931 W | 0.352 |
| IL-27+ | 11.64 | 7.19 | 1.80 | 10.10 | 2.65 | 23.59 | |||
| CD19+ (B) | IL-27– | 46.82 | 28.57 | 6.93 | 35.65 | 7.18 | 87.80 | 0.686 W | 0.492 |
| IL-27+ | 51.02 | 28.40 | 6.89 | 41.55 | 6.54 | 99.60 |
| Cell Population | Cell Culture | Mean | SD | SEM | Median | Min. | Max. | Test Statistic | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| TIM-3+ total | IL-27– | 5.77 | 3.50 | 0.85 | 4.86 | 2.28 | 16.58 | 2.533 W | 0.011 |
| IL-27+ | 7.54 | 5.24 | 1.27 | 5.99 | 2.42 | 23.41 | |||
| CD4+/TIM-3+ | IL-27– | 0.66 | 0.33 | 0.08 | 0.58 | 0.19 | 2.28 | 0.781 W | 0.435 |
| IL-27+ | 0.71 | 0.48 | 0.12 | 0.55 | 0.13 | 2.52 | |||
| CD8+/TIM-3+ | IL-27– | 2.18 | 1.32 | 0.32 | 2.02 | 0.32 | 5.15 | 2.627 W | 0.009 |
| IL-27+ | 3.09 | 2.04 | 0.49 | 2.27 | 0.44 | 6.55 | |||
| CD19+/TIM3+ | IL-27– | 2.41 | 3.62 | 0.88 | 1.20 | 0.36 | 15.80 | 0.213 W | 0.831 |
| IL-27+ | 2.82 | 5.06 | 1.23 | 1.77 | 0.25 | 21.99 |
| Cell Population | Cell Culture | Mean | SD | SEM | Median | Min. | Max. | Test Statistic | p-Value |
|---|---|---|---|---|---|---|---|---|---|
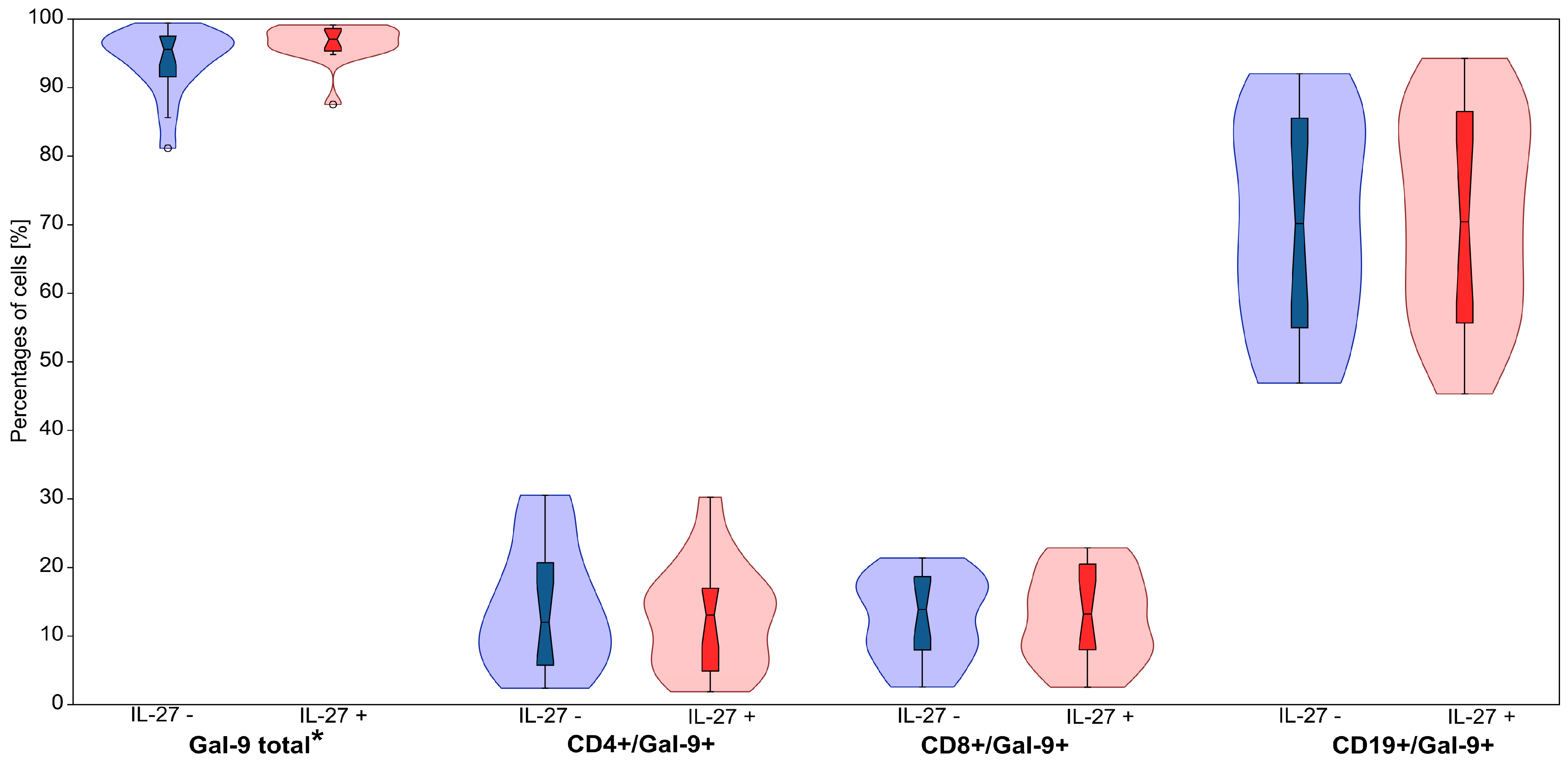
| Gal-9+ total | IL-27– | 93.91 | 4.83 | 1.17 | 95.58 | 81.16 | 99.42 | 2.817 W | 0.005 |
| IL-27+ | 96.55 | 2.77 | 0.67 | 97.06 | 87.54 | 99.13 | |||
| CD4+/Gal-9+ | IL-27– | 14.00 | 9.05 | 2.19 | 12.01 | 2.40 | 30.54 | 1.065 W | 0.287 |
| IL-27+ | 12.81 | 7.74 | 1.88 | 13.08 | 1.89 | 30.27 | |||
| CD8+/Gal-9+ | IL-27– | 12.65 | 6.12 | 1.48 | 13.89 | 2.58 | 21.41 | 0.355 W | 0.723 |
| IL-27+ | 12.67 | 6.63 | 1.61 | 13.22 | 2.53 | 22.86 | |||
| CD19+/Gal-9+ | IL-27– | 70.40 | 15.57 | 3.78 | 70.17 | 46.92 | 92.02 | 0.970 W | 0.332 |
| IL-27+ | 71.80 | 15.11 | 3.66 | 70.42 | 45.33 | 94.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wędrowska, E.; Wandtke, T.; Ulaszewski, B.; Cichocka, E.; Dębski, R.; Kopiński, P.; Styczyński, J.; Przybylski, G. Expression of TIM-3 and Gal-9 Immune Checkpoints in Chronic Lymphocytic Leukemia: The Potential Role of Interleukin-27. Curr. Issues Mol. Biol. 2025, 47, 881. https://doi.org/10.3390/cimb47110881
Wędrowska E, Wandtke T, Ulaszewski B, Cichocka E, Dębski R, Kopiński P, Styczyński J, Przybylski G. Expression of TIM-3 and Gal-9 Immune Checkpoints in Chronic Lymphocytic Leukemia: The Potential Role of Interleukin-27. Current Issues in Molecular Biology. 2025; 47(11):881. https://doi.org/10.3390/cimb47110881
Chicago/Turabian StyleWędrowska, Ewelina, Tomasz Wandtke, Bartosz Ulaszewski, Edyta Cichocka, Robert Dębski, Piotr Kopiński, Jan Styczyński, and Grzegorz Przybylski. 2025. "Expression of TIM-3 and Gal-9 Immune Checkpoints in Chronic Lymphocytic Leukemia: The Potential Role of Interleukin-27" Current Issues in Molecular Biology 47, no. 11: 881. https://doi.org/10.3390/cimb47110881
APA StyleWędrowska, E., Wandtke, T., Ulaszewski, B., Cichocka, E., Dębski, R., Kopiński, P., Styczyński, J., & Przybylski, G. (2025). Expression of TIM-3 and Gal-9 Immune Checkpoints in Chronic Lymphocytic Leukemia: The Potential Role of Interleukin-27. Current Issues in Molecular Biology, 47(11), 881. https://doi.org/10.3390/cimb47110881






