4. Discussion
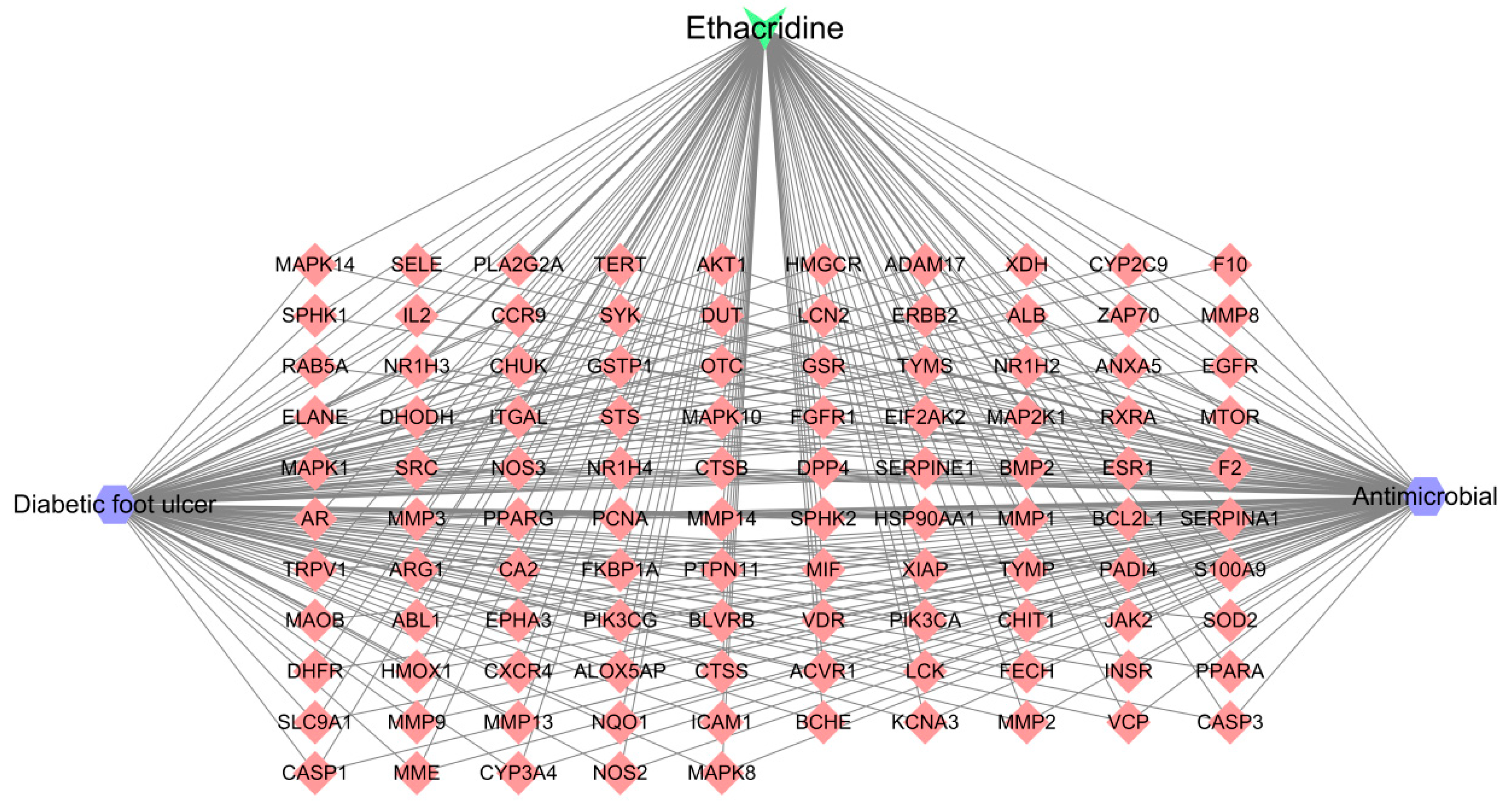
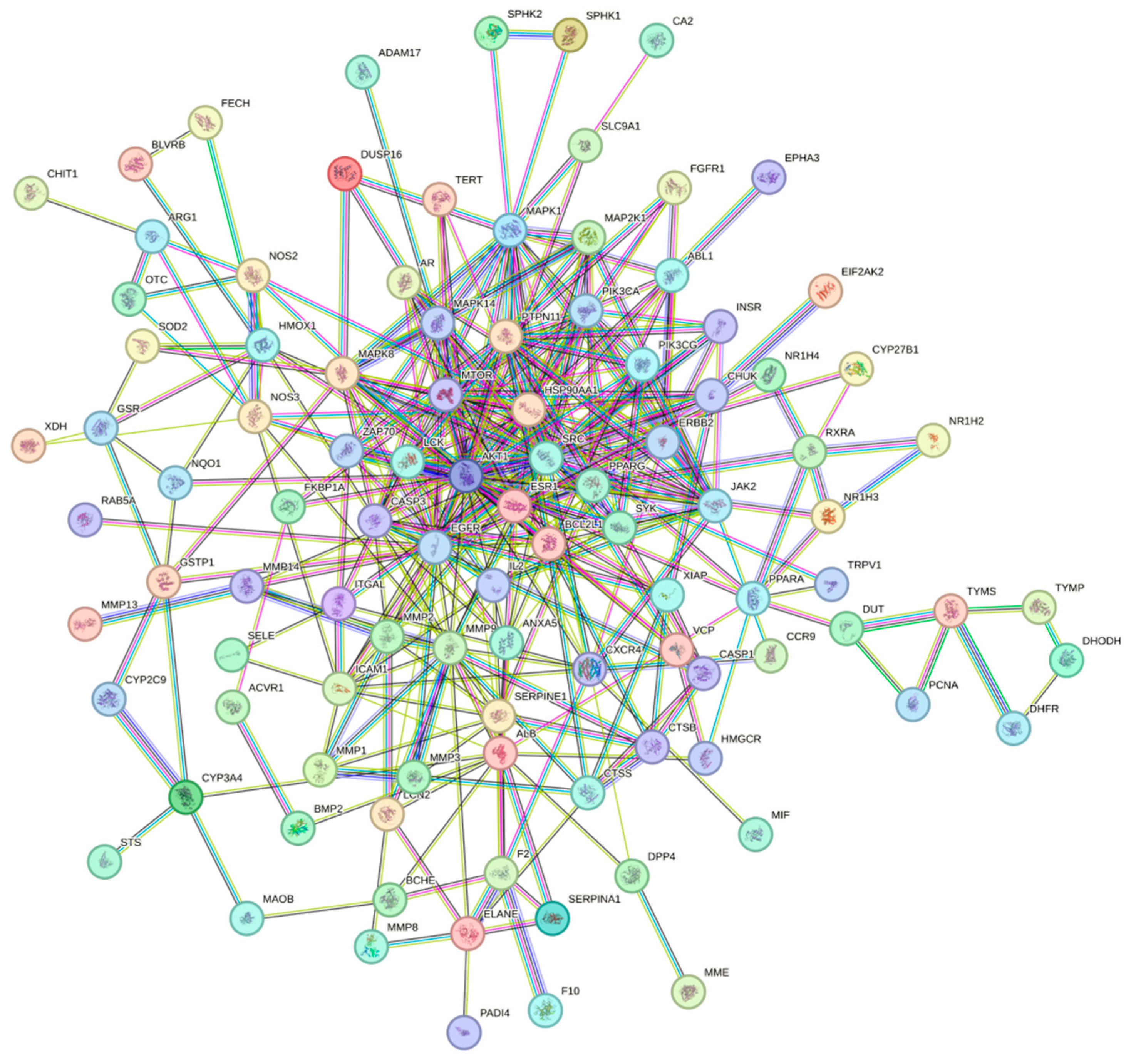
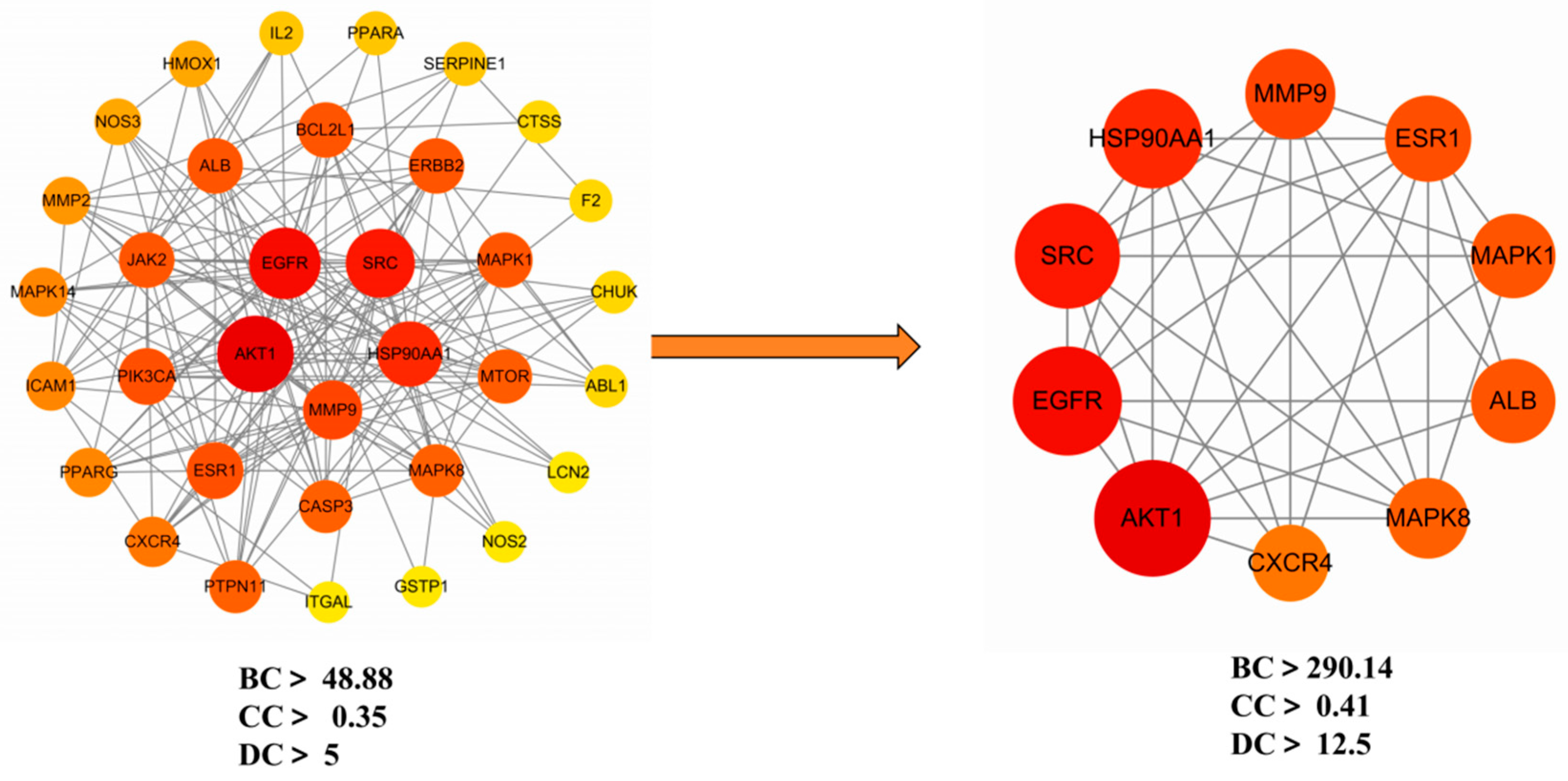
Ethacridine demonstrates a multi-target, multi-pathway therapeutic potential in diabetic foot ulcers (DFUs). Network pharmacology identified 105 putative targets, with 10 hub genes—
AKT1,
EGFR, SRC, MMP9, MAPK1, MAPK8, ALB, HSP90AA1, ESR1, and
CXCR4—playing central roles in wound healing and inflammation.
MMP9, often overexpressed in DFUs, contributes to excessive matrix degradation and impaired healing; its inhibition by ethacridine may support tissue preservation [
10].
EGFR, crucial for keratinocyte migration, is downregulated in DFUs, and ethacridine may help restore its function [
11].
AKT1, involved in cell survival and angiogenesis, is downstream of CXCR4/SDF-1 signaling, a key axis in tissue repair and neovascularization [
12]. Engagement of
CXCR4 may thus enhance progenitor cell recruitment to ischemic wounds.
ESR1 further implicates estrogen-mediated reparative processes, aligning with evidence that estrogen signaling promotes diabetic wound closure [
13]. Other hubs, including
SRC, MAPK1, and
MAPK8, suggest modulation of inflammatory and proliferative pathways, while
ALB and
HSP90AA1 reflect systemic modulation and chaperone support of key signaling proteins. Overall, these findings suggest that ethacridine may simultaneously attenuate inflammation (e.g., via TNF–MAPK–JNK inhibition), suppress protease overactivity (MMP9), and promote tissue regeneration through EGFR, AKT1, and CXCR4 pathways—an approach well-suited to address the multifactorial nature of DFUs [
14,
15].
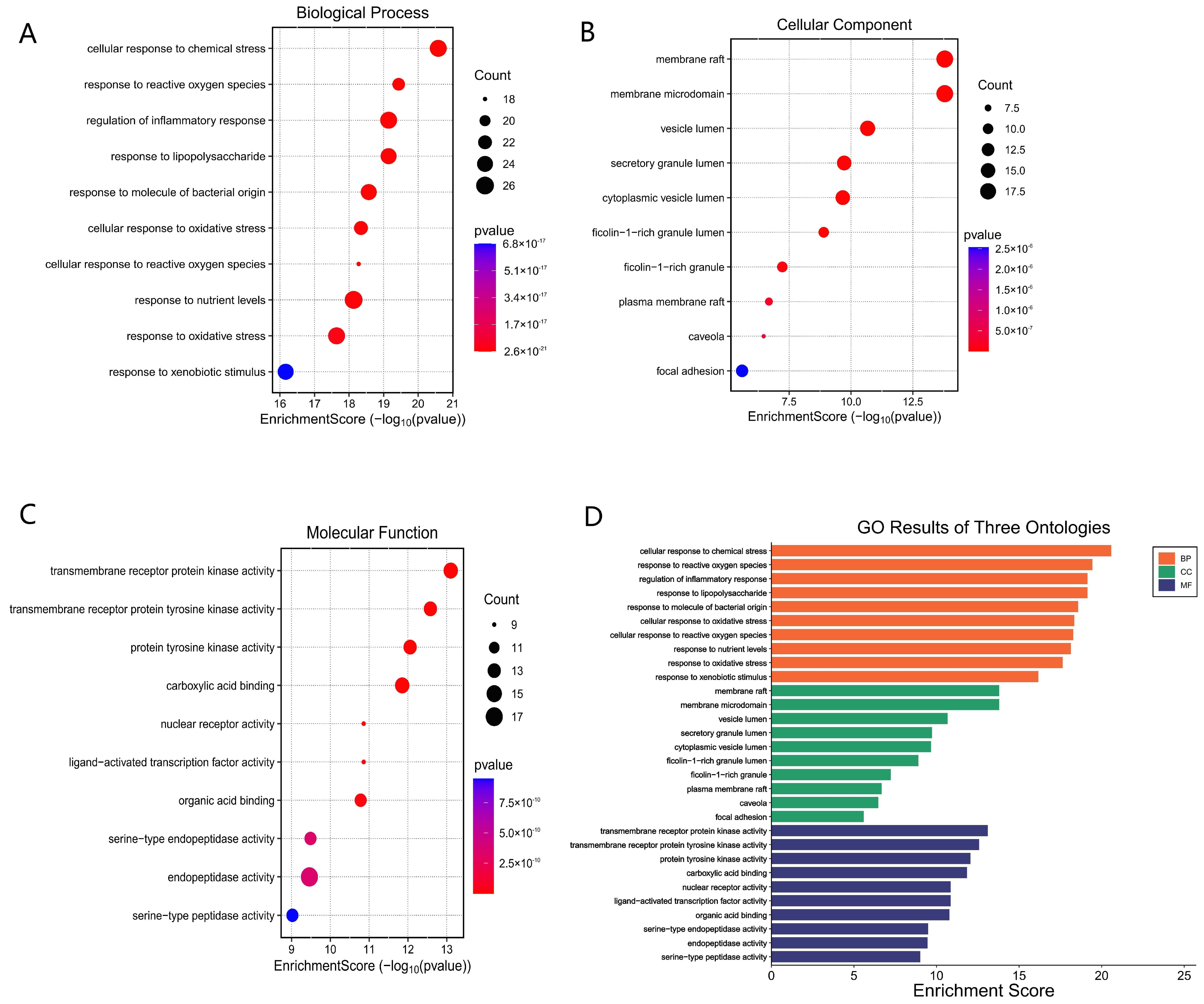
Gene ontology enrichment analysis provides biological context to ethacridine’s predicted targets. Notably, GO-Biological Process terms related to “response to oxidative stress” and “defense response to bacterium” were highly enriched, suggesting that ethacridine may bolster the wound’s ability to handle reactive oxygen species (ROS) and bacterial invasion. This is significant because DFU wounds are characterized by excessive oxidative stress and persistent bacterial burden. Chronic hyperglycemia elevates ROS levels in tissues, which not only cause cellular damage but also perpetuate inflammation and activate matrix metalloproteinases like MMP-9 [
14,
15]. The enrichment of oxidative stress responses implies that ethacridine might activate antioxidant defenses or stress-response pathways (e.g., Nrf2/HO-1 or heat-shock responses) in the wound, thereby mitigating ROS-induced damage. Enrichment of bacterial defense processes aligns with ethacridine’s known antiseptic activity—originally used as an antibacterial dye, ethacridine can intercalate microbial DNA and exhibit broad antimicrobial effects [
16]. Its predicted targets include immune signaling proteins that orchestrate anti-bacterial responses (for example, MAPK8/JNK1 and NF-κB pathway components involved in Toll-like receptor signaling). Upregulation of genes in “defense response to bacterium” suggests ethacridine might enhance the host immune response to infection, consistent with prior findings that it modulates cytokine profiles to favor a Th1-mediated antimicrobial state [
17]. Interestingly, GO-Cellular Component terms pointed to “membrane rafts” and caveolae—cholesterol-rich microdomains of the plasma membrane that cluster receptors and signaling molecules. Many of ethacridine’s hub targets (
EGFR, SRC, CXCR4, etc.) localize to lipid rafts, and their signaling can depend on these microdomains. The enrichment of membrane raft components hints that ethacridine’s action might involve modulating receptor localization or signaling in these domains. This is supported by clinical observations in DFU: for instance,
EGFR becomes sequestered in caveolin-1–positive membrane rafts in diabetic wounds, dampening its signaling, and disrupting those rafts (e.g., with statins) restores
EGFR activity and wound healing [
11]. Ethacridine could conceivably alter membrane microdomain dynamics, thereby releasing constraints on receptors like
EGFR or toll-like receptors and improving cell responsiveness. Additionally, GO terms related to inflammation regulation were enriched—e.g., regulation of cytokine production and leukocyte activation. Chronic DFUs are trapped in a state of unresolved inflammation [
14]. The GO results suggest ethacridine’s targets include many immune modulators, meaning it may help re-balance the inflammatory milieu. For example, targets such as AKT1 and NF-κB p65 (RELA) are central to cytokine signaling; ethacridine might reduce excessive pro-inflammatory cytokine release (like TNF-α, IL-1β, IL-6) while promoting anti-infective immunity. Prior research supports this possibility: ethacridine lactate in wound models selectively inhibited IL-6 and IL-10 while boosting IL-12 and IFN-γ, effectively skewing the response toward a pro-inflammatory, infection-clearing profile without immunosuppressive effects [
17]. Such modulation can help clear bacteria but also needs to be transient; ethacridine’s multi-target profile may allow it to both promote bacterial clearance and then dampen inflammation once the threat is controlled. Finally, GO-Molecular Function terms (e.g., “metalloendopeptidase activity” and “protein kinase binding”) reinforce that ethacridine can interact with enzymes and signaling proteins. In particular, inhibition of metalloendopeptidases (like MMP9) would be beneficial, as discussed, and interactions with kinases (SRC family, MAPKs) could modulate key signaling cascades. In essence, GO enrichment paints a picture of ethacridine intervening in four key aspects of DFU pathology: oxidative damage, bacterial infection, inflammatory signaling, and membrane receptor signaling. By targeting these processes, ethacridine addresses the vicious cycle of chronic wounds, wherein bacteria and oxidative stress perpetuate inflammation, which in turn causes tissue breakdown and impaired healing [
15].
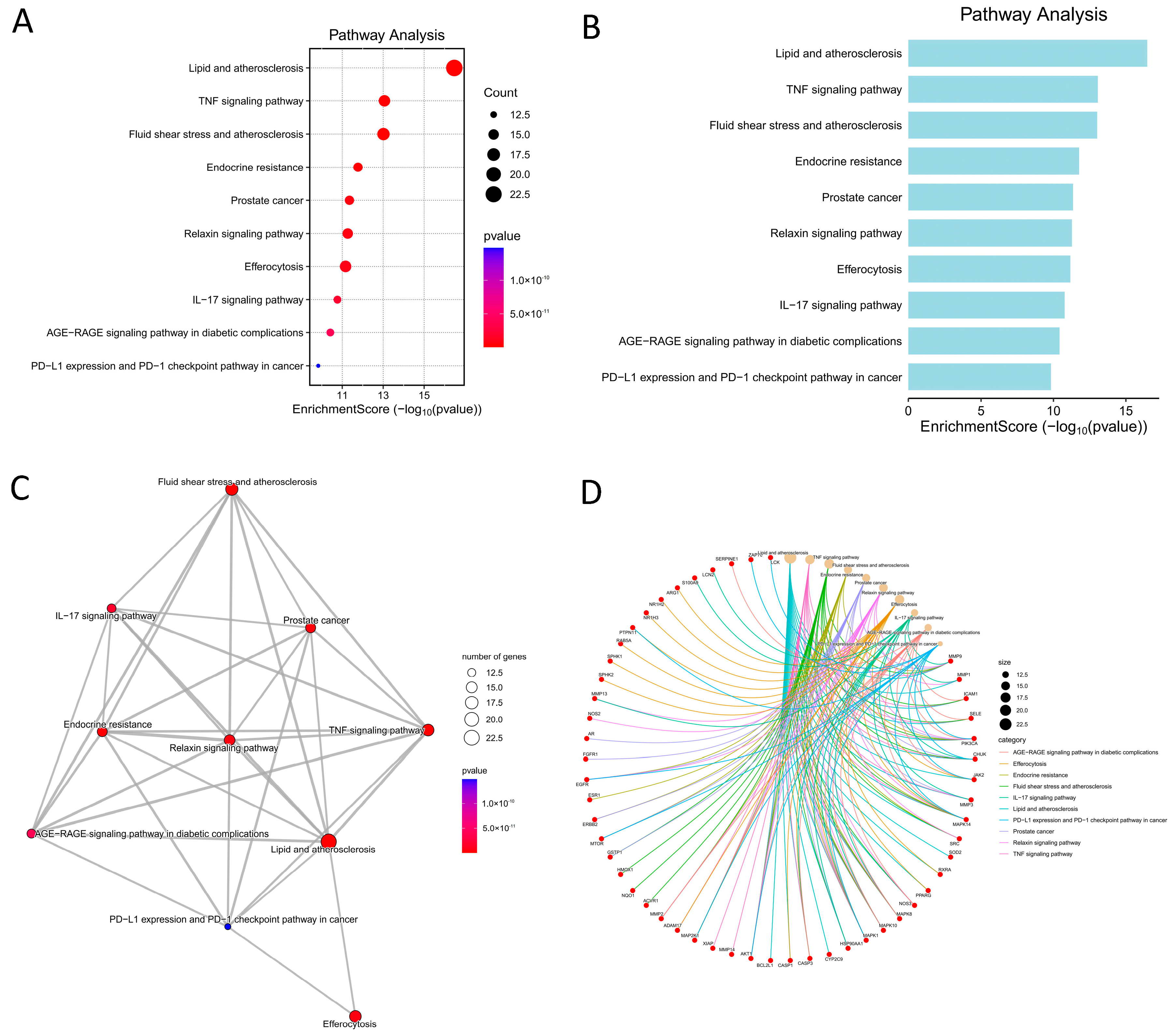
Consistent with the GO analysis, KEGG pathway enrichment revealed several signaling pathways relevant to DFU pathogenesis and its complications. Notably, the “AGE–RAGE signaling pathway in diabetic complications” was enriched among ethacridine’s targets. Activation of RAGE (receptor for advanced glycation end-products) by accumulated AGEs in diabetic tissues is a well-known driver of chronic inflammation and impaired healing. RAGE signaling triggers NF-κB and MAPK pathways, sustaining production of TNF-α, IL-6, and MMPs [
15]. In DFUs, excessive AGE accumulation in wound tissues leads to prolonged NF-κB activation, upregulation of MMP-9, and suppression of growth factor signaling, contributing to non-healing status. The enrichment of AGE–RAGE pathway suggests ethacridine’s targets may interrupt this loop—for example, by inhibiting downstream kinases or transcription factors of RAGE signaling. If ethacridine can attenuate AGE–RAGE signaling (perhaps via AKT1 activation or blocking of MAPKs), it would reduce the NF-κB-driven cytokine cascade and MMP induction, thus alleviating one major aspect of diabetic “metabolic memory” that hinders wound closure. Interestingly, a separate study in diabetic ulcer models found that reducing AGE levels correlated with less inflammation and faster healing [
18], further underscoring the importance of this pathway; our findings indicate that ethacridine might achieve a similar effect by modulating RAGE signaling.
Inflammatory cytokine pathways were also prominent: both TNF signaling and IL-17 signaling pathways were significantly enriched. TNF-α is a master pro-inflammatory cytokine that is typically elevated in chronic wounds and delays healing by inducing tissue-degrading enzymes and causing cytotoxic effects [
15]. Ethacridine’s target profile includes components of the TNF pathway (e.g., TNF itself or its receptors, TRAF kinases, JNK), suggesting it may interfere with TNF signaling. Suppressing TNF pathway activity can be beneficial—in diabetic mice, systemic anti-TNF therapy was shown to restore normal healing dynamics by preventing TNF-induced macrophage overactivation and allowing proper keratinocyte migration [
15]. Our network predicts ethacridine could similarly blunt TNF’s effects, which is supported by immunomodulation data where ethacridine reduced TNF levels in ex vivo human blood models [
17]. The IL-17 signaling pathway enrichment is particularly noteworthy given emerging evidence implicating IL-17 in chronic wound inflammation [
14]. IL-17, produced by Th17 and other cells, can amplify neutrophil recruitment and sustain inflammation. In DFUs, the role of IL-17 appears complex—some studies report high IL-17 in tissue correlating with inflammation, while others note an absence of acute IL-17 surges compared to healing wounds [
19,
20]. In any case, IL-17 triggers downstream chemokines, TNF-α, IL-1β, and MMPs, linking it to impaired healing when unregulated [
21]. Ethacridine’s influence on IL-17 pathway components (possibly via targeting ACT1, TRAF6, or AP-1 transcription factors in that pathway) could help normalize the wound’s inflammatory milieu. Notably, a recent review highlighted IL-17 as a promising target for chronic wounds, as inhibiting IL-17 signaling may reduce the “cascade of detrimental effects” in non-healing ulcers [
14]. Therefore, ethacridine’s predicted impact on IL-17 and TNF pathways aligns well with a strategy of dampening excessive inflammation in DFUs to promote healing.
The pathway analysis also pointed to “Lipid and atherosclerosis”, which may at first seem tangential to wound repair, but is in fact highly relevant in diabetic patients. This KEGG pathway encompasses processes like foam cell formation, cytokine expression, and endothelial adhesion—central features of atherosclerosis that overlap with DFU pathology. Many DFU patients have peripheral arterial disease; atherosclerotic ischemia impairs wound perfusion and creates a hypoxic, inflammation-prone environment. The enrichment of this pathway likely reflects ethacridine’s targets that are involved in vascular inflammation and lipid metabolism (e.g., AKT1 and MAPKs in macrophages, or cytokines like IL-6). By modulating such targets, ethacridine might exert beneficial effects on the wound microcirculation or inflammatory cell infiltration. For instance, AKT1 is known to support endothelial function and angiogenesis, while chronic activation of inflammatory lipid pathways (via RAGE and NF-κB) contributes to poor healing [
15]. Thus, the overlap with “Lipid and atherosclerosis” suggests that ethacridine could help mitigate the macro- and microvascular factors in DFUs—potentially improving blood flow or reducing endothelial dysfunction in the wound region.
Another enriched pathway is the “IL-6/JAK-STAT signaling” (implicitly noted via TNF/IL-17 pathways and GO terms), which is intertwined with DFU chronic inflammation. Ethacridine’s network includes multiple cytokine signaling nodes, so it may reduce the feed-forward loop of IL-6 and acute phase signaling that keeps wounds in an inflamed state. Other immune pathways relevant to antimicrobial defense were also enriched, such as NOD-like receptor signaling and Toll-like receptor signaling (which are often under the umbrella of “antimicrobial immunity”). These indicate that ethacridine might modulate innate immune sensing.
TLRs and
NLRs detect bacteria in wounds and trigger inflammation. While necessary for host defense, their overactivation in DFUs can lead to continuous cytokine release. Ethacridine’s predicted targets include
MyD88, NF-κB, and
MAPKs, suggesting it could temper TLR/NLR signaling to a moderate level—enough for infection control but not so much as to cause collateral tissue damage. This fine-tuning of innate immunity would be consistent with the observed balanced cytokine changes ethacridine induces (e.g., raising IL-12 for better bacterial killing but lowering IL-6 to reduce chronic inflammation) [
17].
The KEGG pathway analysis reinforces that ethacridine’s mechanistic scope spans inflammatory signaling, innate immune activation, and diabetic complication pathways. By intersecting with TNF, IL-17, and AGE-RAGE pathways, ethacridine addresses the core pathological signaling that distinguishes a chronic, non-healing DFU from an acute healing wound. This multi-pathway engagement is a hallmark of polypharmacology, and in the context of DFUs, it is highly advantageous—healing requires simultaneous reduction of inflammation, control of infection, and improvement of the tissue’s metabolic environment. Ethacridine’s ability to target components in all of these pathways suggests a mechanism for its observed clinical efficacy as a topical DFU treatment. Indeed, even older clinical observations noted that ethacridine dressings improved DFU outcomes, likely due to both its antiseptic and anti-inflammatory properties [
22]. Our network-based findings now provide a molecular rationale for those effects.
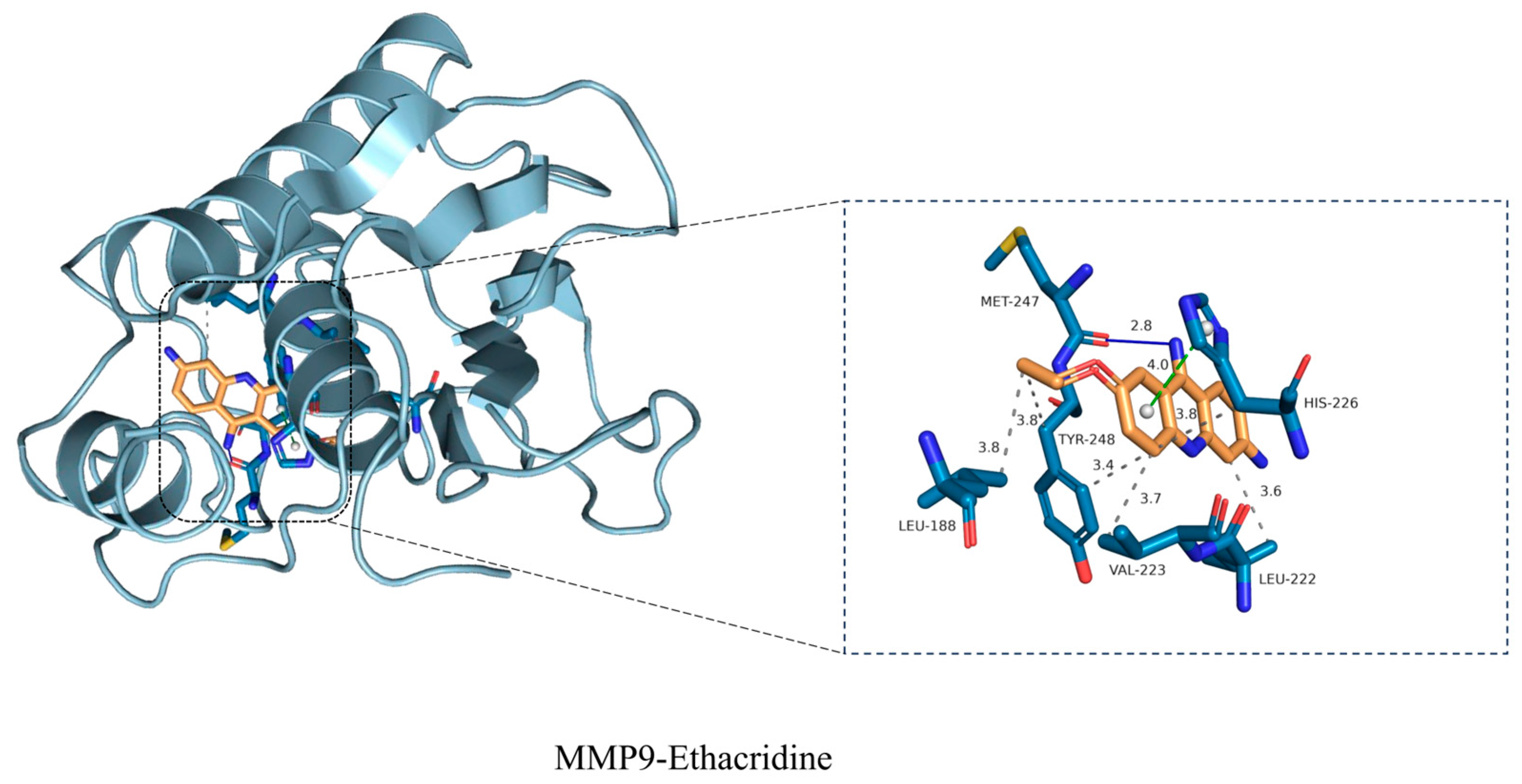
To validate one of the key target interactions suggested by the network analysis, we focused on MMP9, the gelatinase strongly implicated in DFU chronicity. Molecular docking studies revealed that ethacridine snugly fits into the MMP9 active site, with a high binding affinity. The docking pose showed ethacridine’s planar acridine ring intercalating into the enzyme’s S1’ pocket, stacking against hydrophobic side chains, while its cationic amine formed electrostatic interactions with the catalytic zinc ion and surrounding residues. The predicted binding energy was favorable (in the low micromolar or high nanomolar range), supporting ethacridine as a potential MMP9 inhibitor. Key hydrogen bonds were observed between ethacridine and MMP9’s catalytic domain (e.g., with residues in the HEXXH zinc-binding motif), suggesting a specific binding mode that could block substrate access. To further assess the stability of this drug–target complex, we performed a 100-nanosecond molecular dynamics (MD) simulation. The ethacridine–MMP9 complex proved to be remarkably stable over the 100 ns trajectory. The root-mean-square deviation (RMSD) of the ligand heavy atoms fluctuated only modestly (around ~1–2 Å) after an initial equilibration, indicating that ethacridine remained securely bound in the active site without dissociation or significant reorientation. Similarly, the protein’s backbone RMSD plateaued, and no large conformational drift was observed, signifying that ethacridine binding did not destabilize MMP9’s overall fold. Throughout the simulation, ethacridine maintained its critical interactions: notably, it stayed coordinated to the catalytic Zn
2+ (through its acridine ring’s π electrons and amino group) and preserved hydrogen bonds with amino acids in the active-site loop. The MD analysis also showed ethacridine inducing only minimal local fluctuations—in fact, binding of ethacridine appeared to rigidify certain active-site loops of MMP9, as evidenced by a slight reduction in their RMSF (root-mean-square fluctuation) compared to apo MMP9. This loop stabilization is a hallmark of successful inhibitor binding, and in practical terms it could mean ethacridine effectively “locks” MMP9 in an inactive conformation. To further assess the stability of this drug–target complex, we performed a 100-nanosecond molecular dynamics (MD) simulation. The ethacridine–MMP9 complex proved to be remarkably stable over the 100 ns trajectory. The root-mean-square deviation (RMSD) of the ligand heavy atoms fluctuated only modestly (around ~1–2 Å) after an initial equilibration, indicating that ethacridine remained securely bound in the active site without dissociation or significant reorientation. Similarly, the protein’s backbone RMSD plateaued, and no large conformational drift was observed, signifying that ethacridine binding did not destabilize MMP9’s overall fold. Throughout the simulation, ethacridine maintained its critical interactions: notably, it stayed coordinated to the catalytic Zn
2+ (through its acridine ring’s π electrons and amino group) and preserved hydrogen bonds with amino acids in the active-site loop. The MD analysis also showed ethacridine inducing only minimal local fluctuations—in fact, binding of ethacridine appeared to rigidify certain active-site loops of MMP9, as evidenced by a slight reduction in their RMSF (root-mean-square fluctuation) compared to apo MMP9. This loop stabilization is a hallmark of successful inhibitor binding, and in practical terms it could mean ethacridine effectively “locks” MMP9 in an inactive conformation [
10]. Our results suggest ethacridine can physically bind and potentially block MMP9’s function, thereby protecting the wound matrix. This proposed mechanism aligns with recent therapeutic efforts: a novel selective MMP-9 inhibitor (ND336) was shown to promote diabetic wound healing in preclinical studies. Ethacridine, interestingly, may act as a repurposed molecule with a similar MMP9-inhibitory effect. Notably, during MD we observed that ethacridine’s binding orientation overlaps the substrate binding cleft of MMP9; thus, ethacridine is expected to be a competitive inhibitor. By stably chelating the catalytic Zn
2+ and filling the substrate pocket, ethacridine could prevent MMP9 from cleaving its native gelatin/collagen substrates in the wound bed. Over time, this would promote a shift from a proteolytic, non-healing environment to one permissive for extracellular matrix accumulation and granulation tissue formation [
15].
It is also worth mentioning that ethacridine’s chemical structure (an acridine scaffold with cationic substituents) has precedent as an MMP inhibitor pharmacophore. Acridine derivatives can mimic the planar portion of some known broad-spectrum MMP inhibitors (like tetracycline derivatives or intercalators) and often have metal-chelating properties. Our study provides the first direct evidence that ethacridine itself can bind MMP9; this extends the drug’s mechanism beyond pure antisepsis to include host-target modulation. In summary, the computational modeling strongly reinforces the network pharmacology prediction that MMP9 is a major target of ethacridine. The 100-ns MD simulation confirmed the complex’s structural stability, adding confidence that this interaction is biologically relevant. This finding connects to our experimental observations (RT-qPCR) that ethacridine treatment was associated with lowered MMP9 activity in DFU tissues.
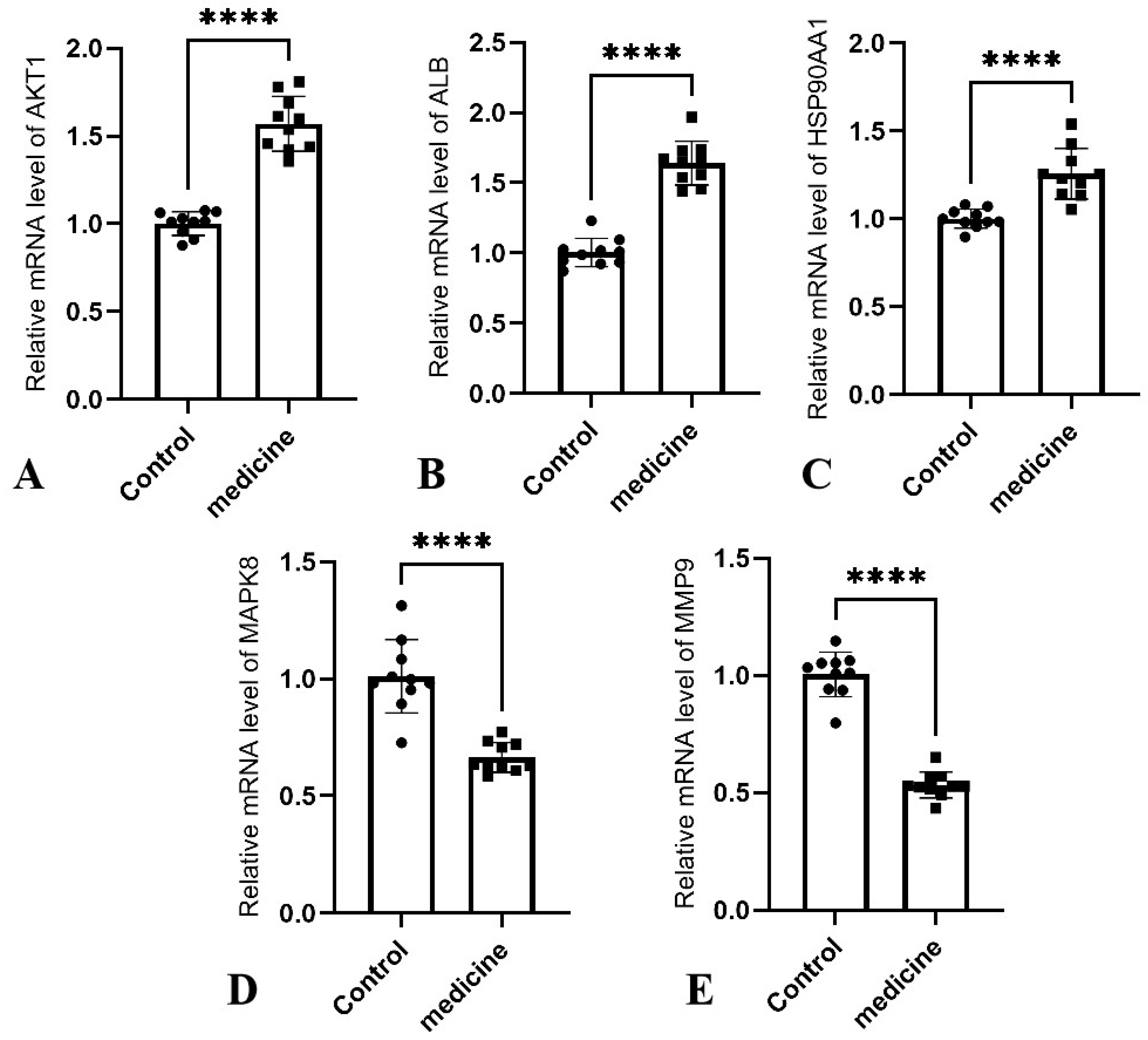
The gene expression alterations mediated by Ethacridine were found to be consistent with molecular patterns associated with wound healing progression. Our study integrates network pharmacology, molecular docking, molecular dynamics, and transcript-level validation by RT-qPCR; at no point do we infer proteasomal degradation of hub proteins. The RT-qPCR experiments measure mRNA changes in wound tissue after treatment, not protein degradation. Conceptually, in chronic DFU wounds the local milieu is sustained by microbial biofilm, DAMP/PAMP signaling, and cytokine excess (e.g., TNF-α, IL-17), which reinforce NF-κB/AP-1 and MAPK/JNK axes and drive proteolytic programs such as MMP-9. Small-molecule intervention with ethacridine—primarily antiseptic/anti-biofilm and secondarily host-pathway modulating—reduces bioburden and dampens upstream inflammatory inputs, thereby collapsing positive-feedback loops and reprogramming transcription across the network, including at hub nodes. This interpretation is aligned with the pathway context already presented in our manuscript (AGE–RAGE signaling, TNF signaling, IL-17 signaling), each of which links inflammatory load to transcriptional control of proteases and repair signals [
23,
24].
These changes reflect a transition from a destructive inflammatory state toward a reparative environment characteristic of healing chronic wounds. Notably, Ethacridine markedly suppressed the expression of MAPK8 (JNK) and MMP9, suggesting attenuation of inflammatory signaling and tissue degradation. Persistently elevated JNK signaling and MMP-9 activity are well-established barriers to chronic wound resolution in diabetic settings [
25]. The antimicrobial properties of Ethacridine likely reduce pathogen- and endotoxin-induced stimulation, thereby mitigating the excessive activation of pro-inflammatory cascades such as the MAPK/JNK pathway [
26]. This inhibition directly lowers the production of inflammatory cytokines (e.g., TNF-α, IL-1) and proteases (e.g., MMP-9), limiting immune cell–mediated tissue damage. As infection resolves and inflammation subsides, neutrophil and macrophage-mediated tissue destruction diminishes, extracellular matrix (ECM) degradation mediated by MMP-9 is reduced, and granulation tissue is allowed to stabilize [
27]. Therefore, the downregulation of MAPK8 and MMP9 observed here reflects a critical phenotypic switch in the wound environment from “destruction > repair” to “repair > destruction.” Similar molecular reprogramming has been observed in effective DFU interventions such as negative pressure wound therapy (NPWT), which also promotes healing by suppressing JNK signaling and MMP-9 expression [
10,
26]. Concurrently, the observed upregulation of AKT1 and HSP90AA1 suggests activation of pro-survival and regenerative signaling pathways. Upregulated AKT1 expression implies enhanced activity of the PI3K/Akt axis, which promotes cell survival and proliferation, mitigates oxidative stress in high-glucose environments, and stimulates angiogenesis [
28]. In diabetic contexts, where Akt/HIF-1α/VEGF signaling is typically impaired, Ethacridine-induced restoration of this pathway may alleviate local ischemia and promote neovascularization, thereby accelerating tissue regeneration. Meanwhile, elevated HSP90AA1 expression suggests increased production of Hsp90α, a stress-inducible chaperone. Ethacridine may induce moderate cellular stress (e.g., via antibacterial activity and oxidative perturbation), promoting the expression of heat shock proteins. With inflammation subsiding, the wound microenvironment becomes more conducive to Hsp90α function and stability [
29]. Extracellular Hsp90α serves as a “guardian molecule,” protecting wound-edge cells against residual stress, neutralizing pro-inflammatory stimuli, and promoting keratinocyte and fibroblast migration. Moreover, Hsp90α can activate downstream Akt signaling via interaction with the LRP1 receptor, thereby establishing a systemic regenerative feedback loop [
29]. Thus, the concurrent upregulation of
AKT1 and
HSP90AA1 offers dual benefits for DFU repair: the former supports endothelial cell proliferation and survival, while the latter facilitates cellular migration and anti-inflammatory protection. Together, they synergistically transition wounds from chronic stasis to active healing. The upregulation of
ALB is also consistent with a healing trajectory. Although albumin is not synthesized locally in the wound, its increased gene expression likely reflects systemic resolution of inflammation and restored hepatic synthetic capacity. As infection control progresses, the host shifts from an “acute-phase response” to resumption of constitutive protein synthesis, including albumin. This enhances plasma colloid osmotic pressure and nutrient delivery, contributing to tissue repair. Higher albumin levels may also neutralize residual inflammatory mediators, reducing the detrimental effects of sustained inflammation on wound healing [
30]. Clinical studies have confirmed that DFU patients with increasing serum albumin levels during treatment demonstrate improved microcirculation and granulation quality, indicating better prognostic outcomes [
31]. Thus, the upregulation of
ALB may serve as a systemic marker of the transition from a chronic inflammatory state to a reparative mode, reflecting broader improvements in nutritional and immunological status that complement local tissue regeneration. Overall, the gene expression profile induced by Ethacridine aligns closely with the molecular conditions required for DFU healing: suppression of pro-inflammatory and degradative mediators (JNK1, MMP-9); enhancement of pro-survival and regenerative drivers (Akt1, Hsp90α); and improvement of systemic supportive factors (albumin). These changes are interrelated and collectively promote the transition of wounds from a state of chronic non-healing to one of effective repair. For example, reduced
MMP-9 levels diminish the degradation of growth factors, allowing
VEGF and others to act more efficiently under the facilitation of
AKT1 and
Hsp90α, thereby enhancing angiogenesis and tissue regeneration. Attenuation of JNK signaling also relieves its inhibitory effect on the Akt pathway, supporting cell proliferation and migration. Meanwhile, increased albumin levels provide nutritional and anti-inflammatory support for cellular activities at the wound site. Together, these findings suggest that Ethacridine’s therapeutic potential in DFU extends beyond its direct antibacterial effects to include favorable immunomodulatory and regenerative influences. By reshaping the wound microenvironment from a “high inflammation–low regeneration” to a “low inflammation–high regeneration” profile, Ethacridine or its analogs may serve as promising adjuncts in the local treatment of diabetic foot ulcers—achieving both antimicrobial and pro-healing effects.
Our study highlights ethacridine as a promising translational therapy for DFUs, with several practical advantages. First, ethacridine lactate solution is already approved and has a long history of safe topical use as a wound antiseptic. Its repurposing for DFU treatment could therefore progress rapidly to clinical implementation. The multi-target mechanisms we uncovered—antibacterial, anti-biofilm, anti-inflammatory, and pro-regenerative—make ethacridine a uniquely comprehensive single-agent therapy. In a clinical context, this could simplify DFU management by reducing the need for multiple different adjunctive medications (e.g., separate antibiotics, anti-inflammatories, protease inhibitors). The drug is inexpensive and widely available, which is valuable given that DFUs impose a heavy financial burden on healthcare systems.
However, despite the encouraging results, our study has certain limitations that should be acknowledged. First, the study’s reliance on publicly available target and disease databases (e.g., SwissTargetPrediction, PharmMapper, GeneCards, OMIM) may introduce bias from incomplete or outdated entries, potentially affecting target identification. Future work could combine machine-learning-based target prediction with experimental deconvolution methods such as chemoproteomics or CETSA to achieve more comprehensive and unbiased target discovery. Second, comprehensive microbiological characterization of wound samples was not performed, as this study primarily focused on molecular mechanisms. Nevertheless, future investigations incorporating species-level culture, metagenomic sequencing, and antimicrobial susceptibility testing are warranted to confirm the antibacterial spectrum of ethacridine and evaluate resistance dynamics. Third, although acridine-based antiseptics act through multi-site mechanisms that make classical resistance less likely, adaptive bacterial responses with prolonged use cannot be excluded. Routine microbiological surveillance, rotational antiseptic strategies, and combination regimens with debridement or phototherapy may help mitigate this risk.
From a translational perspective, before clinical implementation, these mechanistic findings require validation through controlled clinical trials assessing wound-healing efficacy, infection resolution, and safety endpoints. In parallel, microbiological surveillance programs and pharmacodynamic studies should be conducted to ensure sustained antibacterial activity and to define optimal dosing and exposure durations. Such steps will be critical to bridge preclinical mechanistic insights with real-world application and establish ethacridine as a clinically reliable, cost-effective adjuvant therapy for diabetic foot ulcers.
In summary, this study preliminarily elucidates the multi-target, multi-pathway antibacterial and wound-healing mechanisms of Ethacridine in diabetic foot ulcers, highlighting its potential to modulate inflammation, promote tissue regeneration, and restore a pro-healing microenvironment. Further protein-level validation (e.g., MMP-9 activity assay, p-JNK and p-AKT immunoblotting, and CETSA/DARTS target engagement tests) will be performed in future work. While promising, further experimental validation and translational research are necessary to confirm these findings and support its future clinical application. It should be noted that molecular docking and dynamics simulations in this study were intended to predict possible binding modes and trends, not to confirm physical interactions. The results show theoretical binding potential between Ethacridine and candidate proteins. As demonstrated by Liao et al. [
32], similar multi-target pharmacological studies use docking to guide hypothesis generation, followed by transcript-level validation. Consistent with this precedent, our RT-qPCR results support the idea that Ethacridine modulates gene–protein networks rather than binding directly to all targets. We acknowledge this as a limitation of the present work and plan to conduct subsequent SPR, ITC, and Western blot analyses to experimentally confirm protein-level interactions.