Pathophysiological Links Between Stroke and Prediabetes: A Systematic Review
Abstract
1. Introduction
1.1. Research Question
1.2. Objectives
- To summarise the evidence on blood biomarker changes associated with stroke risk in individuals with prediabetes.
- To evaluate the reliability and clinical relevance of blood biomarkers for assessing stroke risk and outcomes in individuals with prediabetes.
2. Materials and Methods
2.1. Eligibility Criteria for the Study
2.2. Ethics Approval and Consent to Participate
2.3. Diagnostic Criteria
2.4. Search Strategy
2.5. Identification of Eligible Studies
2.6. Data Extraction
2.7. Risk of Bias and Quality Appraisal
2.8. Data Synthesis and Analysis
3. Results
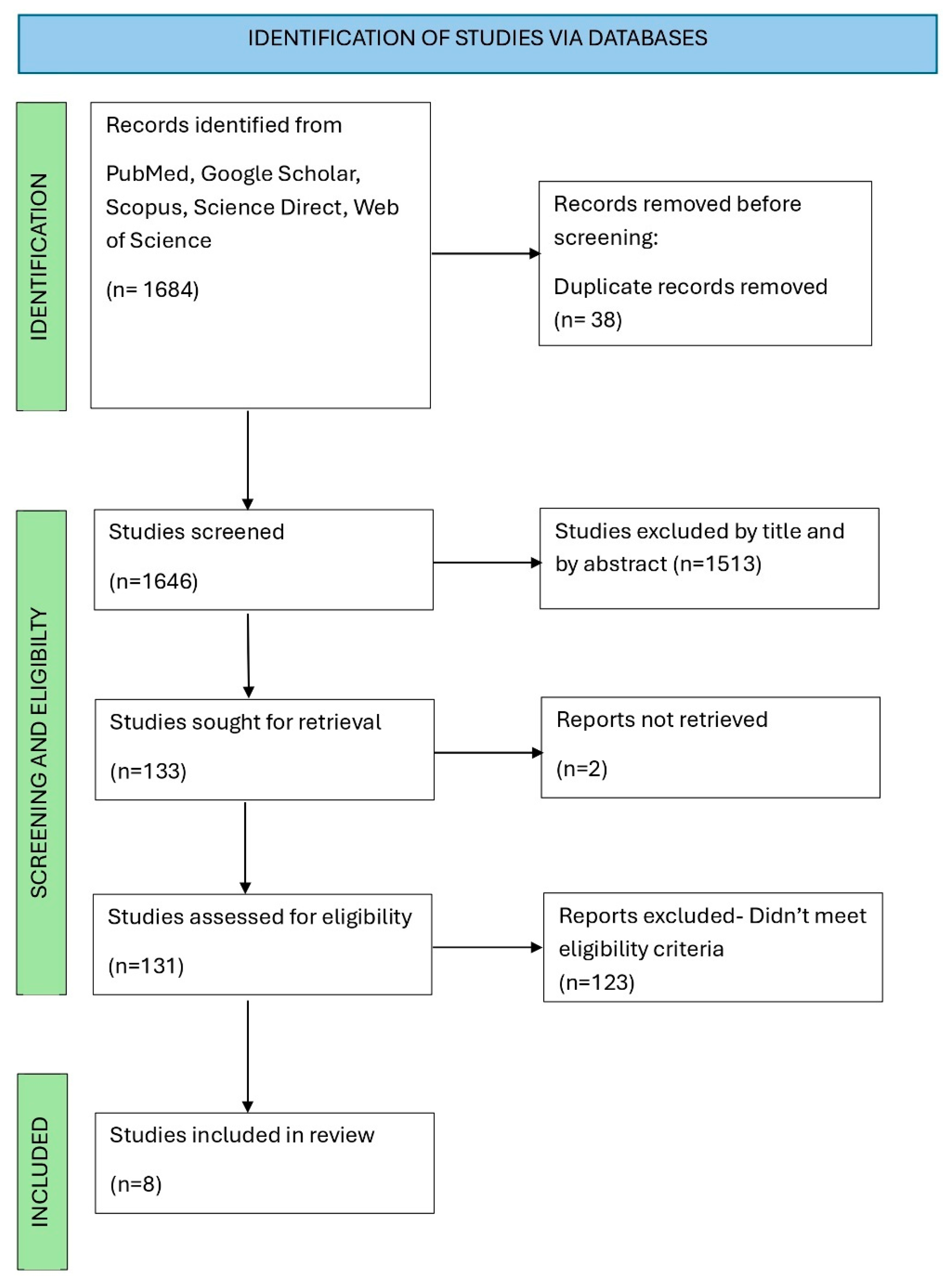
3.1. Search Report Results and Eligible Reports
3.2. Study Characteristics
4. Discussion
Pathophysiology Mechanisms Linking Stroke and Type 2 Diabetes Mellitus
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| AHA | American Heart Association |
| BBB | Blood–brain barrier |
| CT | Computed tomography |
| GFAP | Glial fibrillary acidic protein |
| HbA1c | Glycated Haemoglobin |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| IL-6 | Interleukin 6 |
| MRI | Magnetic resonance imaging |
| NSE | Neuron-specific enolase |
| PRISMA | Prepared Reporting Items for Systematic Reviews and Meta-Analyses |
| S100B | Calcium-binding protein B |
| T2DM | Type 2 diabetes mellitus |
| UKZN | University of KwaZulu-Natal |
| WHO | World Health Organization |
References
- Smushkin, G.; Vella, A. What is type 2 diabetes? Medicine 2010, 38, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prsevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Schlienger, J.-L. Complications du diabète de type 2. La Presse Médicale 2013, 42, 839–848. [Google Scholar] [CrossRef]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Milian, M.; Oliveres, M.; García-Eroles, L.; Massons, J. Impact of Female Gender on Prognosis in Type 2 Diabetic Patients with Ischemic Stroke. Eur. Neurol. 2006, 56, 6–12. [Google Scholar] [CrossRef]
- Ménégaut, L.; Laubriet, A.; Crespy, V.; Leleu, D.; Pilot, T.; Van Dongen, K.; de Barros, J.-P.P.; Gautier, T.; Petit, J.-M.; Thomas, C.; et al. Inflammation and oxidative stress markers in type 2 diabetes patients with Advanced Carotid atherosclerosis. Cardiovasc. Diabetol. 2023, 22, 248. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.F.; Zhang, H.S.; Wang, J.; Gao, Y.Y.; Mao, J.N.; Hang, C.H.; Li, W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front. Endocrinol. 2022, 13, 1092431. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. Neuromol. Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Alvarez, S.; Coffey, R.; Mathias, P.M.; Algotar, A.M. Prediabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Copyright © 2024; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef]
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol. Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- Mosenzon, O.; Cheng, A.Y.; Rabinstein, A.A.; Sacco, S. Diabetes and Stroke: What Are the Connections? J. Stroke 2023, 25, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.L.; Hong, K.-S.; Song, S.; Chang, K.-H.; Ovbiagele, B. Effect of pre-diabetes on future risk of stroke: Meta-analysis. BMJ Br. Med. J. 2012, 344, e3564. [Google Scholar] [CrossRef]
- Merino, J.G.; Warach, S. Imaging of acute stroke. Nat. Rev. Neurol. 2010, 6, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Waje-Andreassen, U.; Kråkenes, J.; Ulvestad, E.; Thomassen, L.; Myhr, K.-M.; Aarseth, J.; Vedeler, C.A. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005, 111, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Tian, B.-L.; Li, G.; Cui, Q.; Wang, C.-f.; Zhang, Q.; Peng, B.; Gao, Y.; Zhan, Y.-Q.; Hu, D.; et al. Elevated plasma D-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: A prospective, observational study. BMC Neurol. 2019, 19, 175. [Google Scholar] [CrossRef]
- di Biase, L.; Bonura, A.; Pecoraro, P.M.; Carbone, S.P.; Di Lazzaro, V. Unlocking the Potential of Stroke Blood Biomarkers: Early Diagnosis, Ischemic vs. Haemorrhagic Differentiation and Haemorrhagic Transformation Risk: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 11545. [Google Scholar] [CrossRef]
- Sowers, J.R.; Standley, P.R.; Ram, J.L.; Jacober, S.; Simpson, L.; Rose, K. Hyperinsulinemia, insulin resistance, and hyperglycemia: Contributing factors in the pathogenesis of hypertension and atherosclerosis. Am. J. Hypertens 1993, 6, 260s–270s. [Google Scholar] [CrossRef]
- Parikh, N.S.; Merkler, A.E.; Iadecola, C. Inflammation, Autoimmunity, Infection, and Stroke: Epidemiology and Lessons from Therapeutic Intervention. Stroke 2020, 51, 711–718. [Google Scholar] [CrossRef]
- Huang, K.; Liang, Y.; Ma, Y.; Wu, J.; Luo, H.; Yi, B. The Variation and Correlation of Serum Adiponectin, Nesfatin-1, IL-6, and TNF-α Levels in Prediabetes. Front. Endocrinol. 2022, 13, 774272. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Sun, Z.; Wang, X.; Miao, J.; Zheng, Z. The predictive value of TNF-α and IL-6 and the incidence of macrovascular complications in patients with type 2 diabetes. Acta Diabetol. 2012, 49, 3–7. [Google Scholar] [CrossRef]
- Pickup, J.C.; Chusney, G.D.; Thomas, S.M.; Burt, D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000, 67, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Kim, J.-H.; Kim, J.-M.; Kang, K.-W.; Lee, C.; Kim, J.-T.; Choi, S.-M.; Park, M.-S.; Cho, K.-H. D-dimer Level as a Predictor of Recurrent Stroke in Patients with Embolic Stroke of Undetermined Source. Stroke 2021, 52, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Fu, Q.; Zhou, L.; Fan, Y.; Liu, F.; Fan, Y.; Zhang, X.; Lin, W.; Wu, X. D-dimer as a predictor of cardiovascular outcomes in patients with diabetes mellitus. BMC Cardiovasc. Disord. 2022, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Žurek, J. Chapter 33—Biomarkers in Traumatic Brain Injury. In Essentials of Neuroanesthesia; Prabhakar, H., Ed.; Academic Press: Waltham, MA, USA, 2017; pp. 587–591. [Google Scholar]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Ayala-Guerrero, L.; García-delaTorre, P.; Sánchez-García, S.; Guzmán-Ramos, K. Serum Levels of Glial Fibrillary Acidic Protein Association with Cognitive Impairment and Type 2 Diabetes. Arch. Med. Res. 2022, 53, 501–507. [Google Scholar] [CrossRef]
- Gayger-Dias, V.; Vizuete, A.F.; Rodrigues, L.; Wartchow, K.M.; Bobermin, L.; Leite, M.C.; Quincozes-Santos, A.; Kleindienst, A.; Gonçalves, C.A. How S100B crosses brain barriers and why it is considered a peripheral marker of brain injury. Exp. Biol. Med. 2023, 248, 2109–2119. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Huang, Z.X.; Liu, Z. Biomarkers and the outcomes of ischemic stroke. Front. Mol. Neurosci. 2023, 16, 1171101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Xie, M.; Yan, L.; Chen, J.; Wang, H. NSE, a potential biomarker, is closely connected to diabetic peripheral neuropathy. Diabetes Care 2013, 36, 3405–3410. [Google Scholar] [CrossRef]
- Roshdy, N.; Zakaria, A.; Kahla, H.; Samy, S. Diagnostic value of initial s-100b and neuron- specific enolase levels in diabetic patients with ischemic stroke. Bull. Egypt. Soc. Physiol. Sci. 2007, 27, 275–290. [Google Scholar] [CrossRef]
- Pandey, A.; Saxena, K.; Verma, M.; Bharosay, A. Correlative study between neuron-specific enolase and blood sugar level in ischemic stroke patients. J. Neurosci. Rural. Pract. 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Nayak, A.R.; Badar, S.R.; Lande, N.; Kawle, A.P.; Kabra, D.P.; Chandak, N.H.; Raje, D.V.; Singh, L.R.; Daginawala, H.F.; Kashyap, R.S. Prediction of Outcome in Diabetic Acute Ischemic Stroke Patients: A Hospital-Based Pilot Study Report. Ann. Neurosci. 2016, 23, 199–208. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Liu, X.; Du, X.; Deng, B. Levels of serum S100B are associated with cognitive dysfunction in patients with type 2 diabetes. Aging 2020, 12, 4193–4203. [Google Scholar] [CrossRef] [PubMed]
- Mochol, M.; Taubøll, E.; Aukrust, P.; Ueland, T.; Andreassen, O.A.; Svalheim, S. Serum Markers of Neuronal Damage and Astrocyte Activity in Patients with Chronic Epilepsy: Elevated Levels of Glial Fibrillary Acidic Protein. Acta Neurol. Scand. 2023, 2023, 7246373. [Google Scholar] [CrossRef]
- Žurek, J.; Fedora, M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. 2012, 154, 93–103, discussion 103. [Google Scholar] [CrossRef] [PubMed]
| Criteria | ADA | WHO |
|---|---|---|
| Fasting Plasma glucose (IFG) | 5.6–6.9 mmol/L | 6.1–6.9 mmol/L |
| 2 Hour plasma glucose (IGT) | 7.8–11.0 mmol/L | 7.8–11.0 mmol/L |
| HbA1c % | 5.7–6.4% | - |
| Search | Search Terms |
|---|---|
| 1 | Search (“Prediabetes”[MeSH Terms] OR “Impaired Glucose Tolerance” OR “Type 2 Diabetes Mellitus”) |
| 2 | Search (“Stroke”[MeSH Terms] OR “Stroke Risk” OR “Cerebrovascular Accident”) |
| 3 | Search (“NSE” OR “Neuron-Specific Enolase” OR “D-dimer” OR “GFAP” OR “Glial Fibrillary Acidic Protein” OR “IL-6” OR “Interleukin-6” OR “S100B” OR “Fibrinogen” OR “Stroke Biomarkers”) |
| 4 (Additional Filters) | Humans, Age 18+, English, Free Full Text, Publication Date: 2003–2023 |
| 5 | Search (#1 AND #2 AND #3 AND #4) |
| Biomarker | Author (Year) | Study Design | Country | Population (n) | Avg. Age | Diabetes Status | Stroke Type/Condition | Key Findings | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Neurovascular Injury | |||||||||
| NSE | Pandey et al. (2011) | Cross-sectional case–control | India | 191 | 60.5 | Diabetes Mellitus | Ischaemic Stroke | Stroke: 18.0 ± 4.5 ng/mL Controls: 7.5 ± 1.5 ng/mL (p = 0.001) Normoglycemic Ischaemic Stroke Patients: 15.2 ± 2.4 ng/mL Hyperglycaemic Ischaemic Stroke Patients: 19.7 ± 4.7 ng/mL (p = 0.05). | Low |
| NSE | Nayak et al. (2016) | Cohort | India | 104 | 50 | Type 2 Diabetes Mellitus | Ischaemic Stroke | NSE < 0.65 µg/L in controls. Significant group differences but specific p-values not given. | High |
| NSE | Roshdy et al. (2007) | Cross-sectional | Egypt | 66 | 52.3 | Type 2 Diabetes Mellitus | Ischaemic Stroke | Diabetic Stroke: 14.42 ± 4.09 µg/L Controls: 6.71 ± 1.29 µg/L (p < 0.001) Strong positive correlation with infarct size (r = 0.938). | Low |
| S100B | Roshdy et al. (2007) | Cross-sectional | Egypt | 66 | 52.3 | Type 2 Diabetes Mellitus | Ischaemic Stroke | Diabetic Stroke: 115.18 ± 7.45 µg/L vs. Controls: 28.22 ± 7.9 µg/L (p < 0.001) Strong correlation with infarct size (r = 0.9816). | Low |
| S100B | Yu et al. (2020) | Case–control | Turkey | 164 | 49.67 | Type 2 Diabetes Mellitus | Cognitive Dysfunction | Lower levels in T2DM with cognitive dysfunction (0.117 µg/L) vs. controls (0.344 µg/L) (p < 0.05). | Moderate |
| GFAP | Ayala-Guerrero et al. (2022) | Comparative study | Mexico | 138 | 72.3 | Type 2 Diabetes Mellitus | Neurocognitive Disorders | GFAP higher in NCD with T2D (2046 pg/mL) vs. control without T2D (583.2 pg/mL) (p < 0.0001). | Moderate |
| Inflammation | |||||||||
| IL-6 | Pickup et al. (2000) | Case–control | UK | 37 | 55.4 | Type 2 Diabetes | Diabetic Complications | T2D: 1.8 pg/mL vs. Controls: 1.1 pg/mL (p < 0.01); No difference between diabetics with and without complications. | Low |
| IL-6 | Wu et al. (2012) | Case–control | China | 327 | 55 | Type 2 Diabetes Mellitus | Atherosclerosis | Higher IL-6 in both diabetic and non-diabetic groups with atherosclerosis vs. without (p = 0.007 and p = 0.004, respectively). | Low |
| Coagulation | |||||||||
| D-Dimer | Cheng et al. (2022) | Retrospective cohort | China | 1976 | 59.6 | Type 2 Diabetes Mellitus | Stroke Events (general) | Higher quartiles of D-dimer levels associated with more stroke events. p < 0.001 across all quartile comparisons. | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naicker, Y.; Khathi, A. Pathophysiological Links Between Stroke and Prediabetes: A Systematic Review. Curr. Issues Mol. Biol. 2025, 47, 854. https://doi.org/10.3390/cimb47100854
Naicker Y, Khathi A. Pathophysiological Links Between Stroke and Prediabetes: A Systematic Review. Current Issues in Molecular Biology. 2025; 47(10):854. https://doi.org/10.3390/cimb47100854
Chicago/Turabian StyleNaicker, Yerushka, and Andile Khathi. 2025. "Pathophysiological Links Between Stroke and Prediabetes: A Systematic Review" Current Issues in Molecular Biology 47, no. 10: 854. https://doi.org/10.3390/cimb47100854
APA StyleNaicker, Y., & Khathi, A. (2025). Pathophysiological Links Between Stroke and Prediabetes: A Systematic Review. Current Issues in Molecular Biology, 47(10), 854. https://doi.org/10.3390/cimb47100854







