Abstract
Chronickidney disease (CKD) poses a major global public health challenge, driven by a complex pathogenesis involving multiple interconnected processes—including metabolic disturbances, chronic inflammation, oxidative stress, endoplasmic reticulum stress, and ferroptosis—which collectively contribute to progressive and often irreversible loss of renal function. Although current standard therapies can ameliorate CKD progression, a substantial number of patients still advance to end-stage renal disease, highlighting the urgent need for innovative treatment strategies. Natural products have shown great promise in the prevention and management of CKD, largely attributable to their multi-target and multi-pathway synergistic effects. This review systematically outlines the core pathogenic mechanisms underlying CKD and elucidates the molecular mechanisms through which bioactive natural compounds exert renoprotective effects. Despite robust preclinical evidence, the clinical translation of these compounds remains hindered by limitations such as poor bioavailability and a lack of large-scale clinical trials. Moving forward, research should prioritize clinical translation of these compounds, aiming to provide novel therapeutic perspectives for CKD management.
1. Introduction
Chronic kidney disease (CKD) is a clinical syndrome characterized by irreversible changes in kidney structure and/or function, caused by various etiologies. It has a long course, poor prognosis, and multiple complications, making it one of the urgent public health issues worldwide. With the rapid development of society and the increasing aging population, the prevalence of metabolic diseases, cardiovascular diseases, and other conditions that can lead to CKD has been rapidly increasing. According to statistics, CKD affects an estimated 850 million people globally, with a subset of patients progressing to end-stage renal disease that requires renal replacement therapy [1,2]. The pathophysiology of CKD involves a complex network of interrelated mechanisms. Recent socioeconomic development has been associated with dietary patterns characterized by excessive nutrient intake and sedentary lifestyles, which collectively contribute to metabolic dysregulation. This dysregulation promotes ectopic lipid deposition in the kidney and damages renal parenchyma [3,4]. Furthermore, chronic inflammation, oxidative stress, endoplasmic reticulum stress, and ferroptosis are closely implicated in the development and progression of CKD [5,67]. These interconnected mechanisms collectively drive renal fibrosis, leading to irreversible loss of kidney function.
Current therapeutic strategies, including intensive lifestyle interventions and pharmacological approaches such as renin–angiotensin–aldosterone system inhibitors (RAASis) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) for managing blood pressure, glucose levels, and proteinuria, can partially slow the progression of chronic kidney disease (CKD). Nevertheless, a considerable number of patients still inevitably progress to end-stage renal disease (ESRD), ultimately becoming dependent on renal replacement therapy for survival [8,9]. Therefore, exploring other potentially effective novel treatments to ameliorate renal impairment in CKD constitutes an urgent research priority.
Within the complex pathological network of CKD, natural products demonstrate unique advantages due to their multi-target and multi-pathway synergistic effects. A systematic evaluation of clinical trials by Josa et al. confirmed that bioactive compounds such as curcumin, sulforaphane, and emodin exert nephroprotective effects in CKD patients through anti-inflammatory and antioxidant activities [10]. Furthermore, a 2024 meta-analysis indicated that Cordyceps sinensis alleviates renal inflammation and reduces plasma creatinine and urea nitrogen levels, suggesting its potential as an adjunct therapy for CKD patients undergoing renal replacement therapy [11]. These findings collectively support the unique and broad therapeutic potential of natural compounds in CKD management. This review systematically examines the key pathogenic mechanisms of CKD, details the renoprotective effects and molecular mechanisms of various natural products, and discusses current research limitations and future directions, aiming to provide novel insights into therapeutic strategies for CKD.
2. Methods
A comprehensive literature search was conducted using electronic databases, including PubMed, Web of Science, Google Scholar, and Embase, to identify publications from January 2000 to August 2025 related to the pathogenesis of chronic kidney disease and natural product interventions. The search strategy incorporated a combination of free-text terms and subject headings, such as “chronic kidney disease”, “renal fibrosis”, “nephropathy”, “kidney disease”, “renal failure”, “renal injury”, “pathogenesis”, “natural product”, “herb”, as well as specific compound names, such as “Curcumin” and “Resveratrol”. Inclusion criteria encompassed original research (both basic and clinical studies) and systematic reviews. Exclusion criteria consisted of case reports, conference abstracts, studies with incomplete or inaccessible data, and publications with uninterpretable outcomes. For duplicate publications, only the version with the most comprehensive data or the earliest publication date was retained. Literature screening and data extraction were performed independently by two researchers, with any discrepancies resolved through consensus discussion.
3. Pathogenesis
3.1. Metabolic Dysregulation
A genome-wide association analysis of 157 European CKD patients revealed that metabolic dysregulation is one of the primary pathways contributing to CKD progression (Figure 1) [12]. Under physiological conditions, substances such as glucose, uric acid, and lipids maintain dynamic equilibrium, and disruption of this balance—due to either overproduction or impaired excretion—constitutes a major cause of metabolic dysregulation. Prolonged consumption of a high-sugar, high-fat, or high-purine diet (where intake exceeds expenditure), as well as abnormal expression of metabolic enzymes, can lead to the accumulation of these metabolites, ultimately resulting in renal impairment.
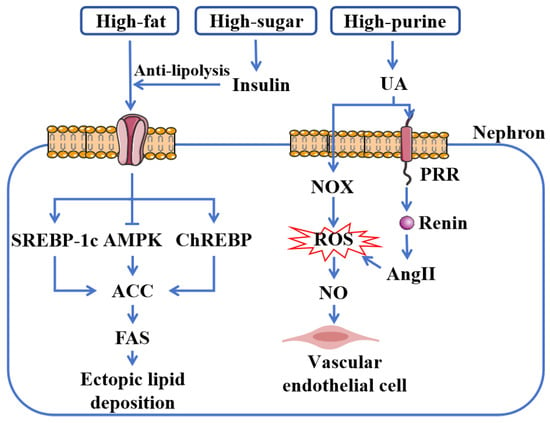
Figure 1.
Metabolic dysregulation in CKD. A high-fat and high-sugar diet contributes to renal ectopic lipid deposition by elevating FFAs. Insulin, which exerts an anti-lipolytic effect, plays a pivotal role in this process. These fatty acids subsequently promote lipid deposition by upregulating SREBP-1c and ChREBP and downregulating AMPK, which in turn leads to the activation of ACC and the upregulation of FAS, further driving lipid synthesis. Furthermore, a high-purine diet induces renal endothelial cell injury through a mechanism mediated by AngII and NOX-derived ROS. Abbreviations: SREBP-1c, sterol regulatory element-binding protein-1c; AMPK, AMP-activated protein kinase; ChREBP, carbohydrate response element-binding protein; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; UA, uric acid; NOX, NADPH oxidase; ROS, reactive oxygen species; NO, nitric oxide; PRR, pro-renin receptor; AngII, angiotensin II.
With the growing population of obese individuals, we have observed that diet and other lifestyle factors play a crucial role in the development and progression of CKD. Prolonged high-calorie diets contribute to “ectopic lipid accumulation”—a condition in which excess lipids deposit in non-adipose tissues when energy intake surpasses adipose storage capacity [13]. Studies indicate that lipids can accumulate in virtually all renal cell types, including mesangial cells, podocytes, and proximal tubular cells [3,14]. This ectopic lipid deposition is considered “lipotoxicity” and can induce the toxic effects in cellular injury, collectively promoting renal fibrosis [15,16,17].
The upregulation of key transcriptional regulators, including sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate response element-binding protein (ChREBP), coupled with downregulation of AMP-activated protein kinase (AMPK), promotes renal ectopic lipid deposition. This cascade subsequently activates acetyl-CoA carboxylase (ACC), leading to increased fatty acid synthase (FAS) expression and enhanced lipogenesis, thereby exacerbating renal lipid accumulation [18,19,20,21]. Animal studies have confirmed that mice deficient in AMPK activity develop lipid droplets in the proximal renal tubules [22]. Alterations in the expression or function of these molecules have also been consistently observed in CKD patients [23].
Furthermore, insulin resistance, frequently associated with CKD, exacerbates glomerular hyperfiltration, leading to glomerulosclerosis and renal dysfunction [24,25]. Additionally, insulin’s anti-lipolytic effect further promotes lipid accumulation, establishing a vicious cycle that perpetuates metabolic dysregulation [26].
Uric acid also contributes directly to renal injury. Derived mainly from dietary sources and nucleotide metabolism, uric acid is the end product of purine metabolism. As a pro-oxidant, it activates NADPH oxidase (NOX), inducing oxidative reactions and generating free radicals [27,28]. Additionally, uric acid stimulates the pro-renin receptor (PRR) in proximal tubular cells, upregulating the expression of renin and excessively activating the renin–angiotensin system (RAS). This leads to increased angiotensin II (AngII) production, elevated intracellular reactive oxygen species (ROS), inducing oxidative stress, suppression of nitric oxide (NO) release, and mediating endothelial cell injury, collectively contributing to renal dysfunction [29,30].
3.2. Chronic Inflammation and Oxidative Stress
Chronic inflammation is widely recognized as a pivotal driver in the initiation and progression of CKD, forming a self-reinforcing vicious cycle through its interplay with oxidative stress and mitochondrial dysfunction. Distinct from acute inflammation, chronic inflammation represents a maladaptive response triggered by persistent activation of pro-inflammatory signaling pathways. When renal resident cells are exposed to metabolic toxins, ischemia, or hypoxia, sustained inflammatory responses are activated, ultimately leading to renal interstitial fibrosis [31] (Figure 2). Hyperactivation of the key pro-inflammatory transcription factor nuclear factor kappa B (NF-κB) competitively suppresses nuclear factor erythroid 2-related factor 2 (Nrf2) and promotes immune cell infiltration [32,33]. This process upregulates pivotal pro-fibrotic factors such as transforming growth factor-beta (TGF-β), facilitating the transition of epithelial and mesangial cells into fibroblasts and myofibroblasts, which in turn stimulates collagen and extracellular matrix (ECM) protein secretion, thereby accelerating renal fibrosis.
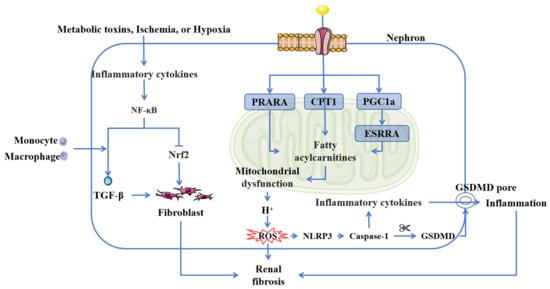
Figure 2.
Inflammation and oxidation in CKD. Chronic inflammation and oxidative stress are related to the NF-κB inflammatory signaling pathway and mitochondrial function. NF-κB activation further exacerbates oxidative stress by suppressing Nrf2. Mitochondrial fatty acid metabolism is central to this process. The reduced expression of CPT1, coupled with suppressed PPARA and PGC1a activity, initiates a pathogenic cascade. Furthermore, the downregulation of PGC1a inhibits ESRRA expression, leading to the accumulation of fatty acylcarnitines and subsequent mitochondrial dysfunction. This dysfunction promotes excessive ROS production, which triggers the assembly and activation of the NLRP3 inflammasome. Subsequent caspase-1 activation cleaves GSDMD, forming membrane pores and ultimately inducing pyroptosis. Abbreviations: TGF-β, transforming growth factor-beta; Nrf2, Nuclear factor erythroid-2-related factor 2; NF-κB, nuclear factor kappa-B; PPARA, peroxisome proliferator-activated receptor alpha; CPT1, carnitine palmitoyltransferase-1; PGC1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ESRRA, estrogen-related receptor alpha; ROS, reactive oxygen species; GSDMD, gasdermin D.
Chronic inflammation is closely linked with oxidative stress. The kidney, particularly renal tubular cells, is rich in mitochondria to meet its high energy demands, making mitochondrial health essential for renal function [34]. Mitochondria primarily generate energy through fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS). However, mitochondrial dysfunction is prevalent in CKD progression [35]. Studies of CKD have revealed significant downregulation of genes associated with FAO and OXPHOS [36,37]. Key manifestations include reduced expression of carnitine palmitoyltransferase-1 (CPT1), a rate-limiting enzyme in mitochondrial FAO, along with suppressed activity of peroxisome proliferator-activated receptor alpha (PPARA) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a) [38,39,40]. Downregulated PGC1a further inhibits estrogen-related receptor alpha (ESRRA) expression, impairing fatty acid oxidation and resulting in the accumulation of medium-chain and short-chain acylcarnitines [41]. Additionally, PGC1a and ESRRA serve as critical regulators of mitochondrial biogenesis and function, reflecting mitochondrial dysfunction [42].
Mitochondrial dysfunction disrupts the proton gradient across the inner mitochondrial membrane, leading to hydrogen ion leakage that reacts with oxygen and triggers a chain reaction of reactive oxygen species (ROS) generation [43,44]. As a key mediator of kidney injury, ROS activates the NLRP3 inflammasome, promotes caspase-1-mediated cleavage of gasdermin D (GSDMD), and induces pyroptosis—a form of programmed cell death characterized by pore formation in the cell membrane. This process facilitates the release of cellular contents and pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), thereby amplifying inflammatory responses and accelerating CKD progression [45,46]. In summary, chronic inflammation, oxidative stress, and mitochondrial dysfunction are causally interrelated and collectively drive the pathogenesis and progression of CKD.
3.3. Endoplasmic Reticulum Stress
Endoplasmic reticulum stress (ERS) represents a significant pathological mechanism in the progression of CKD. Under physiological conditions, the endoplasmic reticulum serves as an intricate membrane-bound organelle responsible for maintaining protein homeostasis—including synthesis, folding, processing, and trafficking—as well as regulating lipid synthesis and distribution [47,48]. However, during CKD progression, endogenous and exogenous factors such as tissue ischemia, hypoxia, oxidative stress, environmental toxins, and medications disrupt ER homeostasis, leading to sustained ERS (Figure 3). Persistent ERS leads to the continuous accumulation of misfolded or unfolded proteins, which, beyond a critical threshold, activate the unfolded protein response (UPR) and subsequently contribute to renal injury [6,49].
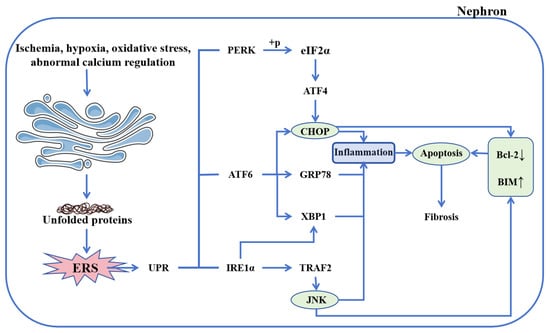
Figure 3.
Endoplasmic reticulum stress in CKD. The imbalance of endoplasmic reticulum homeostasis triggered by various factors initiates UPR, which is mediated by three key sensors: PERK, ATF6, and IRE1α. PERK activation phosphorylates eIF2α, leading to ATF4 upregulation and subsequent induction of CHOP expression. ATF6 enhances both CHOP and GRP78 expression, while also acting synergistically with IRE1α to promote XBP1 activation. Furthermore, IRE1α recruits TRAF2 to activate JNK, thereby initiating inflammatory signaling and promoting apoptosis. Both activated CHOP and JNK converge to suppress Bcl-2 while stimulating BIM, collectively driving apoptosis. Abbreviations: ERS, endoplasmic reticulum stress; UPR, unfolded protein response; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ATF6, activating transcription factor 6; IRE1α, inositol-requiring enzyme 1 alpha; ATF4, activating transcription factor 4; CHOP, CCAAT/enhancer-binding protein homologous protein; GRP78, glucose-regulated protein 78; XBP1, X-box binding protein 1; TRAF2, tumor necrosis factor receptor-associated factor 2; JNK, c-Jun N-terminal kinase; Bcl-2, B-cell lymphoma-2; BIM, Bcl-2 interacting mediator of cell death.
The UPR is mediated through three primary signaling branches involving protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 alpha (IRE1α), and activating transcription factor 6 (ATF6), which collectively function as sensors of ER stress [50]. Sustained ERS promotes PERK activation, resulting in phosphorylation of eukaryotic initiation factor 2α (eIF2α), upregulation of ATF4 expression, and induction of C/EBP homologous protein (CHOP) [51,52,53]. Concurrently, unmitigated ERS enhances IRE1α expression, leading to activation of X-box binding protein 1 (XBP1) and recruitment of TNF receptor-associated factor 2 (TRAF2), activating c-Jun N-terminal kinase (JNK), thereby initiating inflammatory signaling [54,55]. ATF6, a type II ER transmembrane protein, upregulates the expression of glucose-regulated protein 78 (GRP78), XBP1, and CHOP under persistent ERS conditions [54,56]. Activated CHOP and JNK further suppress the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2) while promoting expression of the pro-apoptotic Bcl-2 interacting mediator of cell death (BIM), collectively driving apoptosis [57,58,59].
Furthermore, ERS is closely associated with other CKD pathogenic mechanisms, including oxidative stress and inflammation. The accumulation of misfolded proteins in the ER enhances reactive oxygen species (ROS) generation and depletes glutathione (GSH), ultimately inducing oxidative stress [60,61]. Interestingly, GRP78, an ER molecular chaperone, not only facilitates protein folding and calcium binding but also modulates innate and adaptive immune responses, thereby inducing an inflammatory response [62]. Chronic ER stress also leads to overactivation of XBP1s and CHOP, which exacerbate inflammatory signaling, induce proliferation and deposition of extracellular matrix, accelerating the progression of renal fibrosis [63,64]. These findings collectively indicate that ERS contributes to the progression of CKD to end-stage renal disease (ESRD) and highlight its potential as a therapeutic target in chronic kidney disease.
3.4. Ferroptosis
In the early 21st century, Dolma et al. identified a novel compound named erastin, which selectively kills cancer cells through a non-apoptotic mechanism that can be significantly inhibited by iron chelators [65,66]. In 2012, Dixon termed this iron-dependent form of cell death “ferroptosis” [67]. The core mechanism involves intracellular iron accumulation, which triggers lipid peroxidation of polyunsaturated fatty acids in cell membranes, leading to toxic lipid peroxide buildup, elevated reactive oxygen species (ROS), and subsequent cell death [68]. Recent studies indicate that ferroptosis is co-regulated by multiple metabolic pathways, including iron metabolism, lipid metabolism, amino acid metabolism, redox homeostasis, and mitochondrial activity [69,70,71,72].
During the progression of CKD, dysregulations in lipid and iron metabolism are commonly present, providing a compelling rationale for investigating ferroptosis in CKD (Figure 4) [13,73]. System-Xc, composed of SLC7A11 and SLC3A2 subunits, is an amino acid antiporter that mediates the exchange of extracellular L-cystine and intracellular L-glutamate. It plays a vital role in glutathione (GSH) synthesis, a key antioxidant essential for maintaining cellular oxidative balance [74]. Inhibition of System-Xc reduces GSH levels, impairs glutathione peroxidase 4 (GPX4) expression, exacerbates lipid peroxide accumulation, and ultimately induces ferroptosis [75]. Studies have confirmed that inhibition of GPX4 in CKD promotes renal ferroptosis and accelerates kidney fibrosis progression [76,77].
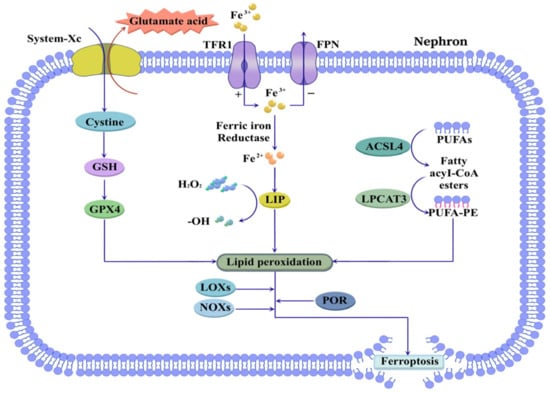
Figure 4.
Ferroptosis in CKD. Ferroptosis is closely associated with dysregulation of iron and lipid metabolism. Increased TFR1 activity and decreased FPN expression contribute to intracellular iron overload, resulting in the formation of LIP that catalyzes free radical generation. Concurrently, GSH depletion via System-Xc inhibition suppresses GPX4 activity. Furthermore, ACSL4 and LPCAT3 mediate the esterification of PUFAs into PUFA-PE, and further promote ferroptosis through LOXs, NOXs, and POR. Abbreviations: TFR1, transferrin receptor 1; FPN, ferroportin; LIP, labile iron pool; GSH, glutathione; GPX4, glutathione peroxidase 4; ACSL4, acyl-CoA synthetase long chain family member 4; LPCAT3, lysophosphatidylcholine acyltransferase 3; PUFA, polyunsaturated fatty acid; PUFA-PE, polyunsaturated fatty acid-phosphatidyl ethanolamine; LOX, lipoxygenase; NOX, NADPH oxidase; POR, cytochrome P450 oxidoreductase.
Under normal physiological conditions, iron regulatory proteins maintain iron homeostasis, preventing iron deposition in the kidneys. However, in CKD patients, dysregulation of iron metabolism-related proteins leads to abnormal intracellular iron accumulation [78]. Circulating iron, primarily in the Fe3+ form, is taken up by cells via transferrin receptor 1 (TFR1) and exported through ferroportin (FPN). Upregulation of TFR1 and downregulation of FPN result in elevated intracellular iron content [79,80]. Intracellular iron is reduced from Fe3+ to Fe2+ by ferric reductases; excess Fe2+ forms an unstable labile iron pool (LIP), where it catalyzes the conversion of hydrogen peroxide into hydroxyl radicals and other ROS via the Fenton reaction. This promotes lipid peroxidation, generates substantial lipid peroxides, damages membrane systems, and ultimately induces cell death [81]. Therefore, targeting iron metabolism-related proteins has emerged as a potential therapeutic strategy for CKD. Restoring renal iron homeostasis and mitigating iron-dependent ferroptosis may delay the onset and progression of CKD.
Ectopic lipid deposition is observed in nearly all renal cell types in CKD and correlates positively with disease progression [13,82,83]. The core mechanism of ferroptosis is iron-dependent lipid peroxidation, in which polyunsaturated fatty acids (PUFAs) serve as key substrates. During ferroptosis, PUFAs are activated by two membrane-remodeling enzymes: acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3). ACSL4 catalyzes the esterification of free PUFAs to acyl-CoA esters, which are then converted to polyunsaturated fatty acid-phosphatidyl ethanolamine (PUFA-PE) by LPCAT3 [84,85,86]. PUFA-PE, as the lipid peroxidation substrate, is further oxidized by lipoxygenases (LOXs), NADPH oxidases (NOXs), and cytochrome P450 oxidoreductase (POR), generating abundant radicals that drive ferroptosis [87,88,89,90]. In summary, targeting key proteins involved in the ferroptosis pathway represents a promising therapeutic strategy for CKD patients. This approach can effectively ameliorate intracellular iron overload, inhibit the accumulation of lipid peroxidation substrate PUFA-PE, improve oxidative stress, and block the lipid peroxidation cascade, thereby ameliorating renal injury.
4. Treatment
In recent years, natural plant-extracted compounds have gained significant attention as promising therapeutic agents for CKD. These compounds demonstrate considerable therapeutic potential in the treatment of renal pathologies, characterized by multi-target and multi-pathway synergistic effects, and demonstrate renal protective activities through mechanisms including regulation of metabolic dysregulation, anti-inflammatory actions, antioxidant effects, amelioration of endoplasmic reticulum stress, and suppression of ferroptosis (Table 1).
4.1. Flavonoids
Quercetin (QR), a naturally occurring flavonoid abundant in various vegetables and fruits, possesses multiple pharmacological properties, including anti-lipid deposition, anti-inflammatory, and antioxidant activities. Studies indicate that quercetin activates AMPK mRNA expression, upregulates PPARA and CPT1, and suppresses SREBP-1 expression, thereby reducing triglyceride levels in renal tubular epithelial cells, ameliorating lipid deposition, and decreasing urinary albumin (ALB) and β2-microglobulin (β2-MG) excretion, effectively attenuating lipid deposition-induced renal injury [91]. Furthermore, in vitro experiments demonstrate that quercetin inhibits NF-κB activation and reduces expression of tumor necrosis factor-alpha (TNF-α) and TGF-β1, significantly alleviating renal damage mediated by chronic inflammation [92]. Quercetin also exhibits protective effects against endoplasmic reticulum stress (ERS) by activating Sirtuin-1 (SIRT1), suppressing acetylation of eIF2α and XBP1, and consequently reducing CHOP mRNA expression, thereby protecting against CdCl2-induced renal injury [93]. Another animal study corroborates these findings, showing that quercetin significantly downregulates ERS markers, including GRP78, CHOP, PERK, IRE1α, and ATF6 in renal tissues, effectively ameliorating ERS in CKD [94]. Additionally, quercetin upregulates SLC7A11 expression, increases GSH and GPX4 levels, scavenges ROS, and decreases malondialdehyde (MDA) level, thereby mitigating ferroptosis-induced renal fibrosis [95].
Baicalin (BAI), a flavonoid obtained from the roots of Scutellaria baicalensis Georgi, regulates lipid metabolism disorders and demonstrates significant anti-inflammatory and antioxidant properties. Research shows that BAI activates the SIRT1/AMPK signaling pathway, alleviating lipid deposition in renal podocytes induced by high glucose conditions, thereby exerting renoprotective effects [96]. Additionally, BAI reduces urinary albumin-to-creatinine ratio (UACR) and urinary albumin excretion rate (UAER), while ameliorating pathological renal changes, including glomerular hypertrophy and mesangial matrix expansion. Its mechanisms involve activation of the Nrf2 signaling pathway, enhanced expression of antioxidant enzymes heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1), and suppression of NF-κB and MAPK signaling pathways, collectively mitigating chronic inflammation and oxidative stress [97]. Furthermore, considering the critical importance of strict glycemic control in CKD management, studies have demonstrated that the combination of baicalin with metformin produces a synergistic interaction, significantly enhancing the expression of antioxidant enzymes SOD and GPX, promoting ROS scavenge, and more effectively improving blood glucose and lipid profiles in diabetic rats compared to monotherapy, thereby providing protective effects against long-term hyperglycemia-induced renal injury [98]. However, the low oral bioavailability of BAI presents a limitation to clinical application. Zheng et al. demonstrated that a baicalin–lysozyme conjugate facilitates kidney-targeted delivery, increasing renal drug concentration, suppressing inflammation via NF-κB signaling, and inhibiting the TGF-β/Smad3 pathway to reduce extracellular matrix accumulation and renal fibrosis progression [99]. Notably, high-dose BAI treatment may abnormally activate the TGF-β/Smad pathway, potentially exacerbating renal injury and fibrosis [100].
Dihydromyricetin (DMY), extracted from Ampelopsis grossedentata (Hand. Mazz.) W. T. Wang, exhibits broad pharmacological activity against renal injury induced by insulin resistance and lipid accumulation. DMY enhances phosphorylation of insulin receptor substrate-1 (IRS1), restoring impaired insulin signaling pathways, improving insulin resistance, and reducing serum glucose and triglyceride concentrations, thereby ameliorating glucose and lipid metabolism disorders [101]. Moreover, DMY induces autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway, reducing renal interstitial fibrosis and preserving renal function [102]. DMY also suppresses TGF-β1 expression and downregulates miR-34a, consequently promoting expression of Klotho, an endogenous inhibitor of renal fibrosis, thus improving renal fibrosis [103]. In vitro studies further confirm that DMY activates the Nrf2/HO-1 signaling pathway and upregulates NQO1 expression, inhibiting high glucose-induced extracellular matrix accumulation in human mesangial cells (HMCs) and reducing fibronectin (FN) expression, effectively attenuating renal fibrosis progression [104].
Chrysin, a flavonoid found abundantly in the propolis of Populus przewalskii Maxim., possesses notable anti-inflammatory and antioxidant properties. In gentamicin (GM)-induced renal injury models, chrysin upregulates the antioxidant system GSH/GPX activity and suppresses the NF-κB/kidney injury molecule-1 (KIM-1) signaling pathway, demonstrating significant anti-inflammatory and antioxidant capacities and alleviating renal pathological changes [105]. Additionally, chrysin enhances the Nrf2/HO-1-mediated antioxidant system, inhibits the RAGE/NLRP3 signaling pathway, attenuates CdCl2-induced renal inflammatory damage, suppresses pro-apoptotic proteins caspase-3/Bcl-2-associated X protein (Bax), and upregulates Bcl-2 expression, thereby reducing renal cell apoptosis and effectively delaying CKD progression [106].
Fisetin, a natural antioxidant abundant in fruits and vegetables, inhibits NF-κB-mediated inflammatory signaling and upregulates antioxidant enzymes, including GSH, NQO1, and SOD. It restores activities of mitochondrial respiratory chain enzymes, ameliorating mitochondrial dysfunction and alleviating renal inflammation and oxidative stress damage [107]. In CKD models, fisetin upregulates GSH and GPX4 levels, while suppressing overexpression of ACSL4 and cyclooxygenase-2 (COX-2), thereby inhibiting ferroptosis. Concurrently, it significantly reverses elevated expression of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and pro-fibrotic factors (α-SMA and fibronectin), effectively ameliorating tubular injury and tubulointerstitial fibrosis [108].
Isoliquiritigenin (ISL), isolated from the roots and rhizomes of Glycyrrhiza uralensis Fisch, suppresses expression of the innate immune receptor macrophage-inducible C-type lectin (Mincle), inhibits M1 macrophage polarization, upregulates SIRT1 expression, promotes NF-κB deacetylation, inhibits NLRP3 expression, and blocks secretion of inflammatory cytokines IL-1β, IL-6, and TNF-α, demonstrating significant anti-inflammatory effects in chronic kidney disease [109,110].
4.2. Polyphenols
Curcumin (CUR), extracted from the traditional medicinal plant Curcuma longa L., is one of the most widely used natural edible pigments globally. Research has revealed its broad pharmacological activities, including anti-inflammatory, antioxidant, and lipid-regulating effects. In CKD mice, CUR functions as a natural Nrf2 activator, scavenging free radicals and alleviating renal oxidative stress. Additionally, it suppresses NF-κB signaling and inhibits IL-1β release, thereby ameliorating inflammatory infiltration, tubular atrophy, and dilation. CUR also modulates apoptosis by downregulating Bcl-2-associated X protein (Bax) and upregulating Bcl-2 expression [111]. A double-blind randomized controlled trial demonstrated that curcumin supplementation downregulated NF-κB mRNA expression in peripheral blood and reduced plasma high-sensitivity C-reactive protein levels in hemodialysis patients, indicating potential anti-inflammatory benefits in CKD management [112]. Despite favorable patient compliance with oral administration, its clinical application is limited by its poor bioavailability. To address this limitation, a curcumin-phospholipid complex utilizing a novel phospholipid drug delivery system significantly enhances curcumin bioavailability, demonstrates a good safety profile, markedly reduces chronic inflammation in stage 3–4 CKD patients, and shows substantial therapeutic benefits [113]. Furthermore, given the multi-component, multi-target nature of natural medicines, their interactions warrant careful consideration. Studies indicate that curcumin and rhein exhibit significant synergistic effects in CKD treatment, with combination therapy reducing metabolic clearance, substantially increasing plasma drug concentrations, and more effectively improving renal fibrosis compared to monotherapy [114]. Notably, considering that conventional CKD management primarily focuses on strict control of blood pressure, glucose, and proteinuria, the potential integration of natural products with standard antihypertensive regimens represents a promising therapeutic approach. In this context, research has demonstrated that the combination of curcumin with amlodipine produces significantly enhanced vasodilatory effects compared to monotherapy, resulting in more effective blood pressure control and consequent renal protection [115].
Resveratrol (RSV), primarily obtained from Reynoutria japonica Houtt (Polygonaceae), promotes AMPK phosphorylation, subsequently activating the SIRT1/PGC1a signaling pathway and PPARA, while inhibiting the expression of SREBP-1—a key regulator of fatty acid synthesis—thereby regulating lipid metabolism disorders and ameliorating renal ectopic lipid deposition [116]. Additionally, resveratrol demonstrates significant anti-inflammatory and antioxidant capacities. Studies show that resveratrol upregulates renal Nrf2 expression; restores levels of antioxidant proteins, including HO-1, superoxide dismutase (SOD), and GPX; suppresses inflammatory mediators TNF-α and IL-6; repairs renal damage induced by inflammation and oxidative stress; and significantly reduces collagen deposition and fibrosis in renal tissues [117]. Furthermore, cotreatment with resveratrol and pioglitazone demonstrates superior efficacy in ameliorating metabolic disorders, significantly reducing hyperglycemia and improving insulin resistance compared to monotherapy, thereby providing enhanced protection against diabetes-induced renal injury [118].
Chlorogenic acid (CGA), a natural polyphenol, is found abundantly in Ilex paraguariensis A. St.-Hil. [119]. CGA inhibits neurogenic locus notch homolog protein 1 (Notch1) and signal transducer and activator of transcription (STAT3) protein expression, consequently attenuating SREBP-1c-mediated fatty acid synthesis while promoting CPT1-mediated fatty acid oxidation. This dual action reduces renal ectopic lipid deposition and suppresses the TGF-β1/Smad signaling pathway, thereby improving renal fibrosis [120]. Moreover, chlorogenic acid possesses potent anti-inflammatory and antioxidant properties. It inhibits Toll-like receptor 4 (TLR4)/NF-κB signal transduction and NLRP3 inflammasome activation, significantly mitigating renal inflammatory damage, while upregulating the Nrf2/HO-1 signaling pathway and enhancing SOD expression, thereby strengthening antioxidant capacity and effectively attenuating renal injury [121,122].
Carnosol, a natural compound extracted from Rosmarinus officinalis L., exhibits diverse biological activities and demonstrates therapeutic potential against various inflammatory diseases. Research confirms that carnosol provides significant protection against renal injury by downregulating pro-oxidant enzymes NOX and LOX, enhancing GSH levels and SOD activity, and exerting potent antioxidant effects to counteract oxidative stress. Simultaneously, it downregulates mRNA or protein expression of UPR signaling components—including GRP78, IRE1α, PERK, ATF4, ATF6, CHOP, XBP1s, and eIF2α—inhibiting endoplasmic reticulum stress, suppressing NF-κB cascade activation, blocking inflammatory cytokine release, and consequently improving renal function impairment [123].
4.3. Glycosides
Dioscin, extracted from Dioscorea nipponica Makino, has been investigated as a potential therapeutic agent for chronic kidney disease (CKD) due to its anti-inflammatory, antioxidant, and anti-fibrotic properties. Studies demonstrate that dioscin reduces phosphorylation of NF-κB both in vivo and in vitro, downregulates inflammatory factors including IL-1β, NLRP3, monocyte chemotactic protein 1 (MCP-1), IL-6, TNF-α, and IL-18, and diminishes inflammatory infiltration and collagen fiber deposition in renal tissues [124]. As key regulators of various biological processes, microRNAs are modulated by dioscin, which suppresses miR-34a expression and upregulates SIRT1, promoting nuclear translocation of Nrf2 and enhancing antioxidant gene transcription [125]. Furthermore, dioscin activates the Nrf2/HO-1 signaling pathway, enhances antioxidant capacity, scavenges ROS, upregulates GSH/GPX4 levels, and inhibits lipid peroxidation, thereby suppressing renal ferroptosis [126].
Astragaloside IV (AS-IV), one of the most bioactive compounds of Astragalus membranaceus (Fisch.) Bunge., attenuates NLRP3 inflammasome overexpression and reduces levels of IL-6, IL-1β, and TNF-α, thereby alleviating renal inflammatory injury [127]. Research indicates that AS-IV activates the Nrf2/HO-1/NQO1 pathway, promotes SOD expression, and eliminates ROS, significantly mitigating high glucose-induced oxidative stress in renal cells [128]. Additionally, AS-IV inhibits ERS-related proteins GRP78, PERK, ATF4, and CHOP, substantially reducing the Bax/Bcl-2 ratio and caspase-3 expression, thereby diminishing ERS-induced apoptosis in tubular epithelial cells and exerting beneficial effects against renal injury [129]. Furthermore, studies demonstrate that intravenous administration of AS-IV in combination with the RAAS inhibitor enalapril produces superior renoprotective effects compared to monotherapy, significantly improving urinary albumin excretion rate, providing better blood pressure control, and markedly reversing pathological changes, including glomerular basement membrane thickening, tubular cell proliferation, and tubular atrophy [130].
Ginsenoside Rb1 (Rb1), a bioactive component of Panax ginseng C. A. Mey., has been used for millennia in East Asian traditional medicine and demonstrates remarkable efficacy in treating various diseases. Rb1 inhibits NOX, thereby reducing renal oxidative stress, while downregulating TGF-β1, collagen I, and FN to suppress renal interstitial fibrosis in UUO rats [131]. It also downregulates GRP78 and eIF2α/CHOP signaling pathway to alleviate ERS and simultaneously suppresses the TGF-β1/Smad3 pathway, positioning it as a potential natural agent for treating renal fibrosis [132]. Clinical studies confirm that Rb1 supplementation upregulates GPX expression, reduces pro-inflammatory cytokines IL-6 and TNF-α, and effectively ameliorates oxidative stress and inflammation in early-stage CKD patients [133].
4.4. Polysaccharides
Astragalus polysaccharide, a key bioactive component of Astragalus membranaceus (Fisch.) Bunge., enhances AMPK activity and suppresses ACC expression, thereby improving glucose uptake and insulin sensitivity [134]. In high glucose-stimulated podocytes, TLR4 expression is significantly upregulated, while Astragalus polysaccharide treatment reverses this effect and inhibits subsequent NF-κB activation, thereby reducing IL-1β, IL-6, and TNF-α levels, suppressing inflammatory responses, and alleviating renal injury [135].
Ginseng polysaccharide, obtained from Panax ginseng C. A. Mey., significantly upregulates GSH and SOD expression, enhances renal antioxidant capacity, and eliminates ROS, thereby inhibiting oxidative stress-induced renal damage [136]. Additionally, it suppresses PERK/eIF2α/ATF4/CHOP signaling cascades, ameliorates ERS, and upregulates anti-apoptosis through the inhibition of Bax and upregulation of Bcl-2, collectively contributing to renal protection [137].
Refined fucose polysaccharide (RFP), extracted from the dry lateral roots of Aconitum carmichaelii Debeaux—commonly used for CKD—exhibits remarkable antioxidant capacity by effectively scavenging free radicals. It upregulates GSH/GPX4 and antioxidant enzyme SOD expression, ameliorates intracellular iron overload in renal cells, inhibits lipid peroxidation, significantly reduces lipid peroxide MDA and 4-hydroxynonenal (4-HNE), thereby effectively alleviating ferroptosis-induced renal injury [138].
4.5. Alkaloids
Nuciferine, extracted from the dried leaves of Nelumbo nucifera Gaertn., has demonstrated beneficial therapeutic effects against kidney diseases. Studies reveal that it activates AMPK, which subsequently downregulates FAS expression, suppresses fatty acid synthesis, and alleviates renal lipid deposition. Furthermore, AMPK activation promotes the Nrf2/HO-1 pathway while suppressing the TLR4/NF-κB pathway, thereby reducing oxidative stress and inflammation in CKD [139]. Nuciferine restores intracellular iron homeostasis by modulating FPN1 and TFR1, thereby attenuating renal iron overload. It also enhances the GSH/GPX4 antioxidant axis, upregulates SLC7A11 expression, and alleviates lipid peroxidation, further inhibiting ferroptosis in CKD, reducing fibronectin (FN) accumulation, and ameliorating renal fibrosis [140].
Berberine, isolated from Coptis chinensis Franch., promotes PGC1a expression, upregulates fatty acid oxidation-related enzymes, including CPT1 and PPARA, while activating the AMPK pathway and promoting ACC phosphorylation to suppress fatty acid synthesis. These processes collectively reduce lipid deposition in proximal tubular cells and ameliorate glomerulosclerosis and tubulointerstitial fibrosis [141]. Furthermore, berberine upregulates the Nrf2 signaling pathway, promotes Bcl-2 expression, downregulates NF-κB pathway, thereby enhancing antioxidant capacity and inhibiting renal apoptosis and inflammation [142].
Boldine, a principal alkaloid extracted from the bark and leaves of the traditional South American medicinal plant Peumus boldus Molina, has been demonstrated to attenuate renal inflammation and oxidative stress by reducing osteopontin (OPN) expression and macrophage infiltration, as well as by scavenging ROS. Boldine also inhibits TGF-β, significantly reducing the expression of α-SMA and collagen III, thus improving renal fibrosis [143]. Moreover, it promotes the expression of gap junction protein Connexin43 (Cx43), enhances intercellular communication among renal mesangial cells, reduces abnormally elevated membrane permeability, and helps maintain renal cellular homeostasis under high-glucose conditions, thereby curbing the localized amplification of inflammation [144].
4.6. Quinones
Emodin, a bioactive anthraquinone extracted from Rheum palmatum L., exerts multifaceted renoprotective effects through several mechanisms. It upregulates the AMPK/mTOR signaling pathway to promote renal autophagy and preserve renal function [145]. Furthermore, emodin enhances antioxidant defense via Nrf2 activation and suppresses inflammation by inhibiting NF-κB signaling [146]. Studies show that emodin can also ameliorate high glucose-induced ERS by inhibiting PERK/eIF2α/ATF4/CHOP pathways, thereby reducing apoptosis and attenuating renal injury [147]. However, findings from the National Toxicology Program indicate a significant dose-dependent safety concern, with mice exposed to high concentrations of emodin showing markedly increased incidence and severity of renal tubule injury and nephropathy [148].
Rhein, isolated from Rheum palmatum L., ameliorates renal fibrosis by inhibiting STAT3 phosphorylation and suppressing the expression of collagen I and α-smooth muscle actin (α-SMA). It also attenuates renal cell apoptosis by upregulating Bcl-2 and downregulating Bax [149]. Additionally, rhein suppresses TFR1 expression, thereby ameliorating intracellular iron overload. Concurrently, it inhibits ACSL4 activity to reduce lipid peroxidation substrate generation and upregulates GSH/GPX4 activity to enhance ROS scavenging, ultimately blocking the onset of ferroptosis [150]. However, caution must be exercised regarding the dose-dependent safety issues associated with rhein. Studies have demonstrated that long-term administration of rhein can significantly reduce SOD and GSH levels, promote the expression of TGF-β1 and TNF-α, increase blood urea nitrogen and serum creatinine levels, and exacerbate pathological renal damage, indicating its potential nephrotoxicity [151].
Tanshinone IIA, extracted from Salvia miltiorrhiza Bunge, exerts renoprotective effects through multi-pathway regulation. It attenuates renal inflammation and fibrosis by inhibiting NF-κB and TGF-β/Smad signaling pathways [152]. Furthermore, it also alleviates oxidative stress and pyroptosis through scavenging ROS, downregulating NLRP3 expression, and suppressing caspase-1-mediated GSDMD cleavage [153]. Additionally, it mitigates endoplasmic reticulum stress in CKD by inhibiting PERK/eIF2α/ATF4/CHOP and reducing GRP78 expression, thereby preserving renal function [154].
4.7. Terpenoids
Oleanolic acid (OA), a natural pentacyclic triterpenoid from Olea europaea L., exhibits broad pharmacological activities. By activating the AMPK/PGC1a pathway to enhance fatty acid metabolism and alleviate renal lipid deposition, while simultaneously suppressing the TLR4/NF-κB axis to inhibit pro-inflammatory mediator production and mitigate inflammatory injury, OA confers renoprotective effects [155]. OA also attenuates oxidative stress by upregulating SOD expression and enhancing ROS scavenging, while concurrently suppressing ERS through inhibition of the PERK/eIF2α/CHOP pathway. Furthermore, OA downregulates TGF-β/Smad signaling to mitigate renal fibrosis and reduces apoptosis by upregulating Bcl-2 expression, collectively attenuating renal injury [156]. Additionally, in diabetic rat models, OA demonstrates synergistic effects with insulin by enhancing insulin signaling pathway activation, promoting glycogen synthase expression, reducing glycogen levels, and consequently ameliorating hyperglycemia-induced organ damage [157]. However, the clinical translation of OA is limited by the poor bioavailability and lack of targeting specificity of conventional oral formulations. To address these challenges, Chen et al. developed a biomimetic drug delivery system consisting of neutrophil membrane-coated liposomes loaded with OA. This innovative platform significantly improves renal targeting, enhances antioxidant and anti-inflammatory efficacy, and promotes renal functional recovery [158].
Withaferin A, a bioactive compound isolated from the traditional Indian medicinal plant Withania somnifera Dunal, demonstrates significant renoprotective effects in CKD. It effectively attenuates renal inflammation by suppressing NF-κB phosphorylation and IL-1β release, while concurrently inhibiting the expression of pro-fibrotic mediators, including TGF-β and fibronectin. Furthermore, withaferin A ameliorates endoplasmic reticulum stress through downregulation of the eIF2α/ATF4/CHOP axis and GRP78 overexpression, collectively contributing to its renal protective activity [159].
Ginkgolide B (GB), a bioactive terpenoid from the leaves of Ginkgo biloba L., alleviates renal ferroptosis and oxidative stress by modulating iron homeostasis and enhancing antioxidant capacity. It upregulates ferritin heavy chain 1 (FTH1) and inhibits TFR1 activity, thereby maintaining intracellular iron homeostasis. Concurrently, GB activates GPX4 and promotes ROS scavenging, effectively ameliorating renal oxidative stress and ferroptosis [160].
4.8. Others
Sulforaphane (SFN), an isothiocyanate compound extracted from Brassica oleracea var. Italica Plenck, demonstrates a renoprotective effect through the regulation of lipid metabolism, mitochondrial function, and oxidative stress. SFN activates AMPK, ameliorating lipid metabolic disorders and attenuating renal lipotoxic injury [161]. It also downregulates the renal scavenger receptor CD36, suppresses SREBP-1 and FAS to inhibit fatty acid synthesis, and effectively reduces renal lipid deposition. Concurrently, SFN upregulates PGC1a and Nrf1, enhances mitochondrial biogenesis, thereby alleviating kidney injury [162]. In vitro studies have further confirmed that sulforaphane (SFN) upregulates Nrf2 activity, promotes NQO1 expression, and scavenges reactive oxygen species (ROS). Concurrently, SFN elevates glutamate-cysteine ligase (GCL) expression, enhances glutathione (GSH) biosynthesis, and thereby synergistically counteracts cisplatin-induced oxidative stress, ultimately alleviating renal injury [163]. Furthermore, a clinical study involving non-dialysis CKD patients revealed that daily supplementation with 400 μg SFN for one month significantly increased Nrf2 and NQO1 expression, enhanced antioxidant capacity, and mitigated oxidative stress-mediated renal injury [164].
Brazilian green propolis (BGP), a natural product extracted from resin collected by bees from Baccharis dracunculifolia DC., is rich in various bioactive components. Clinical studies confirm that supplementation with BGP extract alleviates inflammation and oxidative stress in dialysis patients with CKD, through suppression of NF-κB, reduction in pro-inflammatory cytokines including TNF-α and IL-1β, and activation of the Nrf2 pathway [165,166].
Cordyceps sinensis (Berk.) Sacc. (CS), a fungal–larval complex used traditionally in renal diseases, regulates renal lipid metabolism disorders by activating PPARA and inhibiting FAS [167]. It also attenuates renal fibrosis through suppression of the TGF-β1/Smad signaling pathway and inhibition of renal epithelial–mesenchymal transition (EMT) [168]. Furthermore, in chronic allograft nephropathy patients, CS combined with immunosuppressants significantly reduces the incidence of complications and is beneficial for improving renal function [169].

Table 1.
Potential bioactive components for the treatment of CKD.
Table 1.
Potential bioactive components for the treatment of CKD.
| Categories | Natural Products | Herb | Model | Dose | Effects | Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|
| Flavonoids | Quercetin | Various herbs | Cd-induced SD rats | 50, 100 mg/kg | AMPK↑, PPARA↑, CPT1↑, SREBP-1↓, TG↓; NF-κB↓, TNF-α↓, TGF-β1↓; SIRT1↑, eIF2α↓, XBP1↓, CHOP↓, GRP78↓, PERK↓, IRE1α↓, ATF6↓; SLC7A11↓, GSH↑, GPX4↑, ROS↓, MDA↓ | Improve metabolic dysregulation, anti-inflammation, inhibit endoplasmic reticulum stress, and inhibit ferroptosis | [91] |
| HG-induced SV40 MES 13 cells | 5, 10, 50 μg/L | [92] | |||||
| Cd-induced Wistar rats | 50 mg/kg/d | [93] | |||||
| Adenine andPotassium oxonate-induced SD rats | 50, 100 mg/kg | [94] | |||||
| FA/IRI-induced C57BL/6J mice | 25 mg/kg | [95] | |||||
| RSL3/erastin-induced HK-2/NRK-52E cells | 20 μM | ||||||
| Baicalin | Scutellaria baicalensis Georgi | db/db mice | 100 mg/kg | SIRT1↑, AMPK↑; Nrf2↑, HO-1↑, NQO1↑; NF-κB↓, MAPK↓, TGF-β↓, Smad3↓ | Improve metabolic dysregulation, anti-inflammation, antioxidative stress, and reduce fibrosis | [96] | |
| HG-induced MPC-5 cells | 3, 6, 12 μM | ||||||
| db/db mice | 400 mg/kg | [97] | |||||
| STZ-induced SD rats | 160 mg/kg | [99] | |||||
| Dihydromyricetin | Ampelopsis grossedentata (Hand. Mazz.) W. T. Wang | HFD-induced db/db mice | 500, 1000 mg/kg | IRS1↑, PI3K↓, Akt↓, mTOR↓, TGF-β1↓, Nrf2↑, HO-1↑, NQO1 | Improve metabolic dysregulation, promote autophagy, and antioxidative stress | [101] | |
| STZ-induced SD rats | 100 mg/kg | [102] | |||||
| HG induced NRK-52E/HEK293 cells | 1 μM | ||||||
| UUO C57BL/6J mice | 500 mg/kg | [103] | |||||
| TGF-β1-induced HK-2 cells | 50, 100, 200 μM | ||||||
| HG-induced HMC cells | 10, 20 μM | [104] | |||||
| Chrysin | Populus przewalskii Maxim. | GM-induced albino rats | 100 mg/kg | GSH↑, GPX↑, Nrf2↑, HO-1↑, NF-κB↓, RAGE↓, NLRP3↓, caspase-3↓, Bax↓, Bcl-2↑ | Antioxidative stress, anti-inflammation, and reduce apoptosis | [105] | |
| Cd-induced Wistar albino rats | 25, 50 mg/kg | [106] | |||||
| Fisetin | Various herbs | CIS-induced SD rats | 0.625, 1.25 mg/kg | NF-κB↓, GSH↑, NQO1↑, SOD↑, GPX4↑, ACSL4↓, COX-2↓, IL-1β↓, IL-6↓, TNF-α↓, α-SMA↓, FN↓ | Antioxidative stress, anti-inflammation, inhibit ferroptosis, reduce fibrosis | [107] | |
| Adenine/UUO induced C57BL/6J mice | 50, 100 mg/kg | [108] | |||||
| Adenine/TGF-β1 induced TCMK-1 cells | 20 μM | ||||||
| Isoliquiritigenin | Glycyrrhiza uralensis Fisch | UUO induced C57BL/6J mice | 7.5, 30 mg/kg | SIRT1↑, NF-κB↓, NLRP3↓, IL-1β↓, IL-6↓, TNF-α↓ | Anti-inflammation | [109] | |
| STZ-induced SD rats | 20 mg/kg | [110] | |||||
| Polyphenols | Curcumin | Curcuma longa L. | GM-induced albino rats | 200 mg/kg | Nrf2↑, ROS↓, NF-κB↓, IL-1β↓, Bax↓, Bcl-2↑ | Antioxidative stress, anti-inflammation, and reducing apoptosis | [111] |
| Hemodialysis CKD patients | 2.5 g (95% purity) post-dialysis | [112] | |||||
| CKD 3–4 patients | 500 mg twice a day | [113] | |||||
| Resveratrol | Reynoutria japonica Houtt | C57BLKS/J db/db mice | 20 mg/kg | SIRT1↑, PGC1a↑, PPARA↑, SREBP-1↓, Nrf2↑, HO-1↑, SOD↑, GPX↑, TNF-α↓, IL-6↓ | Improve metabolic dysregulation, antioxidative stress, and anti-inflammation | [116] | |
| HFD-induced Wistar rats | 100 mg/kg | [117] | |||||
| Chlorogenic acid | Ilex paraguariensis A. St.-Hil. | HFD+STZ-induced C57BL/6J mice | 50 mg/kg | Notch1↓, STAT3↓, SREBP-1c↓, CPT1↑, TGF-β1↓, Smad↓, TLR4↓, NF-κB↓, NLRP3↓, Nrf2↑, HO-1↑, SOD↑ | Improve metabolic dysregulation, antioxidative stress, anti-inflammation, and reduce fibrosis | [120] | |
| HG+PA induced HK-2 cells | 20, 40, 80 μM | ||||||
| IRI-induced Swiss mice | 3.5, 7, 14 mg/kg | [121] | |||||
| HFD+STZ-induced Wistar rats | 10 mg/kg | [122] | |||||
| HG-induced HK-2 cells | 20, 50, 100 μM | ||||||
| Carnosol | Rosmarinus officinalis L. | UUO-induced C57BL/6J mice | 50 mg/kg | NOX↓, LOX↓, GSH↑, SOD↑, GRP78↓, IRE1α↓, PERK↓, ATF4↓, ATF6↓, CHOP↓, XBP1↓, eIF2α↓, NF-κB↓ | Antioxidative stress, anti-inflammation, and inhibit endoplasmic reticulum stress | [123] | |
| Glycosides | Dioscin | Dioscorea nipponica Makino | UUO-induced C57BL/6J mice | 50, 100 mg/kg | NF-κB↓, IL-1β↓, NLRP3↓, MCP-1↓, IL-6↓, TNF-α↓, IL-18↓, SIRT1↑, Nrf2↑, HO-1↑, ROS↓, GSH↑, GPX4↑ | Antioxidative stress, anti-inflammation, inhibit ferroptosis | [124] |
| TGF-β1-induced HK-2 cells | 3.125, 6.25, 12.5 μM | ||||||
| CIS-induced HK-2/NRK-52E cells | 50,100, and 200 ng/mL | [125] | |||||
| CIS-induced Wistar rats/C57BL/6J mice | 10, 20, 40 mg/kg; 10, 30, 60 mg/kg | ||||||
| CIS-induced Wistar rats | 60 mg/kg | [126] | |||||
| Astragaloside IV | Astragalus membranaceus (Fisch.) Bunge | db/db mice | 40 mg/kg | NLRP3↓, IL-6↓, IL-1β↓, TNF-α↓, Nrf2↑, HO-1↑, NQO1↑, SOD↑, ROS↓, GRP78↓, PERK↓, ATF4↓, CHOP↓, Bax↓, Bcl-2↑, and caspase-3↓ | Antioxidative stress, anti-inflammation, inhibit endoplasmic reticulum stress, and reduce apoptosis | [127] | |
| HG-induced MPC cells | 10, 20, 40 μM | ||||||
| HG-induced HK-2 cells | 10, 20, 40 μM | [128] | |||||
| HFD+STZ-induced SD rats | 20, 40, 80 mg/kg | [129] | |||||
| Ginsenoside Rb1 | Panax ginseng C. A. Mey. | UUO SD rats | 50 mg/kg | NOX↓, TGF-β1↓, Smad3↓, collagen I↓, FN↓, GRP78↓, eIF2α↓, CHOP↓, GPX↑, IL-6↓, TNF-α↓ | Antioxidative stress, anti-inflammation, inhibit endoplasmic reticulum stress, reduce fibrosis | [131] | |
| Bavachin-induced HK-2 cells | 40 μM | [132] | |||||
| CKD 2–3 patients | 500 mg/d | [133] | |||||
| Polysaccharides | Astragalus polysaccharide | Astragalus membranaceus (Fisch.) Bunge. | HFD+STZ-induced SD rats | 700 mg/kg | AMPK↑, ACC↓, TLR4↓, NF-κB↓, IL-1β↓, IL-6↓, TNF-α↓ | Improve metabolic dysregulation, anti-inflammation | [134] |
| STZ-induced SD rats | 200, 400, 800 mg/kg | [135] | |||||
| Ginseng polysaccharide | Panax ginseng C. A. Mey. | Cr-induced ICR mice | 25, 50, 100, 200, 400 mg/kg | GSH↑, SOD↑, ROS↓, PERK↓, eIF2α↓, ATF4↓, CHOP↓, Bax↓, Bcl-2↑ | Antioxidative stress, inhibit endoplasmic reticulum stress, and reduce apoptosis | [136] | |
| CIS-induced ICR mice | 200, 400 mg/kg | [137] | |||||
| Refined fucose polysaccharide | Aconitum carmichaelii Debeaux | CIS-induced Kunming mice | 200, 400, 800 mg/kg | GSH↑, GPX4↑, SOD↑, MDA↓, 4-HNE↓ | Antioxidative stress, and inhibit ferroptosis | [138] | |
| Alkaloids | Nuciferine | Nelumbo nucifera Gaertn. | PA-induced HK-2 cells | 10, 40 μM | AMPK↑, FAS↓, Nrf2↑, HO-1↑, TLR4↓, NF-κB↓, FPN1↑, TFR1↓, GSH↑, GPX4↑, SLC7A11↑, FN↓ | Improve metabolic dysregulation, antioxidative stress, anti-inflammation, and inhibit ferroptosis | [139] |
| FA-induced C57BL/6 mice | 30 mg/kg | [140] | |||||
| RSL3/erastin/FIN56 induced HK-2/HEK293T cells | 2.5, 5, 10, 20, 40 μM | ||||||
| Berberine | Coptis chinensis Franch. | C57BLKS/J db/db mice | 200 mg/kg | PGC1a↑, CPT1↑, PPARA↑, ACC↓, Nrf2↑, Bcl-2↑, NF-κB↓ | Improve metabolic dysregulation, anti-inflammation, antioxidative stress, and reduce apoptosis | [141] | |
| PA-induced MPC-5 cells | 0.4 μM | ||||||
| Methotrexate induced male albino rats | 200 mg/kg | [142] | |||||
| Boldine | Peumus boldus Molina | Two-Kidney, One-Clip induced SD rats | 50 mg/kg | OPN↓, ROS↓, TGF-β↓, α-SMA↓, collagen III, ↓, Cx43↑ | Antioxidative stress, anti-inflammation, and reducing fibrosis | [143] | |
| STZ-induced SD rats | 50 mg/kg | [144] | |||||
| Quinones | Emodin | Rheum palmatum L. | STZ-induced SD rats | 20, 40 mg/kg | AMPK↑, mTOR↓, Nrf2↑, NF-κB↓, PERK↓, eIF2α↓, ATF4↓, CHOP↓ | Promote autophagy, antioxidative stress, anti-inflammation, and inhibit endoplasmic reticulum stress | [145] |
| adenine-induced SD rats | 100 mg/kg | [146] | |||||
| KK-Ay mice | 40, 80 mg/kg | [147] | |||||
| HG-induced MCP-5 cells | 20, 40 μM | ||||||
| Rhein | Rheum palmatum L. | UUO SD rats | 150 mg/kg | STAT3↓, α-SMA↓, Bax↓, Bcl-2↑, TFR1↓, ACSL4↓, GSH↑, GPX4↑, ROS↓ | Antioxidative stress, inhibiting ferroptosis, and reducing apoptosis and fibrosis | [149] | |
| STZ-induced C57BL/6J mice | 150 mg/kg | [150] | |||||
| HG-induced MPC-5 cells | 25 μg/ml | ||||||
| Tanshinone IIA | Salvia miltiorrhiza Bunge | 5/6 nephrectomy induced SD rats | 10 mg/kg | NF-κB↓, TGF-β↓, Smad↓, ROS↓, caspase-1↓, GSDMD↓, PERK↓, eIF2α↓, ATF4↓, CHOP↓, GRP78↓ | Antioxidative stress, anti-inflammation, inhibit endoplasmic reticulum stress, reduce pyroptosis | [152] | |
| db/db mice | 10 mg/kg | [153] | |||||
| HG-induced HRGEC cells | 20 μg/ml | ||||||
| STZ-induced SD rats | 2, 4, 8 mg/kg | [154] | |||||
| Terpenoids | Oleanolic acid | Olea europaea L. | HFD+STZ induced SD rats | 50, 100 mg/kg | AMPK↑, PGC1a↑, TLR4↓, NF-κB↓, SOD↑, ROS↓, PERK↓, eIF2α↓, CHOP↓, TGF-β↓, Smad↓, Bcl-2↑ | Improve metabolic dysregulation, anti-inflammation, antioxidative stress, inhibit endoplasmic reticulum stress, and reduce apoptosis | [155] |
| OLETF rats | 5 μM | [156] | |||||
| H2O2-induced NRK-52E cells | 8 μM | [158] | |||||
| Withaferin A | Withania somnifera Dunal | UUO-induced C57BL/6J mice | 3 mg/kg/d | NF-κB↓, IL-1β↓, TGF-β↓, FN↓, eIF2α↓, ATF4↓, CHOP↓, GRP78↓ | Anti-inflammation, inhibit endoplasmic reticulum stress, reduce fibrosis | [159] | |
| Ginkgolide B | Ginkgo biloba L. | C57BL/KsJ db/db mice | 200 mg/kg | FTH1↑, TFR1↓, GPX4↑, ROS↓ | Antioxidative stress and inhibit ferroptosis | [160] | |
| PA+HG-induced MCP-5 cells | 20, 40, 80 μM | ||||||
| Others | Sulforaphane | Brassica oleracea var. Italica Plenck | HFD+STZ induced C57BL/6J mice | 0.5 mg/kg | AMPK↑, CD36↓, SREBP-1↓, FAS↓, PGC1a↑, Nrf1↑, Nrf2↑, NQO1↑, ROS↓, GCL↑, GSH↑ | Improve metabolic dysregulation, antioxidative stress | [161] |
| UUO Wistar rats | 1 mg/kg | [162] | |||||
| CIS-induced LLC-PK1 cells | 1, 3, 5 μM | [163] | |||||
| CIS-induced Wistar rats | 500 μg/g | ||||||
| Non-dialysis CKD patients | 400 μg/d | [164] | |||||
| Brazilian green propolis | Baccharis dracunculifolia DC. | Peritoneal dialysis CKD patients | 400 mg/d | NF-κB↓, TNF-α↓, IL-1β↓, Nrf2↑ | Antioxidative stress, anti-inflammation | [165] | |
| Hemodialysis CKD patients | 200 mg/d | [166] | |||||
| Cordyceps sinensis extract | Cordyceps sinensis (Berk.) Sacc. | HFD+STZ-induced SD rats | 1.2 g/kg | PPARA↑, FAS↓, TGF-β1↓, Smad↓, EMT↓ | Improve metabolic dysregulation, reduce fibrosis | [167] | |
| 5/6 nephrectomy induced SD rats | 2 g/kg | [168] | |||||
| Chronic allograft nephropathy patients | 2.0 g/d | [169] |
↑ (Upward arrow): represents upregulation. ↓ (Downward arrow): represents downregulation.
5. Conclusions
Chronic kidney disease (CKD) is characterized by a complex pathological network involving interconnected mechanisms such as metabolic disturbances, chronic inflammation, oxidative stress, endoplasmic reticulum stress (ERS), and ferroptosis. These processes engage multiple signaling molecules and pathways, collectively driving renal injury. Rather than stemming from a single etiology, CKD progression is orchestrated by a vicious cycle of these interconnected pathological events. Natural products have recently emerged as promising candidates for therapeutic intervention, largely owing to their multi-target, multi-pathway synergistic properties. Extensive preclinical evidence indicates that various natural compounds can effectively modulate core pathological processes in CKD, thereby producing synergistic benefits in attenuating renal injury, thereby complementing conventional therapies aimed at controlling blood pressure, glucose, and proteinuria.
While the pleiotropic nature of natural products offers therapeutic advantages for multifaceted conditions like CKD, current evidence predominantly derives from preclinical studies. Their clinical translation is hindered by the absence of large-scale, multicenter, randomized controlled trials. Additionally, some natural products present potential toxicities—for instance, high-dose baicalin has been reported to abnormally activate the TGF-β/Smad pathway and aggravate renal fibrosis. Notably, while the potential toxicities of certain natural products have been well-documented in other disease models, direct evidence within the specific physiological condition of CKD is still lacking. Future studies should prioritize the systematic evaluation of their safety profiles under CKD conditions. Beyond safety concerns, significant pharmacokinetic obstacles impede translation. Many natural compounds exhibit poor oral bioavailability, extensive first-pass metabolism, and rapid systemic clearance, resulting in subtherapeutic concentrations at the renal level. Furthermore, the substantial heterogeneity in quality control, extraction methodologies, dosage protocols, and efficacy endpoints across studies creates reproducibility issues and prevents the establishment of standardized, universally applicable treatment regimens. This combination of insufficient clinical validation, uncertain safety profiles, suboptimal pharmacokinetics, and methodological variability collectively hinders the development of evidence-based, standardized natural product therapies for CKD.
Notwithstanding these limitations, the therapeutic potential of natural products in retarding CKD progression remains considerable. Future research should prioritize elucidating the molecular mechanisms of natural products in CKD. A particularly promising direction lies in exploring their synergistic effects with conventional CKD treatment regimens. Investigating these synergies could unlock strategies to enhance therapeutic efficacy, reduce dosages of conventional drugs—thereby minimizing their side effects—and potentially overcome drug resistance, ultimately leading to more comprehensive and personalized treatment strategies for CKD. To overcome pharmacokinetic challenges such as poor oral bioavailability and rapid clearance, innovative delivery systems, such as curcumin-phospholipid complexes, renal-targeted baicalin-lysozyme conjugates, and neutrophil membrane-coated oleanolic acid liposomes, should be developed to enhance both bioavailability and kidney-specific targeting. Concurrently, rigorously designed randomized controlled trials are imperative to systematically evaluate clinical efficacy, long-term safety, and potential side effects of natural products in CKD, thereby providing robust evidence for clinical translation.
In summary, natural products show considerable therapeutic potential in CKD management. With advances in mechanistic elucidation, optimized delivery systems, strengthened clinical evidence, and standardized preparation protocols, natural products are positioned to become integral components of comprehensive CKD treatment strategies, thereby providing novel therapeutic avenues for patients worldwide.
Author Contributions
Y.D. drafted the manuscript. Y.T. discussed and critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | Acetyl-CoA carboxylase |
| ACSL4 | Acyl-CoA synthetase long chain family member 4 |
| ALB | Albumin |
| AMPK | AMP-activated protein kinase |
| AngII | Angiotensin II |
| ATF4 | Activating transcription factor 4 |
| ATF6 | Activating transcription factor 6 |
| α-SMA | Alpha-smooth muscle actin |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| BIM | Bcl-2 interacting mediator of cell death |
| β2-MG | β2-microglobulin |
| CHOP | C/EBP homologous protein |
| ChREBP | Carbohydrate response element-binding protein |
| CKD | Chronic kidney disease |
| COX-2 | Cyclooxygenase-2 |
| CPT1 | Carnitine palmitoyltransferase-1 |
| Cx43 | Connexin43 |
| ECM | Extracellular matrix |
| eIF2α | Eukaryotic initiation factor 2α |
| EMT | Epithelial-mesenchymal transition |
| ERS | Endoplasmic reticulum stress |
| ESRD | End-stage renal disease |
| ESRRA | Estrogen-related receptor alpha |
| FAO | Fatty acid oxidation |
| FAS | Fatty acid synthase |
| FFAs | Free fatty acids |
| FN | Fibronectin |
| FPN | Ferroportin |
| FTH1 | Ferritin heavy chain 1 |
| GCL | Glutamate-cysteine ligase |
| GM | Gentamicin |
| GPX4 | Glutathione peroxidase 4 |
| GRP78 | Glucose-regulated protein 78 |
| GSH | Glutathione |
| GSDMD | Gasdermin D |
| HMCs | Human mesangial cells |
| HO-1 | Heme oxygenase-1 |
| IL-18 | Interleukin-18 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IRE1α | Inositol-requiring enzyme 1 alpha |
| IRS1 | Insulin receptor substrate-1 |
| JNK | C-Jun N-terminal kinase |
| KIM-1 | Kidney injury molecule-1 |
| LIP | Labile iron pool |
| LOX | Lipoxygenase |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| MCP-1 | Monocyte chemotactic protein 1 |
| MDA | Malondialdehyde |
| Mincle | Macrophage-inducible C-type lectin |
| NF-κB | Nuclear factor kappa-B |
| Notch1 | Neurogenic locus notch homolog protein 1 |
| NOX | NADPH oxidase |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OPN | Osteopontin |
| OXPHOS | Oxidative phosphorylation |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| PGC1a | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARA | Peroxisome proliferator-activated receptor alpha |
| POR | Cytochrome P450 oxidoreductase |
| PRR | Pro-renin receptor |
| PUFA | Polyunsaturated fatty acid |
| PUFA-PE | Polyunsaturated fatty acid-phosphatidyl ethanolamine |
| RAASi | Renin–angiotensin–aldosterone system inhibitor |
| RAS | Renin–angiotensin system |
| ROS | Reactive oxygen species |
| SGLT2i | Sodium-glucose cotransporter-2 inhibitor |
| SIRT1 | Sirtuin-1 |
| SOD | Superoxide dismutase |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor-beta |
| TFR1 | Transferrin receptor 1 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-alpha |
| TRAF2 | TNF receptor-associated factor 2 |
| UACR | Urinary albumin-to-creatinine ratio |
| UAER | Urinary albumin excretion rate |
| UPR | Unfolded protein response |
| XBP1 | X-box binding protein 1 |
| 4-HNE | 4-hydroxynonenal |
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- GBD 2023 Kidney Failure with Replacement Therapy Collaborators. Global, regional, and national prevalence of kidney failure with replacement therapy and associated aetiologies, 1990–2023: A systematic analysis for the Global Burden of Disease Study 2023. Lancet Glob. Health 2025, 13, e1378–e1395. [Google Scholar] [CrossRef]
- de Vries, A.P.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Pongrac Barlovic, D.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Stasi, A.; Cosola, C.; Caggiano, G.; Cimmarusti, M.T.; Palieri, R.; Acquaviva, P.M.; Rana, G.; Gesualdo, L. Obesity-Related Chronic Kidney Disease: Principal Mechanisms and New Approaches in Nutritional Management. Front. Nutr. 2022, 9, 925619. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.H.; Lebeau, P.F.; Trink, J.; Uppal, N.; Lanktree, M.B.; Krepinsky, J.C.; Austin, R.C. Endoplasmic reticulum stress as a driver and therapeutic target for kidney disease. Nat. Rev. Nephrol. 2025, 21, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Luo, Y.; Yu, M.; Wang, X.; Zeng, L.; Yang, K. Targeting ferroptosis: A new therapeutic opportunity for kidney diseases. Front. Immunol. 2024, 15, 1435139. [Google Scholar] [CrossRef] [PubMed]
- Rimes-Stigare, C.; Frumento, P.; Bottai, M.; Mårtensson, J.; Martling, C.R.; Bell, M. Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit. Care 2015, 19, 383. [Google Scholar] [CrossRef]
- Fischer, M.J.; Hsu, J.Y.; Lora, C.M.; Ricardo, A.C.; Anderson, A.H.; Bazzano, L.; Cuevas, M.M.; Hsu, C.Y.; Kusek, J.W.; Renteria, A.; et al. CKD Progression and Mortality among Hispanics and Non-Hispanics. J. Am. Soc. Nephrol. 2016, 27, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Josa, E.; Barril, G.; Ruperto, M. Potential Effects of Bioactive Compounds of Plant-Based Foods and Medicinal Plants in Chronic Kidney Disease and Dialysis: A Systematic Review. Nutrients 2024, 16, 4321. [Google Scholar] [CrossRef]
- Liu, M.; Cui, C.; Chang, T.; Zhou, Q.; Cui, Y.; Zhang, S.; Liao, X. Effects and safety of Ophiocordyceps sinensis preparation in the adjuvant treatment for dialysis patients: A systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1360997. [Google Scholar] [CrossRef]
- Martini, S.; Nair, V.; Keller, B.J.; Eichinger, F.; Hawkins, J.J.; Randolph, A.; Böger, C.A.; Gadegbeku, C.A.; Fox, C.S.; Cohen, C.D.; et al. Integrative biology identifies shared transcriptional networks in CKD. J. Am. Soc. Nephrol. 2014, 25, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Merscher, S.; Fornoni, A. Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease. Nat. Rev. Nephrol. 2023, 19, 629–645. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, Z.; Hu, J.; Ding, G. Interplay of lipid metabolism and inflammation in podocyte injury. Metabolism 2024, 150, 155718. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Matsusaka, T.; et al. High-Fat Diet-Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Alix, P.M.; Koppe, L.; Pelletier, C.C.; Kalbacher, E.; Fouque, D.; Soulage, C.O. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013, 95, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cui, H.; Wang, Y.; Ju, F.; Cai, Y.; Gang, X.; Wang, G. The role of lipotoxicity in kidney disease: From molecular mechanisms to therapeutic prospects. Biomed. Pharmacother. 2023, 161, 114465. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, Y.; Wang, S.; Liu, J.; Li, H. From Adipose to Ailing Kidneys: The Role of Lipid Metabolism in Obesity-Related Chronic Kidney Disease. Antioxidants 2024, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Long, J.; Mise, K.; Poungavrin, N.; Lorenzi, P.L.; Mahmud, I.; Tan, L.; Saha, P.K.; Kanwar, Y.S.; Chang, B.H.; et al. The transcription factor ChREBP links mitochondrial lipidomes to mitochondrial morphology and progression of diabetic kidney disease. J. Biol. Chem. 2023, 299, 105185. [Google Scholar] [CrossRef]
- Harley, G.; Katerelos, M.; Gleich, K.; de Souza, D.P.; Narayana, V.K.; Kemp, B.E.; Power, D.A.; Mount, P.F. Blocking AMPK signalling to acetyl-CoA carboxylase increases cisplatin-induced acute kidney injury and suppresses the benefit of metformin. Biomed. Pharmacother. 2022, 153, 113377. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Araki, S.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T.; et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef]
- Ma, H.; Guo, X.; Cui, S.; Wu, Y.; Zhang, Y.; Shen, X.; Xie, C.; Li, J. Dephosphorylation of AMP-activated protein kinase exacerbates ischemia/reperfusion-induced acute kidney injury via mitochondrial dysfunction. Kidney Int. 2022, 101, 315–330. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, G.; Ma, L.; Chen, B.; Zhang, D.; Gao, J.; Deng, S.; Chen, Y. Virgin Camellia Seed Oil Improves Glycolipid Metabolism in the Kidney of High Fat-Fed Rats through AMPK-SREBP Pathway. Nutrients 2023, 15, 4888. [Google Scholar] [CrossRef]
- Alqallaf, A.; Swan, P.; Docherty, N.G. Renal insulin resistance in type 2 diabetes mellitus and progression of chronic kidney disease: Potential pathogenic mechanisms. Expert Rev. Endocrinol. Metab. 2022, 17, 523–532. [Google Scholar] [CrossRef]
- Parvathareddy, V.P.; Wu, J.; Thomas, S.S. Insulin Resistance and Insulin Handling in Chronic Kidney Disease. Compr. Physiol. 2023, 13, 5069–5076. [Google Scholar] [CrossRef]
- Xia, W.; Pessentheiner, A.R.; Hofer, D.C.; Amor, M.; Schreiber, R.; Schoiswohl, G.; Eichmann, T.O.; Walenta, E.; Itariu, B.; Prager, G.; et al. Loss of ABHD15 Impairs the Anti-lipolytic Action of Insulin by Altering PDE3B Stability and Contributes to Insulin Resistance. Cell Rep. 2018, 23, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Jung, S.W.; Kim, S.M.; Kim, Y.G.; Lee, S.H.; Moon, J.Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. Ren. Physiol. 2020, 318, F1327–F1340. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess Uric Acid Induces Gouty Nephropathy Through Crystal Formation: A Review of Recent Insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Wang, L.; Huang, Z.; Feng, Z.; Cui, S.; Fu, B.; Cai, G.; Chen, X.; Wu, D. High Concentrations of Uric Acid and Angiotensin II Act Additively to Produce Endothelial Injury. Mediat. Inflamm. 2020, 2020, 8387654. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Attley, J.; Cuzzocrea, S. Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD). Pharmacol. Res. 2014, 81, 91–102. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Doke, T.; Susztak, K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 2022, 32, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-regulation of the Citric Acid (TCA) Cycle in Non-diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Miguel, V.; Tituaña, J.; Herrero, J.I.; Herrero, L.; Serra, D.; Cuevas, P.; Barbas, C.; Puyol, D.R.; Márquez-Expósito, L.; Ruiz-Ortega, M.; et al. Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis. J. Clin. Investig. 2021, 131, e140695. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.M.; Wingert, R.A. PGC-1α in Disease: Recent Renal Insights into a Versatile Metabolic Regulator. Cells 2020, 9, 2234. [Google Scholar] [CrossRef]
- Chung, K.W.; Lee, E.K.; Lee, M.K.; Oh, G.T.; Yu, B.P.; Chung, H.Y. Impairment of PPARα and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging. J. Am. Soc. Nephrol. 2018, 29, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.N.; Knutti, D.; Brogli, K.; Uhlmann, T.; Kralli, A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J. Biol. Chem. 2003, 278, 9013–9018. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 11253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, S.; Pelegrín, P. Pyroptosis and Redox Balance in Kidney Diseases. Antioxid. Redox Signal 2021, 35, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Huang, E.Y.; Olzmann, J.A. Endoplasmic Reticulum-Associated Degradation and Lipid Homeostasis. Annu. Rev. Nutr. 2016, 36, 511–542. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Lee, S.Y.T.; Yap, W.S.; Thibault, G. Endoplasmic reticulum stress and lipids in health and diseases. Prog. Lipid Res. 2023, 89, 101198. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Chen, K.; Zhang, L.; Shi, W.; Zhang, Y.; Niu, S.; Jia, M.; Cong, B.; Li, Y. Endoplasmic Reticulum Stress Is Involved in Stress-Induced Hypothalamic Neuronal Injury in Rats via the PERK-ATF4-CHOP and IRE1-ASK1-JNK Pathways. Front. Cell. Neurosci. 2019, 13, 190. [Google Scholar] [CrossRef]
- Wek, R.C.; Cavener, D.R. Translational control and the unfolded protein response. Antioxid. Redox Signal 2007, 9, 2357–2371. [Google Scholar] [CrossRef]
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The eIF2α kinases: Their structures and functions. Cell. Mol. Life Sci. 2013, 70, 3493–3511. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Sun, H.; Jiang, C.; Dong, Y.; Shan, Q.; Su, S.; Xie, Y.; Xu, N.; Lou, X.; et al. Ubiquitination of inositol-requiring enzyme 1 (IRE1) by the E3 ligase CHIP mediates the IRE1/TRAF2/JNK pathway. J. Biol. Chem. 2014, 289, 30567–30577. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, H.; Ding, S.; Liu, H.; Liu, C.; Fu, R. Molecular mechanism of ATF6 in unfolded protein response and its role in disease. Heliyon 2024, 10, e25937. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Victor, P.; Umapathy, D.; George, L.; Juttada, U.; Ganesh, G.V.; Amin, K.N.; Viswanathan, V.; Ramkumar, K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones 2021, 26, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lee, A.S. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Naik, I.; Braunstein, Z.; Zhong, J.; Ren, B. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front. Immunol. 2017, 8, 1612. [Google Scholar] [CrossRef]
- Ferrè, S.; Deng, Y.; Huen, S.C.; Lu, C.Y.; Scherer, P.E.; Igarashi, P.; Moe, O.W. Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney Int. 2019, 96, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
- Kalinowski, D.S.; Richardson, D.R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 2005, 57, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.E.; Dixon, S.J. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Kuragano, T.; Nanami, M.; Nagasawa, Y.; Hasuike, Y. Misdistribution of iron and oxidative stress in chronic kidney disease. Free Radic. Biol. Med. 2019, 133, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kong, X.Y.; Yao, Y.; Wang, X.A.; Yang, W.; Wu, H.; Li, S.; Ding, J.W.; Yang, J. The critical role and molecular mechanisms of ferroptosis in antioxidant systems: A narrative review. Ann. Transl. Med. 2022, 10, 368. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Dong, J.; Wang, R.; Bi, G.; Xu, D.; Zhang, Y.; Deng, Y.; Lin, W.; Yang, Z.; et al. HDAC3 aberration-incurred GPX4 suppression drives renal ferroptosis and AKI-CKD progression. Redox Biol. 2023, 68, 102939. [Google Scholar] [CrossRef]
- Sun, X.; Huang, N.; Li, P.; Dong, X.; Yang, J.; Zhang, X.; Zong, W.X.; Gao, S.; Xin, H. TRIM21 ubiquitylates GPX4 and promotes ferroptosis to aggravate ischemia/reperfusion-induced acute kidney injury. Life Sci. 2023, 321, 121608. [Google Scholar] [CrossRef]
- Li, S.; Han, Q.; Liu, C.; Wang, Y.; Liu, F.; Pan, S.; Zuo, L.; Gao, D.; Chen, K.; Feng, Q.; et al. Role of ferroptosis in chronic kidney disease. Cell Commun. Signal 2024, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, W.; Zhang, S.; Liu, S. The cardinal roles of ferroportin and its partners in controlling cellular iron in and out. Life Sci. 2020, 258, 118135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Hydroxyl radical generations form the physiologically relevant Fenton-like reactions. Free Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Li, H.; Dixon, E.E.; Wu, H.; Humphreys, B.D. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 2022, 34, 1977–1998.e1979. [Google Scholar] [CrossRef]
- Kuwata, H.; Tomitsuka, Y.; Yoda, E.; Hara, S. Role of ACSL4 in the chemical-induced cell death in human proximal tubule epithelial HK-2 cells. Biosci. Rep. 2022, 42, BSR20212433. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Ai, Y.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.; Zhang, Z.; Wang, X. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e310. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Hong, Y.; Zhang, Q.Y.; Wang, X.N.; Kong, L.D. Quercetin Protects against Cadmium-Induced Renal Uric Acid Transport System Alteration and Lipid Metabolism Disorder in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 548430. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Prahastuti, S.; Tjokropranoto, R.; Onggowidjaja, P.; Kusuma, H.S.W.; Afifah, E.; Arumwardana, S.; Maulana, M.A.; Rizal, R. Quercetin prevents chronic kidney disease on mesangial cells model by regulating inflammation, oxidative stress, and TGF-β1/SMADs pathway. PeerJ 2022, 10, e13257. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Al-Qahtani, W.H.; AlFaris, N.A.; Albekairi, N.A.; Alqahtani, S.; Eid, R.; Yagoub, A.E.A.; Al-Harbi, L.N.; Yahya, M.A. Quercetin alleviates cadmium chloride-induced renal damage in rats by suppressing endoplasmic reticulum stress through SIRT1-dependent deacetylation of Xbp-1s and eIF2α. Biomed. Pharmacother. 2021, 141, 111862. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Q.; Wang, S.; Wang, T.; Pan, L.; Wang, X.; Chi, Y.; Jin, Z. Quercetin ameliorates renal injury in hyperuricemic rats via modulating ER stress pathways. Front. Pharmacol. 2025, 16, 1660599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, H.; Li, C.; Sun, W.; Chen, X.; Cao, Y.; Liu, Y.; Liu, Y.; Chen, J.; Qi, J.; et al. Gandi Capsule Improved Podocyte Lipid Metabolism of Diabetic Nephropathy Mice through SIRT1/AMPK/HNF4A Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 6275505. [Google Scholar] [CrossRef]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Dev. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Waisundara, V.Y.; Hsu, A.; Tan, B.K.; Huang, D. Baicalin improves antioxidant status of streptozotocin-induced diabetic Wistar rats. J. Agric. Food Chem. 2009, 57, 4096–4102. [Google Scholar] [CrossRef]
- Zheng, X.P.; Nie, Q.; Feng, J.; Fan, X.Y.; Jin, Y.L.; Chen, G.; Du, J.W. Kidney-targeted baicalin-lysozyme conjugate ameliorates renal fibrosis in rats with diabetic nephropathy induced by streptozotocin. BMC Nephrol. 2020, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ma, W.; Xiao, Y.; Wu, B.; Li, X.; Liu, F.; Qiu, J.; Zhang, G. High doses of baicalin induces kidney injury and fibrosis through regulating TGF-β/Smad signaling pathway. Toxicol. Appl. Pharmacol. 2017, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Dong, L.; Dang, X.; Wang, L.; Cheng, L.; Huang, Y. Dihydromyricetin Attenuates Metabolic Syndrome And Improves Insulin Sensitivity By Upregulating Insulin Receptor Substrate-1 (Y612) Tyrosine Phosphorylation In db/db Mice. Diabetes Metab. Syndr. Obes. 2019, 12, 2237–2249. [Google Scholar] [CrossRef]
- Guo, L.; Tan, K.; Luo, Q.; Bai, X. Dihydromyricetin promotes autophagy and attenuates renal interstitial fibrosis by regulating miR-155-5p/PTEN signaling in diabetic nephropathy. Bosn. J. Basic Med. Sci. 2020, 20, 372–380. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, X.; Xiong, J.; Han, W.; Xiao, T.; Xu, X.; Yang, K.; Liu, C.; Jiang, W.; He, T.; et al. MicroRNA-34a Promotes Renal Fibrosis by Downregulation of Klotho in Tubular Epithelial Cells. Mol. Ther. 2019, 27, 1051–1065. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Li, H.; Qiao, Y.; Gao, S. Ampelopsin inhibits high glucose-induced extracellular matrix accumulation and oxidative stress in mesangial cells through activating the Nrf2/HO-1 pathway. Phytother. Res. 2020, 34, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Albukhari, T.A.; Bagadood, R.M.; Bokhari, B.T.; Filimban, W.A.; Sembawa, H.; Nasreldin, N.; Gadalla, H.E.; El-Boshy, M.E. Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways. Biomedicines 2025, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, H.; Akaras, N.; Gür, C.; Küçükler, S.; Kandemir, F.M. Beneficial effects of Chrysin on Cadmium-induced nephrotoxicity in rats: Modulating the levels of Nrf2/HO-1, RAGE/NLRP3, and Caspase-3/Bax/Bcl-2 signaling pathways. Gene 2023, 875, 147502. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Mahesh Kumar, J.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.N.; Yang, L.T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis. Acta Pharmacol. Sin. 2024, 45, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tan, R.Z.; Li, J.C.; Liu, T.T.; Zhong, X.; Yan, Y.; Yang, J.K.; Lin, X.; Fan, J.M.; Wang, L. Isoliquiritigenin Attenuates UUO-Induced Renal Inflammation and Fibrosis by Inhibiting Mincle/Syk/NF-Kappa B Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.; Zaitone, S.A.; Said, E.; El-Sherbiny, M.; Ajwah, S.; Alsharif, S.Y.; Elsherbiny, N.M. Protective effect of isoliquiritigenin on experimental diabetic nephropathy in rats: Impact on Sirt-1/NFκB balance and NLRP3 expression. Int. Immunopharmacol. 2020, 87, 106813. [Google Scholar] [CrossRef]
- Hamdy, S.; Elshopakey, G.E.; Risha, E.F.; Rezk, S.; Ateya, A.I.; Abdelhamid, F.M. Curcumin mitigates gentamicin induced-renal and cardiac toxicity via modulation of Keap1/Nrf2, NF-κB/iNOS and Bcl-2/BAX pathways. Food Chem. Toxicol. 2024, 183, 114323. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva(®)) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- He, X.; Li, G.; Chen, Y.; Xiao, Q.; Yu, X.; Yu, X.; Lu, X.; Xiang, Z. Pharmacokinetics and Pharmacodynamics of the Combination of Rhein and Curcumin in the Treatment of Chronic Kidney Disease in Rats. Front. Pharmacol. 2020, 11, 573118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, C.; Choi, H.Y.; Lee, K. Effect of Co-Administration of Curcumin with Amlodipine in Hypertension. Nutrients 2021, 13, 2797. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lim, J.H.; Youn, H.H.; Hong, Y.A.; Yang, K.S.; Park, H.S.; Chung, S.; Ko, S.H.; Shin, S.J.; Choi, B.S.; et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia 2013, 56, 204–217, Erratum in Diabetologia 2013, 56, 681. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Yasmin, T.; Akter, R.; Islam, M.N.; Hossain, M.M.; Khan, F.; Aldhahrani, A.; Soliman, M.M.; Subhan, N.; Haque, M.A.; et al. Resveratrol treatment modulates several antioxidant and anti-inflammatory genes expression and ameliorated oxidative stress mediated fibrosis in the kidneys of high-fat diet-fed rats. Saudi Pharm. J. 2022, 30, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, L.N.; Zakaraya, Z.; Hailat, M.; Abu Dayyih, W.; Daoud, E.; Abed, A.; Saadh, M.J.; Majeed, B.; Abumansour, H.; Aburumman, A.; et al. Anti-diabetic effect of cotreatment with resveratrol and pioglitazone in diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Jiang, D.; Wang, Y.Z.; Duan, M.Y.; Huang, Y.W.; Wang, X.J.; Xiang, Z.M.; Sheng, J.; Zhu, Q.Q. Chlorogenic acid alleviates renal fibrosis by reducing lipid accumulation in diabetic kidney disease through suppressing the Notch1 and Stat3 signaling pathway. Ren. Fail. 2024, 46, 2371988. [Google Scholar] [CrossRef]
- Arfian, N.; Wahyudi, D.A.P.; Zulfatina, I.B.; Citta, A.N.; Anggorowati, N.; Multazam, A.; Romi, M.M.; Sari, D.C.R. Chlorogenic Acid Attenuates Kidney Ischemic/Reperfusion Injury via Reducing Inflammation, Tubular Injury, and Myofibroblast Formation. Biomed. Res. Int. 2019, 2019, 5423703. [Google Scholar] [CrossRef]
- Bao, L.; Gong, Y.; Xu, W.; Dao, J.; Rao, J.; Yang, H. Chlorogenic acid inhibits NLRP3 inflammasome activation through Nrf2 activation in diabetic nephropathy. PLoS ONE 2025, 20, e0316615. [Google Scholar] [CrossRef]
- Park, J.H.; Leem, J.; Lee, S.J. Protective Effects of Carnosol on Renal Interstitial Fibrosis in a Murine Model of Unilateral Ureteral Obstruction. Antioxidants 2022, 11, 2341. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Ma, G.; Wu, C.; Zhu, W.; Sun, P.; Lu, W.; Yang, X.; Zhang, Y.; Liu, N.; et al. Mechanism of dioscin ameliorating renal fibrosis through NF-κB signaling pathway-mediated inflammatory response. Mol. Med. Rep. 2023, 27, 93. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, X.; Yin, L.; Xu, L.; Xu, Y.; Qi, Y.; Han, X.; Song, S.; Zhao, Y.; Lin, Y.; et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. Br. J. Pharmacol. 2017, 174, 2512–2527, Erratum in Br. J. Pharmacol. 2019, 176, 4787. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Jin, S.; Fu, Y.; Liu, Y. Dioscin Protects against Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis and Apoptosis through Activating Nrf2/HO-1 Signaling. Antioxidants 2022, 11, 2443. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, X.; Tang, Y.; Fu, S.; Kong, B.; Liu, X. Astragaloside IV ameliorates diabetic nephropathy in db/db mice by inhibiting NLRP3 inflammasome-mediated inflammation. Int. J. Mol. Med. 2021, 48, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, H.M. Astragaloside IV ameliorates high glucose-induced HK-2 cell apoptosis and oxidative stress by regulating the Nrf2/ARE signaling pathway. Exp. Ther. Med. 2019, 17, 4409–4416. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Su, Y.; Chen, Q.; Ma, K.; Ji, T.; Wang, Z.; Li, W.; Li, W. Protective effects of Astragaloside IV on endoplasmic reticulum stress-induced renal tubular epithelial cells apoptosis in type 2 diabetic nephropathy rats. Biomed. Pharmacother. 2019, 109, 84–92. [Google Scholar] [CrossRef]
- Zhan, H.; Han, P.; Wang, M.; Wang, Y.; Weng, W.; Yu, X.; Yuan, C.; Li, Y.; Shao, M.; Sun, H. Combination of astragaloside IV and ACEi ameliorates renal injuries in db/db mice. Int. J. Clin. Exp. Pathol. 2020, 13, 827–836. [Google Scholar]
- Xie, X.S.; Liu, H.C.; Yang, M.; Zuo, C.; Deng, Y.; Fan, J.M. Ginsenoside Rb1, a panoxadiol saponin against oxidative damage and renal interstitial fibrosis in rats with unilateral ureteral obstruction. Chin. J. Integr. Med. 2009, 15, 133–140. [Google Scholar] [CrossRef]
- Ni, Y.H.; Deng, H.F.; Zhou, L.; Huang, C.S.; Wang, N.N.; Yue, L.X.; Li, G.F.; Yu, H.J.; Zhou, W.; Gao, Y. Ginsenoside Rb1 Ameliorated Bavachin-Induced Renal Fibrosis via Suppressing Bip/eIF2α/CHOP Signaling-Mediated EMT. Front. Pharmacol. 2022, 13, 872474. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Q.; Wu, J.; Li, Y.; Sun, J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: A randomized, placebo-controlled study. Inflammopharmacology 2017, 25, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Mao, X.Q.; Wang, N.; Liu, J.; Ou-Yang, J.P. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol. Sin. 2009, 30, 1607–1615. [Google Scholar] [CrossRef]
- Guo, M.; Gao, J.; Jiang, L.; Dai, Y. Astragalus Polysaccharide Ameliorates Renal Inflammatory Responses in a Diabetic Nephropathy by Suppressing the TLR4/NF-κB Pathway. Drug Des. Dev. Ther. 2023, 17, 2107–2118. [Google Scholar] [CrossRef]
- Jing, B.; Wei, M.; Chen, H.; Xie, W.; An, S.; Li, J.; Wang, S.; Zhou, X. Pharmacodynamic Evaluation and Mechanism of Ginseng Polysaccharide against Nephrotoxicity Induced by Hexavalent Chromium. Nutrients 2024, 16, 1416. [Google Scholar] [CrossRef]
- Wei, X.M.; Jiang, S.; Li, S.S.; Sun, Y.S.; Wang, S.H.; Liu, W.C.; Wang, Z.; Wang, Y.P.; Zhang, R.; Li, W. Endoplasmic Reticulum Stress-Activated PERK-eIF2α-ATF4 Signaling Pathway is Involved in the Ameliorative Effects of Ginseng Polysaccharides against Cisplatin-Induced Nephrotoxicity in Mice. ACS Omega 2021, 6, 8958–8966. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, L.; Dong, Z.; Wang, X.; Qin, X.; Wang, C.; Wang, J.; Huang, Q. Preparation, structural characterization, antioxidant activity and protection against cisplatin-induced acute kidney injury by polysaccharides from the lateral root of Aconitum carmichaelii. Front. Pharmacol. 2022, 13, 1002774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Si, F.; Hao, R.; Zheng, J.; Zhang, C. Nuciferine Protects against Obesity-Induced Nephrotoxicity through Its Hypolipidemic, Anti-Inflammatory, and Antioxidant Effects. J. Agric. Food Chem. 2023, 71, 18769–18779. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199, Erratum in Br. J. Pharmacol. 2022, 179, 2313–2317. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Shalkami, A.S.; Khalaf, M.M.; Mohamed, W.R.; Cai, Y.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P(38)MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.I.; Velarde, V. Boldine Improves Kidney Damage in the Goldblatt 2K1C Model Avoiding the Increase in TGF-β. Int. J. Mol. Sci. 2018, 19, 1864. [Google Scholar] [CrossRef]
- Hernández-Salinas, R.; Vielma, A.Z.; Arismendi, M.N.; Boric, M.P.; Sáez, J.C.; Velarde, V. Boldine prevents renal alterations in diabetic rats. J. Diabetes Res. 2013, 2013, 593672. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Shi, G.; Yang, W.; Zhang, Y.; Chen, W.; Wan, S.; Xiong, F.; Wang, Z. Emodin Ameliorates Renal Damage and Podocyte Injury in a Rat Model of Diabetic Nephropathy via Regulating AMPK/mTOR-Mediated Autophagy Signaling Pathway. Diabetes Metab. Syndr. Obes. 2021, 14, 1253–1266. [Google Scholar] [CrossRef]
- Luo, L.P.; Suo, P.; Ren, L.L.; Liu, H.J.; Zhang, Y.; Zhao, Y.Y. Shenkang Injection and Its Three Anthraquinones Ameliorates Renal Fibrosis by Simultaneous Targeting IƙB/NF-ƙB and Keap1/Nrf2 Signaling Pathways. Front. Pharmacol. 2021, 12, 800522. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Gao, Y.; Wang, X.; Wu, X.; Zou, D.; Zhu, Z.; Han, Z.; Wang, T.; Shi, Y. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des. Devel Ther. 2018, 12, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of EMODIN (CAS NO. 518-82-1) Feed Studies in F344/N Rats and B6C3F1 Mice. Natl. Toxicol. Program. Tech. Rep. Ser. 2001, 493, 1–278. [Google Scholar]
- Chen, Y.; Mu, L.; Xing, L.; Li, S.; Fu, S. Rhein alleviates renal interstitial fibrosis by inhibiting tubular cell apoptosis in rats. Biol. Res. 2019, 52, 50. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Hu, W.; Han, X.; Cai, Y. Rhein Inhibited Ferroptosis and EMT to Attenuate Diabetic Nephropathy by Regulating the Rac1/NOX1/β-Catenin Axis. Front. Biosci. 2023, 28, 100. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Huang, W.Y.; Li, Y.Q.; Luo, Y.; Jiang, Q.; Liang, Y.S.; Zhu, Z.W.; Wang, P.; Meng, X.L. Mechanism of rhein on renal toxicity of mice. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 54–59. [Google Scholar] [CrossRef]
- Wang, D.T.; Huang, R.H.; Cheng, X.; Zhang, Z.H.; Yang, Y.J.; Lin, X. Tanshinone IIA attenuates renal fibrosis and inflammation via altering expression of TGF-β/Smad and NF-κB signaling pathway in 5/6 nephrectomized rats. Int. Immunopharmacol. 2015, 26, 4–12. [Google Scholar] [CrossRef]
- Wu, Q.; Guan, Y.B.; Zhang, K.J.; Li, L.; Zhou, Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J. Ethnopharmacol. 2023, 316, 116667. [Google Scholar] [CrossRef]
- Xu, S.; He, L.; Ding, K.; Zhang, L.; Xu, X.; Wang, S.; Qian, X. Tanshinone IIA Ameliorates Streptozotocin-Induced Diabetic Nephropathy, Partly by Attenuating PERK Pathway-Induced Fibrosis. Drug Des. Dev. Ther. 2020, 14, 5773–5782. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Z.; Xing, H.; Kang, L.; Chen, X.; Liu, B.; Niu, K. Renoprotective effects of oleanolic acid and its possible mechanisms in rats with diabetic kidney disease. Biochem. Biophys. Res. Commun. 2022, 636, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol. Dial. Transplant. 2016, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Mukundwa, A.; Mukaratirwa, S.; Masola, B. Effects of oleanolic acid on the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic male Sprague-Dawley rats. J. Diabetes 2016, 8, 98–108. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, D.; Wang, Y.; Zhang, Z.; Lin, X.; Ji, Q.; Han, Y.; Yu, L.; Xu, J. Neutrophil-Mimetic oleanolic acid-loaded Liposomes targeted to alleviate oxidative stress for renal ischemia-reperfusion injury treatment. Int. J. Pharm. X 2025, 9, 100344. [Google Scholar] [CrossRef]
- Chen, C.M.; Chung, Y.P.; Liu, C.H.; Huang, K.T.; Guan, S.S.; Chiang, C.K.; Wu, C.T.; Liu, S.H. Withaferin A protects against endoplasmic reticulum stress-associated apoptosis, inflammation, and fibrosis in the kidney of a mouse model of unilateral ureteral obstruction. Phytomedicine 2020, 79, 153352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ou, Z.; Gao, T.; Yang, Y.; Shu, A.; Xu, H.; Chen, Y.; Lv, Z. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 2022, 156, 113953. [Google Scholar] [CrossRef]
- Li, Z.; Guo, H.; Li, J.; Ma, T.; Zhou, S.; Zhang, Z.; Miao, L.; Cai, L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin. Sci. 2020, 134, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; García-Arroyo, F.E.; Amador-Martínez, I.; Orozco-Ibarra, M.; Fernández-Valverde, F.; Pedraza-Chaverri, J. Sulforaphane Protects against Unilateral Ureteral Obstruction-Induced Renal Damage in Rats by Alleviating Mitochondrial and Lipid Metabolism Impairment. Antioxidants 2022, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Martínez-Abundis, E.; Tapia, E.; Zarco-Márquez, G.; Zazueta, C.; Pedraza-Chaverri, J. Protective effect of sulforaphane against cisplatin-induced mitochondrial alterations and impairment in the activity of NAD(P)H: Quinone oxidoreductase 1 and γ glutamyl cysteine ligase: Studies in mitochondria isolated from rat kidney and in LLC-PK1 cells. Toxicol. Lett. 2010, 199, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Coutinho-Wolino, K.S.; Nakao, L.S.; Cardozo, L.F.; Mafra, D. Sulforaphane upregulates the mRNA expression of NRF2 and NQO1 in non-dialysis patients with chronic kidney disease. Free Radic. Biol. Med. 2024, 221, 181–187. [Google Scholar] [CrossRef]
- Baptista, B.G.; Fanton, S.; Ribeiro, M.; Cardozo, L.F.; Regis, B.; Alvarenga, L.; Ribeiro-Alves, M.; Berretta, A.A.; Shiels, P.G.; Mafra, D. The effect of Brazilian Green Propolis extract on inflammation in patients with chronic kidney disease on peritoneal dialysis: A randomised double-blind controlled clinical trial. Phytomedicine 2023, 114, 154731. [Google Scholar] [CrossRef]
- Duarte Silveira, M.A.; Malta-Santos, H.; Rebouças-Silva, J.; Teles, F.; Batista Dos Santos Galvão, E.; Pinto de Souza, S.; Dantas Dutra, F.R.; Dantas Gomes, M.M.; Teixeira, M.B.; Miranda Rebelo da Conceição, L.F.; et al. Effects of Standardized Brazilian Green Propolis Extract (EPP-AF®) on Inflammation in Haemodialysis Patients: A Clinical Trial. Int. J. Nephrol. 2022, 2022, 1035475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Li, M.; Yu, M.; Ping, F. Bailing capsule (Cordyceps sinensis) ameliorates renal triglyceride accumulation through the PPARα pathway in diabetic rats. Front. Pharmacol. 2022, 13, 915592. [Google Scholar] [CrossRef]
- Pan, M.M.; Zhang, M.H.; Ni, H.F.; Chen, J.F.; Xu, M.; Phillips, A.O.; Liu, B.C. Inhibition of TGF-β1/Smad signal pathway is involved in the effect of Cordyceps sinensis against renal fibrosis in 5/6 nephrectomy rats. Food Chem. Toxicol. 2013, 58, 487–494. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, Y.; Ye, G. Effect of Cordyceps sinensis on renal function of patients with chronic allograft nephropathy. Urol. Int. 2011, 86, 298–301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).