Action of Carnosic Acid Against Melanoma: A Strategy for Selective Radiosensitization with Protection of Non-Tumoral Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Carnosic Acid
2.2. MTT and XTT Cytotoxicity Assay
MTT and XTT Assays of Irradiated Cells
2.3. Annexin V
2.4. GSH Assay
2.5. Cell Cycle
2.6. Irradiation
2.7. Statistical Analysis
3. Results
3.1. Cytotoxicity Assay, MTT and XTT Assay
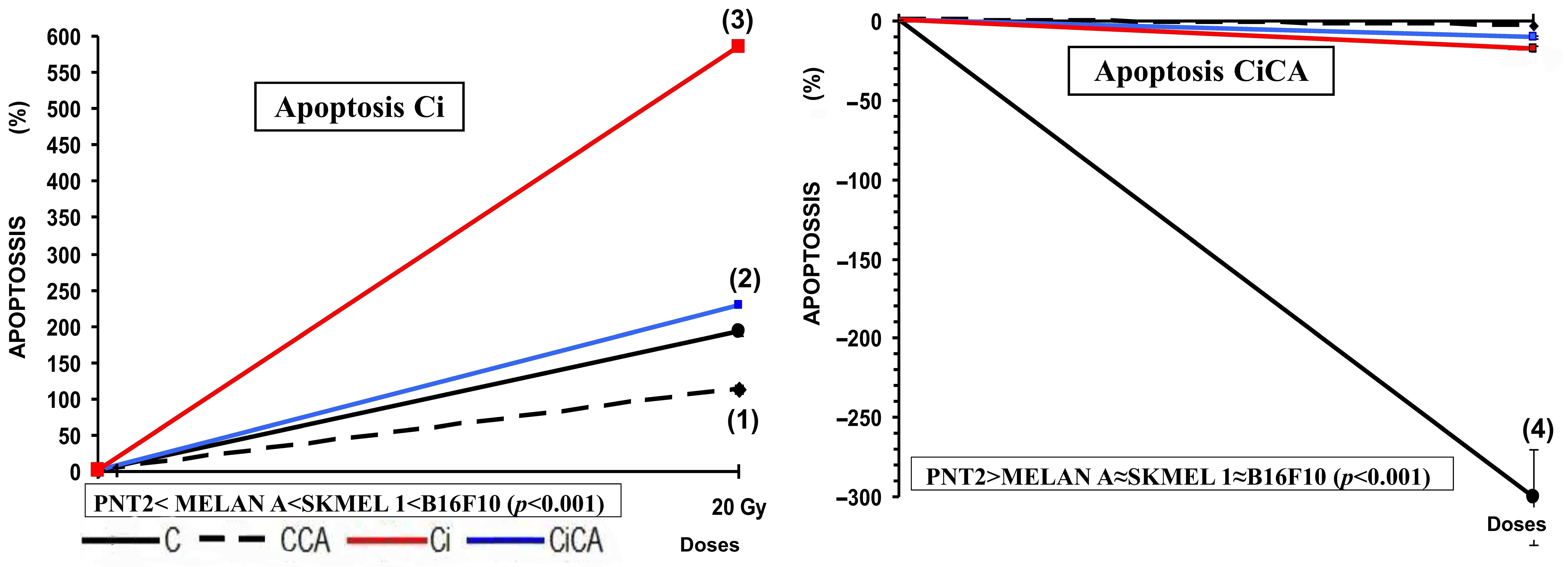
3.2. Apoptosis

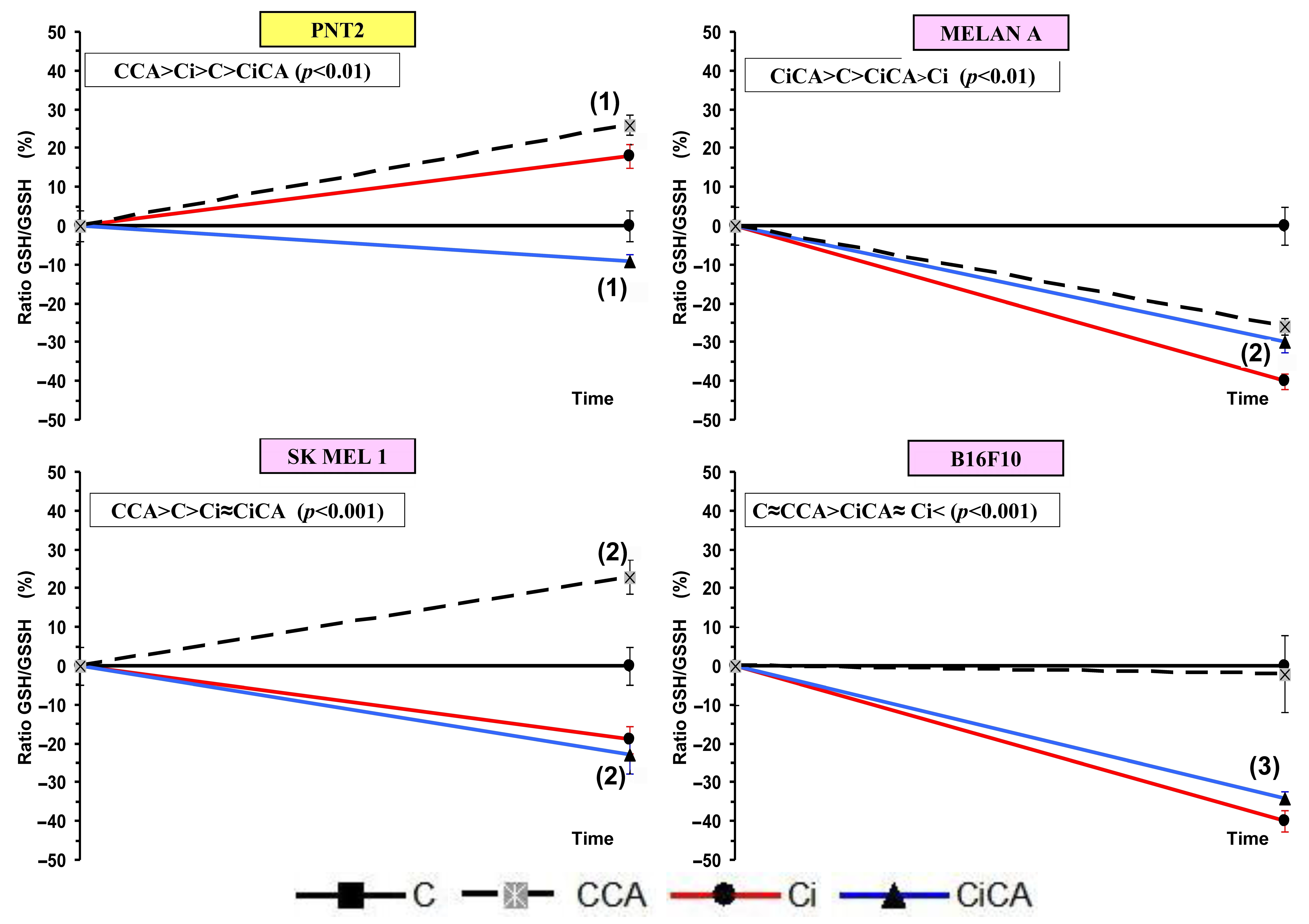
3.3. GSH Assay
3.4. Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbe, C.; Keim, U.; Gandini, S.; Amaral, T.; Katalinic, A.; Hollezcek, B.; Martus, P.; Flatz, L.; Leiter, U.; Whiteman, D. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943–2036. Eur. J. Cancer 2021, 152, 18–25. [Google Scholar] [CrossRef]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Demierre, M.F. Epidemiology and prevention of cutaneous melanoma. Curr. Treat. Options Oncol. 2006, 7, 181–186. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Liu, Q.; Zhang, H.; Jia, L.; Chai, Y.; Jiang, H.; Wu, M.; Li, Y. Global trends in melanoma burden: A comprehensive analysis from the Global Burden of Disease Study, 1990–2021. J. Am. Acad. Dermatol. 2025, 92, 100–107. [Google Scholar] [CrossRef]
- Nagane, M.; Kanai, E.; Shibata, Y.; Shimizu, T.; Yoshioka, C.; Maruo, T.; Yamashita, T. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS ONE 2018, 13, e0195151. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Marmitt, L.P.; Horn, A.P. Subcutaneous injection of multipotent mesenchymal stromal cells admixed with melanoma cells in mice favors tumor incidence and growth: A systematic review and meta-analysis. Arch. Dermatol. Res. 2018, 310, 231–240. [Google Scholar] [CrossRef]
- Stevens, G.; McKay, M.J. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncol. 2006, 7, 575–583. [Google Scholar] [CrossRef]
- Min, Y.; Roche, K.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882, Correction in Nat. Nanotechnol. 2021, 16, 743–744. [Google Scholar] [CrossRef]
- Alcaraz, M.; Achel, D.G.; Olivares, A.; Olmos, E.; Alcaraz-Saura, M.; Castillo, J. Carnosol, radiation and melanoma: A translational possibility. Clin. Transl. Oncol. 2013, 15, 712–719. [Google Scholar] [CrossRef]
- Alcaraz, M.; Alcaraz-Saura, M.; Achel, D.G.; Olivares, A.; López-Morata, J.A.; Castillo, J. Radiosensitizing effect of rosmarinic acid in metastatic melanoma B16F10 cells. Anticancer Res. 2014, 34, 1913–1921. [Google Scholar]
- Olivares, A.; Alcaraz-Saura, M.; Achel, D.G.; Berná-Mestre, J.d.D.; Alcaraz, M. Radiation-induced bystander effect: Loss of radioprotective capacity of rosmarinic acid in vivo and in vitro. Antioxidants 2021, 10, 231. [Google Scholar] [CrossRef]
- Olivares, A.; Alcaraz-Saura, M.; Achel, D.G.; Alcaraz, M. Effect of rosmarinic acid and ionizing radiation on glutathione in melanoma B16F10 cells: A translational opportunity. Antioxidants 2020, 9, 1291. [Google Scholar] [CrossRef]
- Achel, D.G.; Alcaraz-Saura, M.; Castillo, J.; Olivares, A.; Alcaraz, M. Radioprotective and antimutagenic effects of Pycnanthus angolensis Warb seed extract against damage induced by X-rays. J. Clin. Med. 2019, 9, 6. [Google Scholar] [CrossRef]
- Alcaraz, M.; Olivares, A.; Andreu-Gálvez, M.; Achel, D.G.; Mercado, A.M.; Alcaraz-Saura, M. Paradoxical radiosensitizing effect of carnosic acid on B16F10 metastatic melanoma cells: A new treatment strategy. Antioxidants 2022, 11, 2166. [Google Scholar] [CrossRef]
- Hall, E.J. Radiobiology for the Radiologist, 2nd ed.; Harper & Row: Philadelphia, PA, USA, 1978; pp. 93–110. [Google Scholar]
- Yáñez, J.; Vicente, V.; Alcaraz, M.; Castillo, J.; Benavente-García, O.; Canteras, M.; Teruel, J.A.L. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocyte cell lines: Relationship between structure and activity. Nutr. Cancer 2004, 49, 191–199. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sarma, L.; Kesavan, P.C. Protective effects of vitamins C and E against γ-ray-induced chromosomal damage in mouse. Int. J. Radiat. Biol. 1993, 63, 759–764. [Google Scholar] [CrossRef]
- Gorodetska, I.; Schulz, A.; Behre, G.; Dubrovska, A. Confronting Melanoma Radioresistance: Mechanisms and Therapeutic Strategies. Cancers 2025, 17, 2648. [Google Scholar] [CrossRef]
- Becker, A.L.; Indra, A.K. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers 2023, 15, 3038. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; IAEA: Vienna, Austria, 2011; pp. 1–247. [Google Scholar]
- Tagde, A.; Singh, H.; Kang, M.H.; Reynolds, C.P. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma. Blood Cancer J. 2014, 4, e229. [Google Scholar] [CrossRef]
- Clark, E.P.; Epp, E.R.; Biaglow, J.E.; Morse-Gaudio, M.; Zachgo, E. Glutathione depletion, radiosensitization, and misonidazole potentiation in hypoxic Chinese hamster ovary cells by buthionine sulfoximine. Radiat. Res. 1984, 98, 370–380. [Google Scholar] [CrossRef]
- Lin, K.-I.; Lin, C.-C.; Kuo, S.-M.; Lai, J.-C.; Wang, Y.-Q.; You, H.-L.; Hsu, M.-L.; Chen, C.-H.; Shiu, L.-Y. Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Biosci. Rep. 2018, 38, BSR20171092. [Google Scholar] [CrossRef]
- Arakawa, N.; Okubo, A.; Yasuhira, S.; Takahashi, K.; Amano, H.; Akasaka, T.; Masuda, T.; Shibazaki, M.; Maesawa, C. Carnosic acid, an inducer of NAD(P)H quinone oxidoreductase 1, enhances the cytotoxicity of β-lapachone in melanoma cell lines. Oncol. Lett. 2018, 15, 2393–2400. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Takeda, Y. Antioxidant mechanism of carnosic acid: Structural identification of two oxidation products. J. Agric. Food Chem. 2001, 49, 5560–5565. [Google Scholar] [CrossRef]
- Huang, S.-C.; Ho, C.-T.; Lin-Shiau, S.-Y.; Lin, J.-K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem. Pharmacol. 2005, 69, 221–232. [Google Scholar] [CrossRef]
- Patwardhan, R.S.; Sharma, D.; Checker, R.; Thoh, M.; Sandur, S.K. Spatio-temporal changes in glutathione and thioredoxin redox couples during ionizing radiation-induced oxidative stress regulate tumor radio-resistance. Free Radic. Res. 2015, 49, 1218–1232. [Google Scholar] [CrossRef]
- del Marmol, V.; Ito, S.; Bouchard, B.; Libert, A.; Wakamatsu, K.; Ghanem, G.; Solano, F. Cysteine deprivation promotes eumelanogenesis in human melanoma cells. J. Investig. Dermatol. 1996, 107, 698–702. [Google Scholar] [CrossRef]
- Palomares, T.; Alonso-Varona, A.; Alvarez, A.; Castro, B.; Calle, Y.; Bilbao, P. Interleukin-2 increases intracellular glutathione levels and reverses the growth-inhibiting effects of cyclophosphamide on B16 melanoma cells. Clin. Exp. Metastasis 1997, 15, 329–337. [Google Scholar] [CrossRef]
- Rodman, S.N.; Spence, J.M.; Ronnfeldt, T.J.; Zhu, Y.; Solst, S.R.; O’Neill, R.A.; Allen, B.G.; Guan, X.; Spitz, D.R.; Fath, M.A. Enhancement of radiation response in breast cancer stem cells by inhibition of thioredoxin- and glutathione-dependent metabolism. Radiat. Res. 2016, 186, 385–395. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 11, 731. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Bajbouj, K.; Muhammad, J.S.; Vinod, A.; Shafarin, J.; Khoder, G.; Saleh, M.A.; Taneera, J.; Abu-Gharbieh, E. Carnosic acid cooperates with tamoxifen to induce apoptosis associated with caspase-3 activation in breast cancer cells in vitro and in vivo. Pharmaceuticals 2021, 14, 230. [Google Scholar] [CrossRef]
- Kinnaert, E.; Duez, P.; Morandini, R.; Dubois, J.; Van Houtte, P.; Ghanem, G. Cysteine but not glutathione modulates the radiosensitivity of human melanoma cells by affecting both survival and DNA damage. Pigment Cell Res. 2004, 17, 275–280. [Google Scholar] [CrossRef]
- Lonati, L.; Barbieri, S.; Guardamagna, I.; Ottolenghi, A.; Baiocco, G. Radiation-induced cell cycle perturbations: A computational tool validated with flow-cytometry data. Sci. Rep. 2020, 10, 22230. [Google Scholar] [CrossRef]
- Lei, L.; Story, M.; Legerski, R.J. Cellular responses to ionizing radiation damage. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1157–1162. [Google Scholar] [CrossRef]
- Li, M.; You, L.; Xue, J.; Lu, Y. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: A mini review. Front. Pharmacol. 2018, 9, 522. [Google Scholar] [CrossRef]
- Tang, J.-F.; Li, G.-L.; Zhang, T.; Du, Y.-M.; Huang, S.-Y.; Ran, J.-H.; Li, J.; Chen, D.-L. Homoharringtonine inhibits melanoma cell proliferation in vitro and in vivo by inducing DNA damage, apoptosis, and G2/M cell cycle arrest. Arch. Biochem. Biophys. 2021, 708, 108774. [Google Scholar] [CrossRef]
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006, 239, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xiu, H.; Luo, H.; Ding, Y.; Li, Y. A mathematical model for cell cycle control: Graded response or quantized response. Cell Cycle 2022, 21, 735–744. [Google Scholar] [CrossRef]
- Chao, H.-H.; Karagounis, I.V.; Thomas, C.; François, N.B.; Facciabene, A.; Koumenis, C.; Maity, A. Combination of CHEK1/2 inhibition and ionizing radiation results in abscopal tumor response through increased micronuclei formation. Oncogene 2020, 39, 4344–4357. [Google Scholar] [CrossRef]
- Kudugunti, S.K.; Vad, N.M.; Ekogbo, E.; Moridani, M.Y. Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor-bearing C57BL/6 mice. Investig. New Drugs 2011, 29, 52–62. [Google Scholar] [CrossRef]
- Tandogan, B.; Kuruüzüm-Uz, A.; Sengezer, C.; Güvenalp, Z.; Demirezer, L.Ö.; Ulusu, N.N. In vitro effects of rosmarinic acid on glutathione reductase and glucose-6-phosphate dehydrogenase. Pharm. Biol. 2011, 49, 587–594. [Google Scholar] [CrossRef]
- Gülçin, İ.; Scozzafava, A.; Supuran, C.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef]
- Chen, X.; Wei, C.; Zhao, J.; Zhou, D.; Wang, Y.; Zhang, S.; Zuo, H.; Dong, J.; Zhao, Z.; Hao, M.; et al. Carnosic acid: An effective phenolic diterpenoid for prevention and management of cancers via targeting multiple signaling pathways. Pharmacol. Res. 2024, 206, 107288. [Google Scholar] [CrossRef]
- Martínez-Zaguilán, R.; Seftor, E.A.; Seftor, R.E.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef]
- Wahl, M.L.; Owen, J.A.; Burd, R.; Herlands, R.A.; Nogami, S.S.; Rodeck, U.; Berd, D.; Leeper, D.B.; Owen, C.S. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Mol. Cancer Ther. 2002, 1, 617–628. [Google Scholar]
- Arienti, C.; Zoli, W.; Pignatta, S.; Carloni, S.; Paganelli, G.; Ulivi, P.; Romeo, A.; Menghi, E.; Sarnelli, A.; Medri, L.; et al. Efficacy of different sequences of radio- and chemotherapy in experimental models of human melanoma. J. Cell. Physiol. 2014, 229, 1548–1556. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares, A.; de la Fuente, I.; Achel, D.G.; Mercado, A.M.; Garcia-Gamuz, J.A.; del Rosario Tudela, M.; Alcaraz, M. Action of Carnosic Acid Against Melanoma: A Strategy for Selective Radiosensitization with Protection of Non-Tumoral Cells. Curr. Issues Mol. Biol. 2025, 47, 845. https://doi.org/10.3390/cimb47100845
Olivares A, de la Fuente I, Achel DG, Mercado AM, Garcia-Gamuz JA, del Rosario Tudela M, Alcaraz M. Action of Carnosic Acid Against Melanoma: A Strategy for Selective Radiosensitization with Protection of Non-Tumoral Cells. Current Issues in Molecular Biology. 2025; 47(10):845. https://doi.org/10.3390/cimb47100845
Chicago/Turabian StyleOlivares, Amparo, Isabel de la Fuente, Daniel Gyingiri Achel, Ana María Mercado, José Antonio Garcia-Gamuz, María del Rosario Tudela, and Miguel Alcaraz. 2025. "Action of Carnosic Acid Against Melanoma: A Strategy for Selective Radiosensitization with Protection of Non-Tumoral Cells" Current Issues in Molecular Biology 47, no. 10: 845. https://doi.org/10.3390/cimb47100845
APA StyleOlivares, A., de la Fuente, I., Achel, D. G., Mercado, A. M., Garcia-Gamuz, J. A., del Rosario Tudela, M., & Alcaraz, M. (2025). Action of Carnosic Acid Against Melanoma: A Strategy for Selective Radiosensitization with Protection of Non-Tumoral Cells. Current Issues in Molecular Biology, 47(10), 845. https://doi.org/10.3390/cimb47100845







