Microcirculation-Promoting Effect of Escin on Cutaneous Tissue via Gsk3β Down-Regulation
Abstract
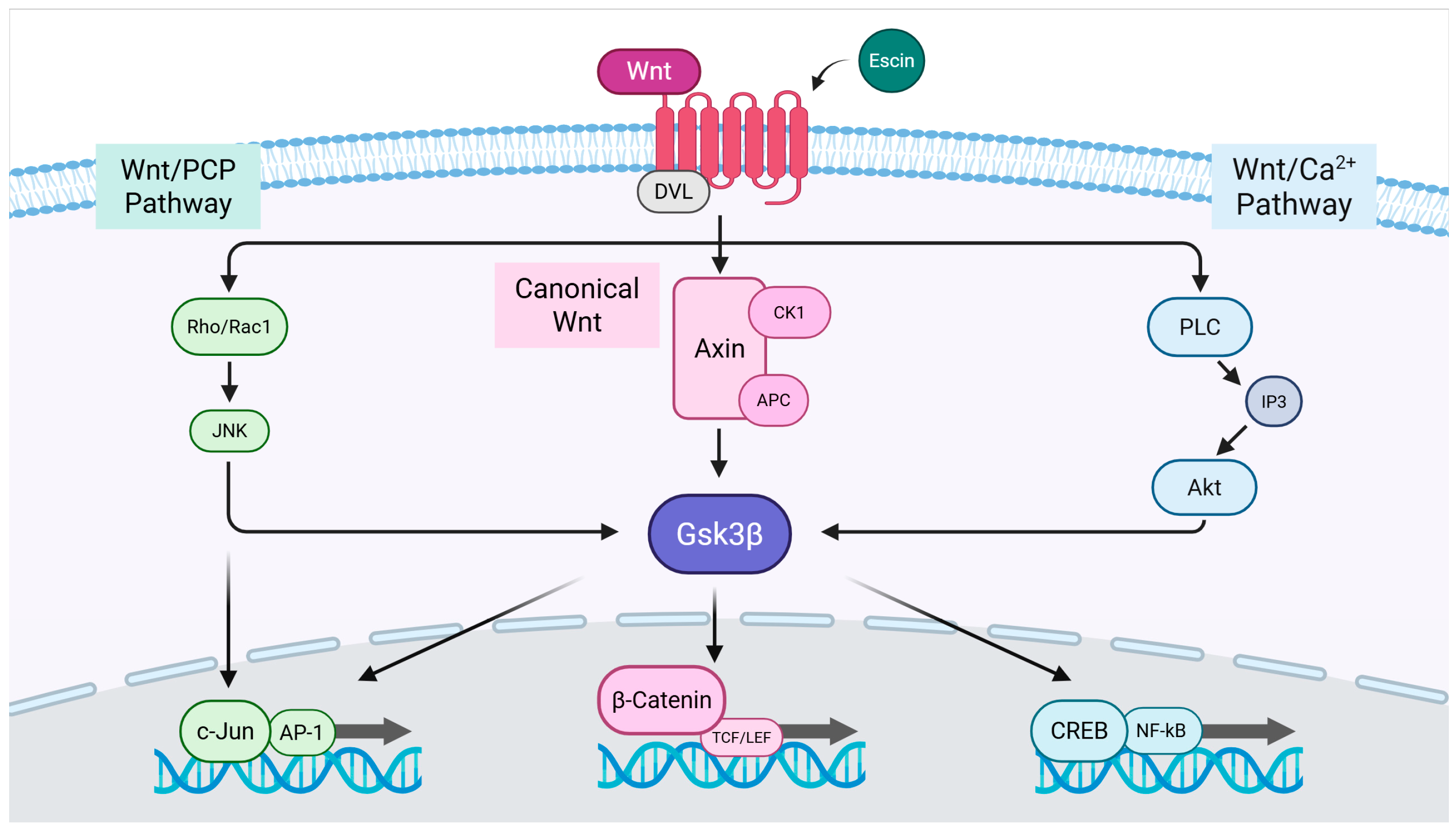
1. Introduction
2. Materials and Methods
2.1. Human Umbilical Vein Endothelial Cell Culture
2.2. Cell Viability Assay for Cultured HUVECs
2.3. Quantitative Real-Time PCR
2.4. TCF/LEF Reporter Assay
2.5. Phosphorylated Protein Dot Blot Analysis for Human Kinase Signaling Transduction
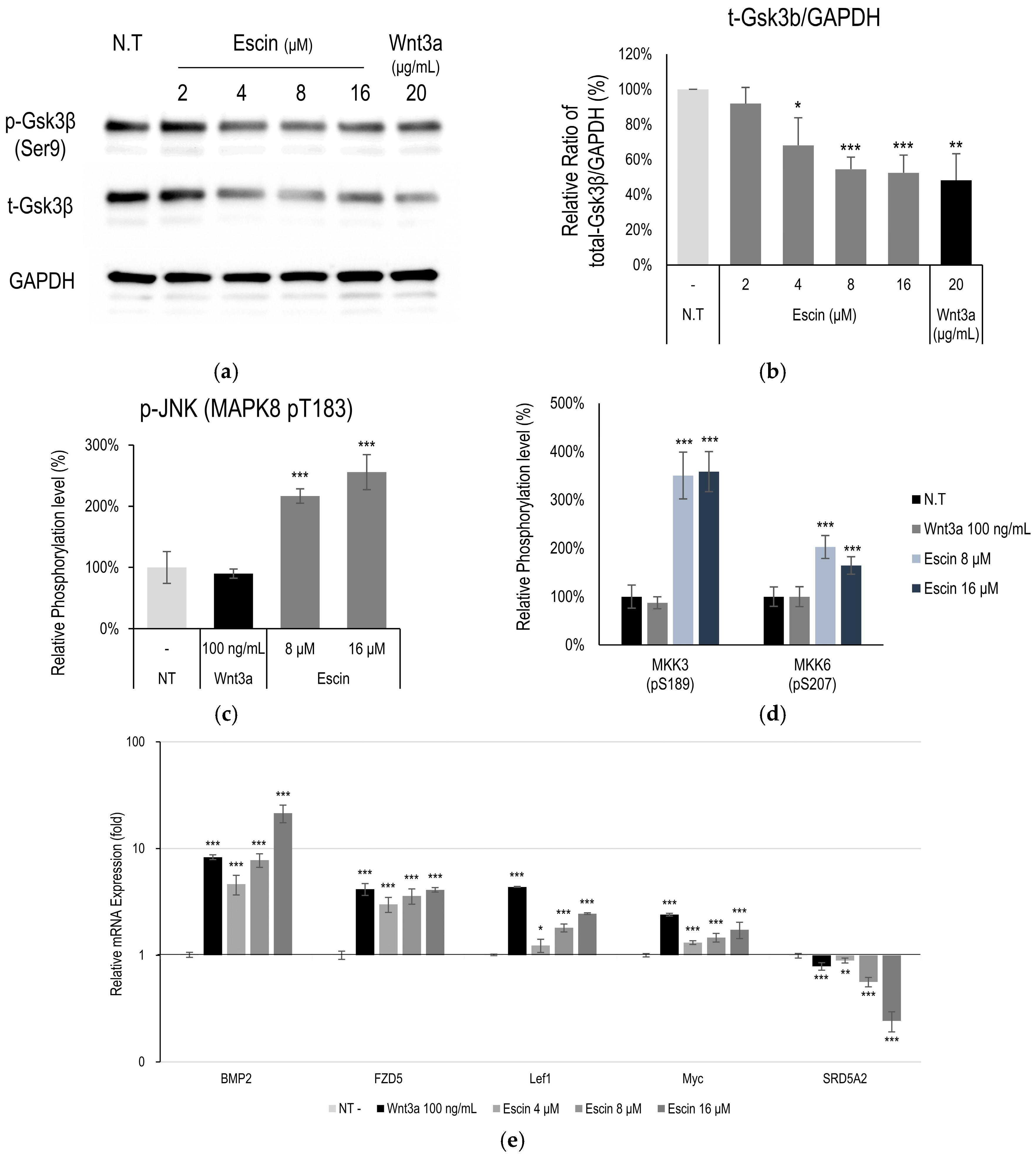
2.6. Western Blotting Analysis
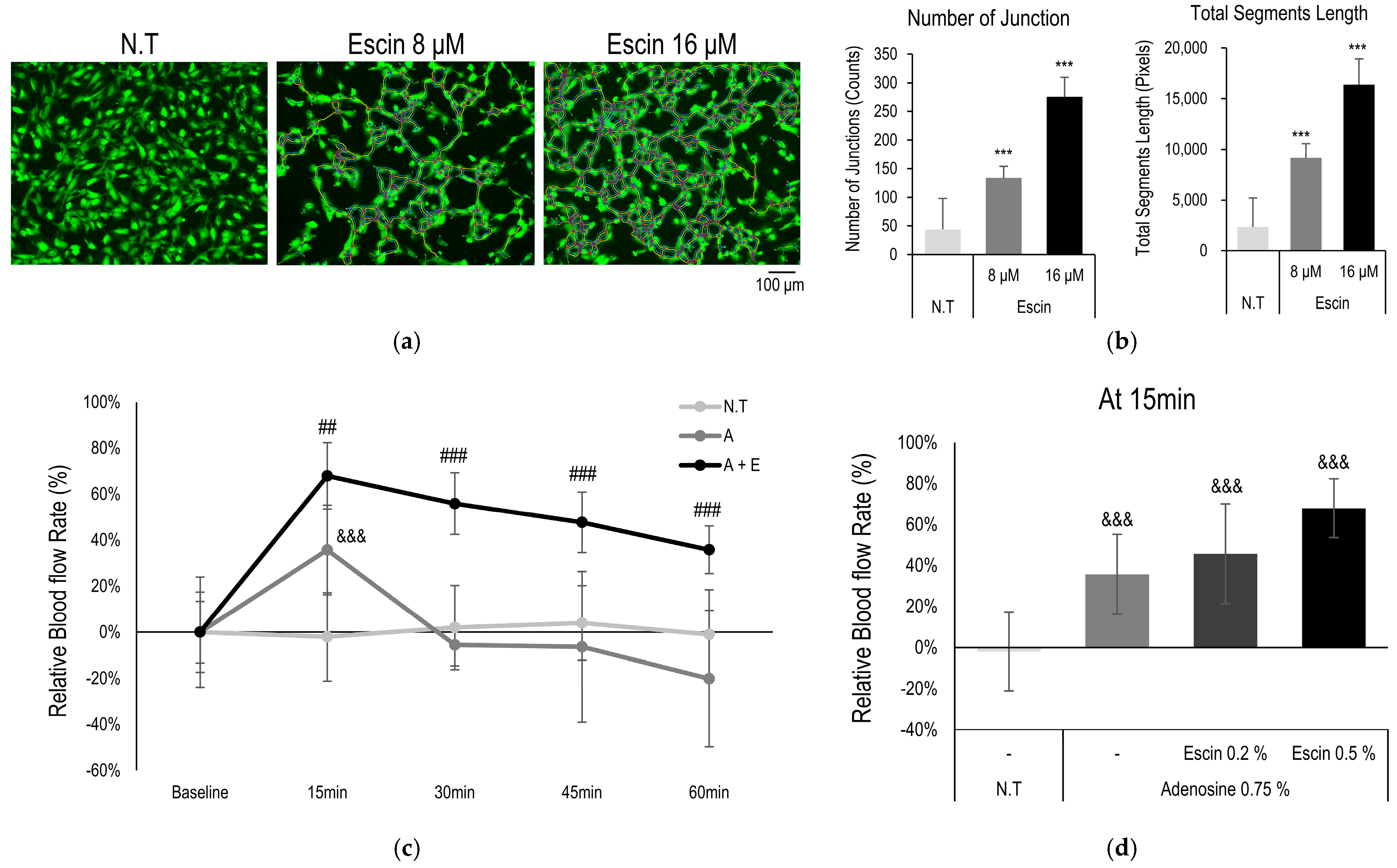
2.7. Blood Vessel Formation Assay
2.8. Evaluation of Skin Blood Flow In Vivo
2.9. Statistical Analysis
3. Results
3.1. Escin Stimulated Wnt/β-Catenin Signaling
3.2. Wnt/β-Catenin Signaling Stimulation by Escin Treatment in Cultured HUVECs
3.3. Gsk3β Down-Regulation by Escin Treatment in Cultured HUVECs
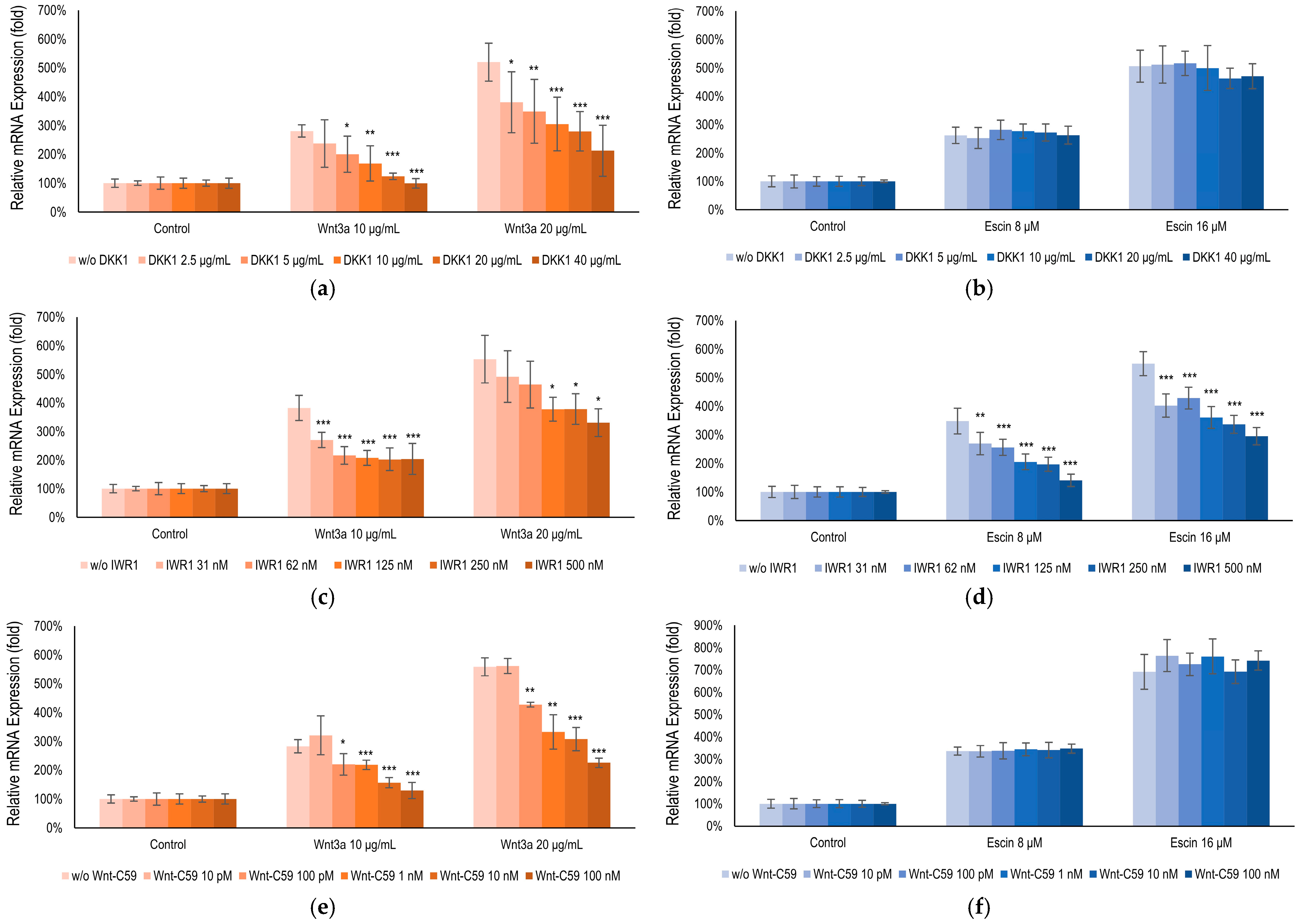
3.4. Escin-Mediated-Wnt/β-Catenin Signaling Was Inhibited by Gsk3β Antagonist IWR1
3.5. Escin Enhanced Vessel Remodeling and Blood Flow In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.-J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.A.; Zhao, G.; Hariharan, A.; Lederer, W.J. Pericytes and the control of blood flow in brain and heart. Annu. Rev. Physiol. 2023, 85, 137–164. [Google Scholar] [CrossRef]
- Fedorovich, A.A.; Rogoza, A.N.; Chikhladze, N.M. Characteristics of functional state of arteriolar and venular skin microvessels in patients with essential arterial hypertension. Microvasc. Res. 2014, 93, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Endothelial ion channels and cell-cell communication in the microcirculation. Front. Physiol. 2022, 13, 805149. [Google Scholar] [CrossRef]
- Shanks, J.; Thomson, S.; Ramchandra, R. Neural control of coronary artery blood flow by non-adrenergic and non-cholinergic mechanisms. Exp. Physiol. 2024, 109, 2011–2016. [Google Scholar] [CrossRef]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.N.; Charkoudian, N. Integrative cardiovascular control in women: Regulation of blood pressure, body temperature, and cerebrovascular responsiveness. FASEB J. 2021, 35, e21143. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Fernández-Varo, G.; Ros, J.; Morales-Ruiz, M.; Cejudo-Martín, P.; Arroyo, V.; Solé, M.; Rivera, F.; Rodés, J.; Jiménez, W. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am. J. Pathol. 2003, 162, 1985–1993. [Google Scholar] [CrossRef]
- Palmer, J.A.; Rosenthal, N.; Teichmann, S.A.; Litvinukova, M. Revisiting Cardiac Biology in the Era of Single Cell and Spatial Omics. Circ. Res. 2024, 134, 1681–1702. [Google Scholar] [CrossRef]
- Holowatz, L.A.; Thompson-Torgerson, C.S.; Kenney, W.L. The human cutaneous circulation as a model of generalized microvascular function. J. Appl. Physiol. 2008, 105, 370–372. [Google Scholar] [CrossRef]
- Dejana, E.; Kühl, M. The Role of Wnt Signaling in Physiological and Pathological Angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; et al. Insights into male androgenetic alopecia using comparative transcriptome profiling: Hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187, 936–947. [Google Scholar] [CrossRef]
- Wen, L.; Fan, Z.; Huang, W.; Miao, Y.; Zhang, J.; Liu, B.; Zhu, D.; Dai, D.; Zhang, J.; Le, D.; et al. Retinoic acid drives hair follicle stem cell activation via Wnt/β-catenin signalling in androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 189–201. [Google Scholar] [CrossRef]
- Cheng, K.; Lin, J.; Wu, M.; Wang, J.; Liu, X.; Yang, K.; Ni, C.; Liu, Q.; Wu, J.; Wu, W. Berberine promotes hair growth by targeting Axin2 and activating Wnt/β-catenin pathway. Phytomedicine 2025, 141, 156669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Wu, L.; Li, Y.; Wang, L.; Zhao, X.; Zhao, T.; Zhang, L.; Yan, Z.; Wei, G. Ameliorative effects of escin on neuropathic pain induced by chronic constriction injury of sciatic nerve. J. Ethnopharmacol. 2021, 267, 113503. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef]
- Elshal, M.; Hazem, S.H. Escin suppresses immune cell infiltration and selectively modulates Nrf2/HO-1, TNF-α/JNK, and IL-22/STAT3 signaling pathways in concanavalin A-induced autoimmune hepatitis in mice. Inflammopharmacology 2022, 30, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Frick, R.W. Three treatments for chronic venous insufficiency: Escin, hydroxyethylrutoside, and Daflon. Angiology 2000, 51, 197–205. [Google Scholar] [CrossRef]
- Peñaranda Figueredo, F.A.; Vicente, J.; Barquero, A.A.; Bueno, C.A. Aesculus hippocastanum extract and the main bioactive constituent β-escin as antivirals agents against coronaviruses, including SARS-CoV-2. Sci. Rep. 2024, 14, 6418. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, T.; Pan, X.; Bian, Y.; Li, J. Escin ameliorates inflammation via inhibiting mechanical stretch and chemically induced Piezo1 activation in vascular endothelial cells. Eur. J. Pharmacol. 2023, 956, 175951. [Google Scholar] [CrossRef]
- Gwozdzinski, L.; Bernasinska-Slomczewska, J.; Hikisz, P.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pieniazek, A. The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study. Biomedicines 2023, 11, 1702. [Google Scholar] [CrossRef]
- Sikorska, M.; Dutkiewicz, M.; Zegrocka—Stendel, O.; Kowalewska, M.; Grabowska, I.; Koziak, K. Beneficial effects of β-escin on muscle regeneration in rat model of skeletal muscle injury. Phytomedicine 2021, 93, 153791. [Google Scholar] [CrossRef]
- Ding, Y.-X.; Eerduna, G.-W.; Duan, S.-J.; Li, T.; Liu, R.-X.; Zhang, L.-M.; Wang, T.; Fu, F.-H. Escin ameliorates the impairments of neurological function and blood brain barrier by inhibiting systemic inflammation in intracerebral hemorrhagic mice. Exp. Neurol. 2021, 337, 113554. [Google Scholar] [CrossRef]
- Liu, P.; Ye, L.; Ren, Y.; Zhao, G.; Zhang, Y.; Lu, S.; Li, Q.; Wu, C.; Bai, L.; Zhang, Z. Chemotherapy-induced phlebitis via the GBP5/NLRP3 inflammasome axis and the therapeutic effect of aescin. Br. J. Pharmacol. 2023, 180, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kim, J.; Choi, Y.-H.; Lee, S.; Kang, N.-G. Escin Activates Canonical Wnt/β-Catenin Signaling Pathway by Facilitating the Proteasomal Degradation of Glycogen Synthase Kinase-3β in Cultured Human Dermal Papilla Cells. Curr. Issues Mol. Biol. 2023, 45, 5902–5913. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, W.-J.; Choi, K.-Y. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell. Signal. 2007, 19, 511–518. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Lee, Y.-C.; You, Y.K.; Lee, S.; Moon, D.H.; Lee, J.-W. Evaluation of blood perfusion using laser doppler flowmetry during endoscopic lumbar sympathectomy in patients with plantar hyperhidrosis: A retrospective observational study. Sci. Rep. 2022, 12, 11456. [Google Scholar] [CrossRef]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; Von Mering, C.; Jensen, L.J.; Bork, P. STITCH 4: Integration of protein–chemical interactions with user data. Nucleic Acids Res. 2014, 42, D401–D407. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Röhrig, U.F.; Goullieux, M.; Bugnon, M.; Zoete, V. Attracting Cavities 2.0: Improving the Flexibility and Robustness for Small-Molecule Docking. J. Chem. Inf. Model. 2023, 63, 3925–3940. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Mu, J.; Li, D.; Ma, Y.; Meng, X. Role of Wnt signaling pathways in type 2 diabetes mellitus. Mol. Cell. Biochem. 2021, 476, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Marković, L.; Bukovac, A.; Varošanec, A.M.; Jakovčević, A.; Tomas, D.; Sonicki, Z.; Puljko, B.; Dumančić, F.; Hrašćan, R.; Pećina-Šlaus, N. Expression of Wnt signaling proteins LEF1, β-catenin, GSK3β, DVL1, and N-myc varies across retinoblastoma subtypes and pRb phosphorylation status. Sci. Rep. 2024, 14, 31725. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Fontes-Ribeiro, C.; Bovee, J.V.; Cleton-Jansen, A.-M.; Gomes, C.M. IWR-1, a tankyrase inhibitor, attenuates Wnt/β-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft. Cancer Lett. 2018, 414, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ahn, V.E.; Chu, M.L.; Choi, H.J.; Tran, D.; Abo, A.; Weis, W.I. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev. Cell 2011, 21, 862–873. [Google Scholar] [CrossRef]
- Proffitt, K.D.; Madan, B.; Ke, Z.; Pendharkar, V.; Ding, L.; Lee, M.A.; Hannoush, R.N.; Virshup, D.M. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013, 73, 502–507. [Google Scholar] [CrossRef]
- Jia, J.; Ye, T.; Cui, P.; Hua, Q.; Zeng, H.; Zhao, D. AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvasc. Res. 2016, 105, 103–108. [Google Scholar] [CrossRef]
- Sancho, M.; Klug, N.R.; Mughal, A.; Koide, M.; Huerta de la Cruz, S.; Heppner, T.J.; Bonev, A.D.; Hill-Eubanks, D.; Nelson, M.T. Adenosine signaling activates ATP-sensitive K+ channels in endothelial cells and pericytes in CNS capillaries. Sci. Signal. 2022, 15, eabl5405. [Google Scholar] [CrossRef]
- Shaulson, E.D.; Cohen, A.A.; Picard, M. The brain-body energy conservation model of aging. Nat. Aging 2024, 4, 1354–1371. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 2015, 11, 95–104, Correction in Organogenesis 2015, 11, 210. [Google Scholar] [CrossRef]
- Adav, S.S.; Ng, K.W. Recent omics advances in hair aging biology and hair biomarkers analysis. Ageing Res. Rev. 2023, 91, 102041. [Google Scholar] [CrossRef]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT–β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Clevers, H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol. 2017, 428, 273–282. [Google Scholar] [CrossRef]
- Rabbani, P.; Takeo, M.; Chou, W.; Myung, P.; Bosenberg, M.; Chin, L.; Taketo, M.M.; Ito, M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 2011, 145, 941–955. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Min, J.K.; Park, H.-S.; Lee, Y.-B.; Kim, J.-G.; Kim, J.-I.; Park, J.-B. Cross-Talk between Wnt Signaling and Src Tyrosine Kinase. Biomedicines 2022, 10, 1112. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Yan, W.; Zhu, L.; Tang, C.; Wang, G. The role of blood flow in vessel remodeling and its regulatory mechanism during developmental angiogenesis. Cell. Mol. Life Sci. 2023, 80, 162. [Google Scholar] [CrossRef]
- Tang, L.; Dai, F.; Liu, Y.; Yu, X.; Huang, C.; Wang, Y.; Yao, W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol. Res. 2018, 133, 201–212. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.-Y.; Li, B.; Tian, X.Y.; Chen, L.-J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016, 540, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular endothelial cells: Heterogeneity and targeting approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Britzen-Laurent, N.; Weidinger, C.; Stürzl, M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 5517. [Google Scholar] [CrossRef]
- Santamaría, R.; González-Álvarez, M.; Delgado, R.; Esteban, S.; Arroyo, A.G. Remodeling of the Microvasculature: May the Blood Flow Be With You. Front. Physiol. 2020, 11, 586852. [Google Scholar] [CrossRef]
- Regazzetti, C.; Joly, F.; Marty, C.; Rivier, M.; Mehul, B.; Reiniche, P.; Mounier, C.; Rival, Y.; Piwnica, D.; Cavalie, M.; et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway: A Promising Target for Repigmenting Vitiligo Patients. J. Investig. Dermatol. 2015, 135, 3105–3114. [Google Scholar] [CrossRef]
- Lien, W.H.; Polak, L.; Lin, M.; Lay, K.; Zheng, D.; Fuchs, E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol. 2014, 16, 179–190. [Google Scholar] [CrossRef]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef]
- Roberts, K.A.; Garcia, L.R.; De Forest, P.R. Proximal End Root Morphology Characteristics in Antemortem Anagen Head Hairs. J. Forensic Sci. 2017, 62, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Uluckan, O.; Jimenez, M.; Karbach, S.; Jeschke, A.; Grana, O.; Keller, J.; Busse, B.; Croxford, A.L.; Finzel, S.; Koenders, M.; et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl. Med. 2016, 8, 330ra37. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Rabbani, P.; Sun, Q.; Hu, H.; Lim, C.H.; Manga, P.; Ito, M. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016, 15, 1291–1302. [Google Scholar] [CrossRef] [PubMed]

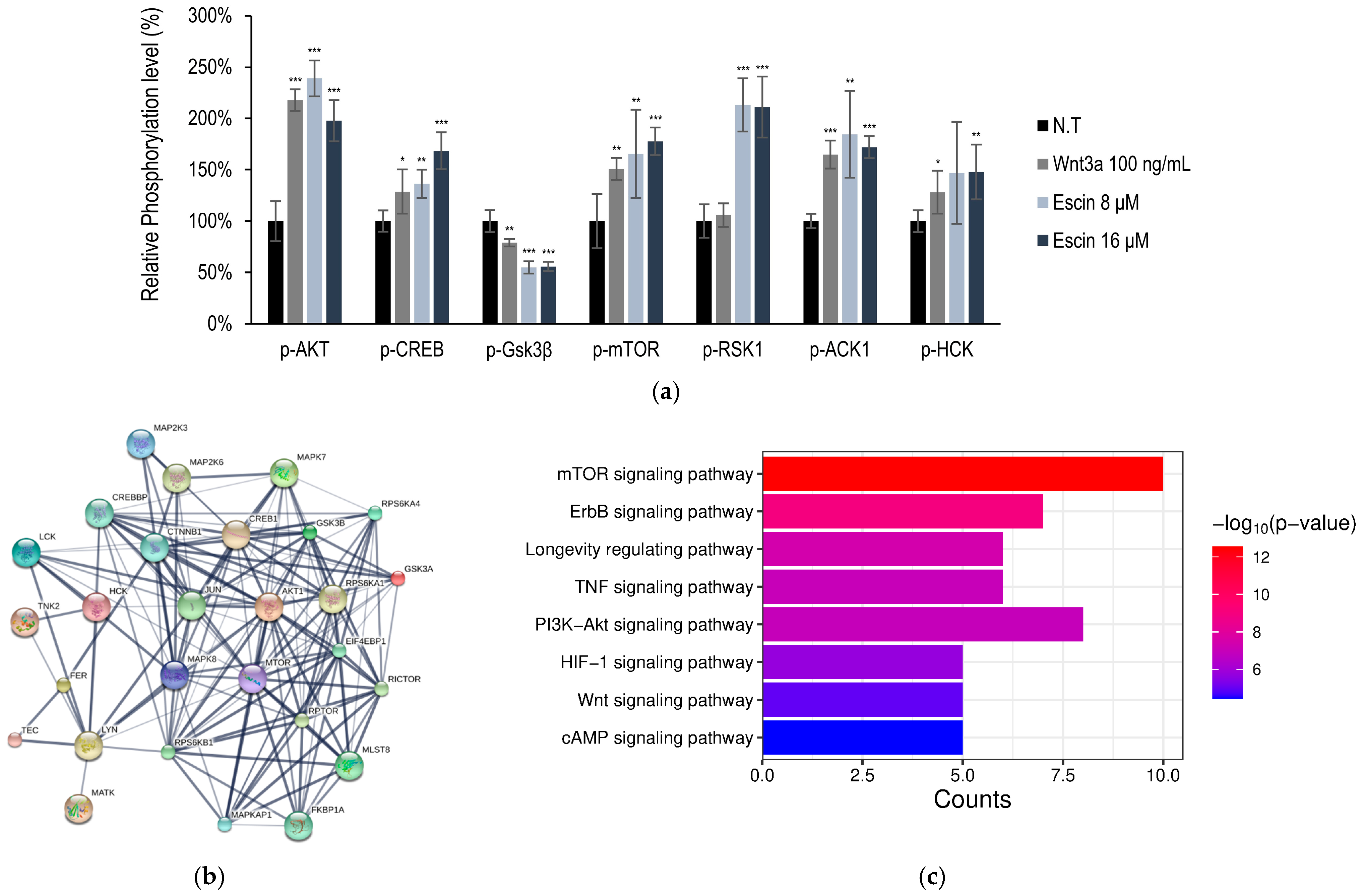
| #node1 | #node2 | Combined Score | #node1 | #node2 | Combined Score | #node1 | #node2 | Combined Score |
|---|---|---|---|---|---|---|---|---|
| c-Jun | AKT1 | 0.977 | CTNNB1 | AKT1 | 0.999 | RICTOR | AKT1 | 0.999 |
| CREBBP | 0.999 | Gsk3β | 0.999 | EIF4EBP1 | 0.999 | |||
| CTNNB1 | 0.992 | CREBBP | 0.999 | RPS6KB1 | 0.999 | |||
| Gsk3β | 0.999 | Gsk3α | 0.988 | MAPKAP1 | 0.999 | |||
| MAP2K3 | 0.78 | FER | 0.977 | RPTOR | 0.998 | |||
| MAPK7 | 0.982 | RPS6KB1 | 0.684 | Gsk3β | 0.929 | |||
| MAPK8 | 0.999 | RPS6KA4 | 0.55 | RPS6KA4 | 0.76 | |||
| mTOR | 0.775 | LCK | 0.407 | MAPK7 | 0.436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Joo, J.H.; Rim, H.; Kim, S.H.; Shin, J.y.; Jun, S.-H.; Kang, N.-G. Microcirculation-Promoting Effect of Escin on Cutaneous Tissue via Gsk3β Down-Regulation. Curr. Issues Mol. Biol. 2025, 47, 840. https://doi.org/10.3390/cimb47100840
Kim J, Joo JH, Rim H, Kim SH, Shin Jy, Jun S-H, Kang N-G. Microcirculation-Promoting Effect of Escin on Cutaneous Tissue via Gsk3β Down-Regulation. Current Issues in Molecular Biology. 2025; 47(10):840. https://doi.org/10.3390/cimb47100840
Chicago/Turabian StyleKim, Jaeyoon, Jang Ho Joo, Heena Rim, Sung Hyun Kim, Jae young Shin, Seung-Hyun Jun, and Nae-Gyu Kang. 2025. "Microcirculation-Promoting Effect of Escin on Cutaneous Tissue via Gsk3β Down-Regulation" Current Issues in Molecular Biology 47, no. 10: 840. https://doi.org/10.3390/cimb47100840
APA StyleKim, J., Joo, J. H., Rim, H., Kim, S. H., Shin, J. y., Jun, S.-H., & Kang, N.-G. (2025). Microcirculation-Promoting Effect of Escin on Cutaneous Tissue via Gsk3β Down-Regulation. Current Issues in Molecular Biology, 47(10), 840. https://doi.org/10.3390/cimb47100840








