Abstract
Diabetes mellitus is a chronic metabolic disorder that facilitates the formation of advanced glycation end products (AGEs), which contribute to oxidative stress, inflammation, and vascular damage, causing complications including nephropathy, neuropathy, and atherosclerosis. AGEs are primarily synthesized through the Maillard reaction, alongside various signaling pathways. Activation of the receptor for AGE (RAGE) triggers inflammatory signaling pathway cascades, exacerbating tissue damage. Phenolic compounds found in plant-based foods, such as quercetin and resveratrol, have shown promise in counteracting AGE-related complications through their antioxidant and anti-inflammatory effects that inhibit AGE formation, reduce oxidative stress, and modulate RAGE signaling, while also enhancing insulin sensitivity and improving glucose homeostasis. Indeed, quercetin can help prevent AGE accumulation and reduce diabetic nephropathy, while resveratrol activates the SIRT1 pathway, improving insulin sensitivity. This review examines the mechanisms through which phenolic compounds mitigate AGE-induced diabetic complications, using computational, in vitro, preclinical, and clinical evidence. This review also explores the synergistic effects of these compounds with conventional antidiabetic drugs, addresses bioavailability challenges, and suggests future research directions. Overall, this review offers a comprehensive understanding of the role of phenolic compounds in managing diabetes, underscoring their potential as complementary agents in diabetes therapy and developing more effective natural treatments.
1. Introduction
Diabetes mellitus (DM) represents a complex, multifactorial metabolic disorder that contributes significantly to the global burden of chronic disease, affecting millions worldwide [1]. The dysregulation of glucose homeostasis is central to the pathophysiology of DM, leading to chronic hyperglycemia [2]. Subsequently, persistently elevated glucose levels result in the accumulation of advanced glycation end products (AGEs), which are formed through the non-enzymatic glycation of proteins, lipids, and nucleic acids [3]. The formation of AGEs is accelerated under conditions of hyperglycemia, while the accumulation of AGEs plays a pivotal role in the development of various diabetic complications, such as nephropathy, retinopathy, neuropathy, and cardiovascular disease [4,5]. The biochemical mechanisms through which AGEs contribute to these complications are complex, involving interactions with cellular receptors, such as the receptor for advanced glycation end products (RAGEs), which trigger inflammatory responses, oxidative stress, and cellular dysfunction [6,7]. These cellular perturbations significantly impact tissue integrity, leading to the progression of diabetic complications across multiple organs, including the kidneys, retina, skin, and vasculature [8].
The impact of AGEs on cellular and molecular pathways is primarily mediated through their interaction with RAGEs, a multi-ligand receptor that initiates a cascade of signaling events upon binding to AGEs. Therefore, the AGE–RAGE axis is crucial in the induction of inflammatory cytokines, matrix metalloproteinases, and various other molecules that facilitate tissue damage and fibrosis, driving the progression of diabetic complications [9]. The activation of downstream signaling pathways, including the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/Akt, leads to the amplification of oxidative stress [10], inflammatory responses, and cellular apoptosis, further contributing to the deterioration of cellular function [11]. Additionally, the deposition of AGEs in tissues causes structural modifications in the extracellular matrix, impairing tissue function and promoting the pathological remodeling observed in diabetic complications [12]. Thus, understanding these molecular mechanisms is crucial for developing therapeutic strategies that aim to prevent or reverse AGE-induced cellular damage, particularly in the context of diabetes management. Polyphenolic compounds, which are abundant in plant-based foods, have attracted significant attention for their potential to mitigate the deleterious effects of AGEs [13]. These compounds are best known for possessing potent antioxidant and anti-inflammatory properties, which enable the compounds to scavenge free radicals and reduce oxidative stress, a process that plays a central role in AGE formation [14]. Polyphenolic compounds are characterized by more than one hydroxyl group attached to an aromatic ring [15]. Some broad polyphenolic compound classes include Flavonoids (quercetin, kaempferol, rutin), phenolic acids (allic acid, chlorogenic acid), stilbenes (resveratrol), lignans (secoisolariciresinol) and Tannins (proanthocyanidins) [16]. Certainly, each compound class has unique structural feature that mainly contribute towards biological activity for instance, in the case of flavonoids, the “flavonoid backbone” that possesses ring A and B, connected by a three-carbon bridge (C ring) is of prime importance [17]. Further, the positioning of OH groups and degree of methylation, hydroxylation and glycosylation also play a vital role in biological activity [18]. In case of phenolic acid, the carboxylic acid group (-COOH) and vinyl group (–CH=CH2) attached to the aromatic ring are of prime importance with respect to biological activities [19]. Similarly, in stilbenes the “stilbene backbone’ and in Lignans, the existence of two phenylpropanoid units that are linked by a central carbon–carbon bond is important. Lastly, in case of Tannins, the occurrence of multiple phenolic groups allows them to interact with various biological targets, including AGEs [20].
The polyphenolic compounds can directly inhibit the Maillard reaction, the process responsible for AGE formation, thus preventing the accumulation of these harmful compounds [21]. In addition to their role in reducing AGE formation, polyphenols also modulate the AGE–RAGE signaling pathway, attenuating the downstream inflammatory responses and oxidative stress induced by AGE accumulation [22]. This dual action, which reduces AGE formation and mitigates AGE-mediated cellular damage, highlights the therapeutic potential of polyphenolic compounds in managing diabetic complications. Several studies have demonstrated that dietary polyphenols, such as flavonoids, phenolic acids and stilbenes, possess the ability to regulate glycemic control, reduce AGE levels, and protect against diabetic nephropathy (DN), retinopathy, and cardiovascular disease [23,24,25]. Further the Anti-AGEs potential of polyphenolic compounds in prediabetic conditions is also important to consider [26]. In a recent investigation, the polyphenolic compounds were analyzed in prediabetic conditions and outstanding outcomes were recorded that show potential of polyphenolic compounds against AGEs [27,].
However, the bioavailability of polyphenolic compounds remains a critical factor in their therapeutic efficacy. Despite the promising biological activities of these polyphenolic compounds, the absorption and metabolism of polyphenols are often limited by factors such as low solubility, rapid metabolism, and poor systemic availability [28]. Hence, significant efforts have been made to enhance the bioavailability of these compounds through various biotechnological approaches. These include the development of advanced drug delivery systems, such as nanoparticles and liposomes, which improve the stability, solubility, and absorption of polyphenols. Furthermore, strategies aimed at improving the pharmacokinetics of polyphenolic compounds, such as modifying the chemical structure or inducing combinations with other bioactive agents, have been explored to maximize the therapeutic potential of these compounds in diabetes management [29,30].
This review aims to provide a comprehensive overview of the pathogenesis of AGEs in diabetes and the role of polyphenolic compounds in modulating AGE formation and mitigating diabetic complications. The scope of the review encompasses a detailed examination of the molecular mechanisms through which AGEs contribute to the development of diabetes-related complications, with particular emphasis on the AGE–RAGE signaling pathway and the subsequent downstream effects. This review also highlights the current state of research and future directions for studying polyphenolic compounds as potential therapeutic agents in the prevention and treatment of diabetic complications associated with AGEs.
2. Literature Search and Methodology
The review is built on a comprehensive investigation of several peer-reviewed journal articles, review papers and book sources that comprised information, findings related to AGEs and the effect of plant polyphenolic compounds in reducing their effects. Several academic databases for instance, Web of Science, ScienceDirect, Wiley, MDPI, Google Scholar and Springer were retrieved for data. The required information was obtained by certain key words including “Diabetes Mellitus”, “Advance glycation”, “Diabetic complications”, “plant polyphenols” “Mechanistic of AGEs inhibition by phenolics”, “AGE-RAGE Signaling pathways”, “AGE-RAGE computational tools”, “ Network pharmacology of phenolics in AGEs” and “Challenges in AGEs research” Use of Boolean operators “AND”, “OR” was accomplished to improve quests and have a more detailed overview. The timeframe for included publications primarily ranged from 1995 through 2025 to include introductory research papers as well as the latest and modern developments in AGEs. The set “inclusion criteria” was delegated to include studies about Diabetes, AGEs and polyphenolic compounds only. All included articles were limited to peer-reviewed works published in the English language only. The “exclusion” criteria were set that limited the use of conference abstracts, literature published before the year 1995, unpublished data, and retracted literature to maintain academic rigor. Initially, a data set of approximately 370 scientific records was saved and curated by “title” and “abstract” for relevance and eminence. Following this, 310 articles were reviewed in full text, and only 280 articles were able to meet the criteria for inclusion in the project. This method warranted a wide but very intensive exposure of the topic. All figures included in this review article were generated by the authors using BioRender.com for more detailed and conceptual illustrations. All information was sensibly vetted to confirm a precise depiction of the discussed content (Table 1).

Table 1.
Key Studies on Phenolic Compounds and AGEs in Diabetes Management.
3. Diabetic Complications and AGEs
AGEs are considered critical in the pathogenesis of diabetes and its allied serious complications. The formation of AGEs is closely linked to hyperglycemia, and these compounds accumulate over time, contributing to the long-term complications observed in diabetic patients [7,31]. AGE accumulation is particularly problematic in tissues with long turnover rates, such as the skin, kidneys, retina, and vascular systems, where the AGE can exacerbate the damage caused by diabetes [32].
3.1. AGE Formation Mechanism and the Mechanistic Role in Diabetic Complications
AGEs form primarily through the Maillard reaction, where reducing sugars bind to the free amino groups of proteins, creating an initial reversible product [33], which eventually undergoes further chemical modifications to form irreversible AGEs [22]. The non-enzymatic glycation of proteins, lipids, and nucleic acids promotes the formation of cross-linked structures, which accumulate in various tissues over time [34]. Under hyperglycemic conditions, the rate of AGE formation is significantly accelerated, resulting in the accumulation of AGEs in tissues such as the skin, kidneys, eyes, and blood vessels [5]. These accumulated AGEs modify the structure and function of macromolecules, such as collagen and elastin, which are crucial for maintaining tissue elasticity and function [35]. Additionally, AGEs interact with specific receptors, most notably the RAGE, leading to the activation of proinflammatory pathways and an increase in oxidative stress [36]. This results in a cascade of molecular events that contribute to the development of diabetic complications, including vascular stiffening, renal damage, and retinal degeneration.
The formation process for AGEs starts with the development of a Schiff base between the carbonyl group of the sugar and the amino group in the target molecule. This Schiff base is rearranged to form an Amadori product, which represents a more stable but still reactive intermediate [37]. Over time, Amadori products undergo further chemical modifications, including oxidation, dehydration, and cross-linking, resulting in the formation of AGEs [38]. These reactions are accelerated in the presence of hyperglycemia, oxidative stress, and chronic inflammation, which are prevalent in diseases such as diabetes and aging [39].
3.2. Major AGE Types
Methylglyoxal (MGO) and other reactive dicarbonyl compounds, such as Nε-carboxymethyllysine (CML) and pentosidine, represent well-known AGEs and are highly reactive intermediates in the glycation pathway. Additionally, several other types of AGEs are recognized for their roles in various diseases [40]. MGO-derived hydroimidazolone (MGO-H1) and glyoxal-derived hydroimidazolone (GH1) are significant AGEs formed from an interaction between MGO and glyoxal with protein residues [41]. Other notable AGEs include pyrraline, a cross-linking AGE formed by the reaction between pentoses that contain a lysine and 3-deoxyglucosone (3-DG), an intermediate that can extensively modify proteins. Ne-(carboxyethyl)valine (CEV) is another important AGE, and arises from the modification of valine residues by reactive carbonyl species [42]. Additionally, the glyoxal-lysine dimer (GOLD) and MGO-lysine dimer (MOLD) are AGEs formed by the reaction between glyoxal and MGO with lysine residues, respectively (Figure 1). These dimeric AGEs have been implicated in the cross-linking of extracellular matrix proteins, contributing to tissue stiffness [43]. These AGEs, along with others such as the imidazolone family, play critical roles in the aging process and are associated with chronic diseases, including cardiovascular disorders, neurodegeneration, and diabetes.
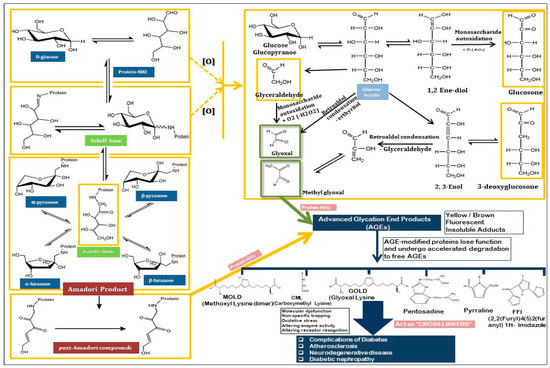
Figure 1.
The mechanism of Maillard reaction through which AGEs are formed, the and the presentation of various AGEs.
3.3. Pathophysiological Consequences of AGE-Induced Diabetic Complications
As explained earlier, an increased accumulation of AGEs in various tissues results in profound alterations in tissue architecture and function, contributing to the pathophysiology of several diabetic complications. AGEs in the kidneys promote the formation of advanced cross-links in collagen and elastin fibers, leading to the thickening of the glomerular basement membrane and mesangial expansion [44]. These changes compromise kidney function, impairing filtration and promoting the development of DN, which can lead to the onset of proteinuria. The increased production of proinflammatory cytokines and growth factors, such as TGF-β, further exacerbates the fibrosis and inflammation in renal tissue, contributing to the progressive nature of the disease. In diabetic retinopathy, AGE accumulation in the retinal vasculature leads to endothelial cell dysfunction, increased vascular permeability, and the formation of microaneurysms [45]. The leakage of fluid from these damaged vessels results in retinal edema, a key feature of diabetic macular edema, which can severely impair vision if left untreated.
Cardiovascular complications related to AGEs are primarily driven by the accumulation of these products in the vascular endothelium and smooth muscle cells, where the AGEs contribute to endothelial dysfunction, inflammation, and the promotion of vascular calcification. AGE-induced modifications of collagen and elastin in the vascular walls cause the arteries to stiffen, which increases blood pressure and contributes to the development of atherosclerosis [46]. These alterations also promote platelet aggregation, increasing the risk of thrombosis and myocardial infarction in diabetic patients [47]. Additionally, AGEs have been shown to activate the renin–angiotensin system, further exacerbating blood pressure regulation and contributing to the vascular complications observed in diabetes [48]. The cumulative effect of AGE-induced modifications in multiple organ systems significantly impairs overall health and accelerates the onset of severe complications, emphasizing the importance of understanding the role of AGEs in diabetes (Figure 2).
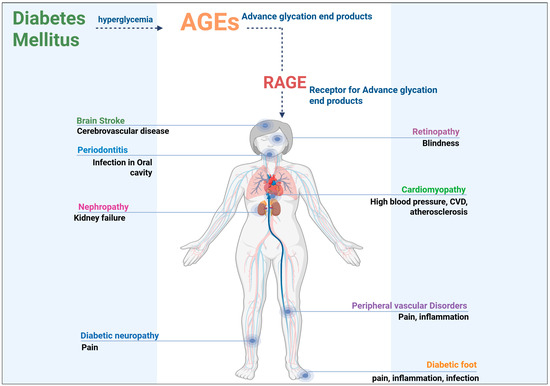
Figure 2.
Pathological considerations of AGEs in diabetic patients.
4. Signaling Pathways Involved in AGE-Induced Diabetic Complications
The pathophysiology of diabetic complications is significantly influenced by the accumulation of AGEs, which, in turn, trigger multiple intracellular signaling pathways that mediate tissue damage and dysfunction [49]. One of the key mechanisms through which AGEs exert their deleterious effects is through an interaction with the RAGE. A detailed discussion regarding important signaling pathways is presented below.
4.1. AGE–RAGE Signaling Pathway
The receptor for advanced glycation end products (RAGE) plays a central role in mediating the effects of AGEs on cellular function [50]. RAGE is a multi-ligand receptor that binds AGEs and triggers a series of intracellular signaling events that contribute to the pathological consequences of AGE accumulation [51]. Upon binding to AGEs, RAGE activates several downstream signaling pathways, including NF-κB, MAPKs, and the PI3K/Akt pathway, all of which promote inflammation, oxidative stress, and cellular dysfunction [52]. The activation of NF-κB, a key regulator of immune responses, leads to the production of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, which further exacerbate tissue inflammation and contribute to the development of diabetic complications [51]. Additionally, RAGE activation increases the production of reactive oxygen species (ROS), leading to oxidative stress, which accelerates cellular damage and promotes fibrosis in affected tissues [51].
The AGE-RAGE interaction also contributes to the development of insulin resistance, a key symbol of type 2 diabetes, by disrupting insulin signaling pathways and impairing glucose uptake in peripheral tissues such as muscle and adipose tissue [53]. In the vasculature, RAGE activation induces the expression of matrix metalloproteinases (MMPs), enzymes that degrade extracellular matrix components contributing to vascular remodeling and endothelial dysfunction [47]. Likewise in the kidneys, RAGE-mediated signaling promotes the accumulation of extracellular matrix proteins, contributing to glomerulosclerosis and tubulointerstitial fibrosis [54]. The interaction between AGEs and RAGE is thus central to the cellular dysfunction observed in diabetic complications, and therapeutic strategies aimed at modulating this interaction hold promise for alleviating AGE-induced damage.
4.2. AGE-Induced Activation of MAPK Pathways
MAPKs, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, are key mediators of AGE-induced cellular dysfunction [55] and are involved in regulating various cellular processes, such as inflammation, apoptosis, and fibrosis [56]. In the context of diabetes, these kinases are activated downstream of the AGE–RAGE axis and contribute to the development of diabetic complications by promoting inflammatory responses and tissue remodeling [57]. The activation of ERK, JNK, and p38 MAPK in response to AGE exposure leads to the phosphorylation of several target proteins, including transcription factors and enzymes involved in cell survival, proliferation, and inflammation [58].
In particular, ERK activation promotes the production of proinflammatory cytokines and enhances cell survival in response to stress [59]. Conversely, JNK and p38 MAPK activation are primarily involved in regulating apoptosis and inflammation [60]. The activation of JNK and p38 MAPK in endothelial cells contributes to endothelial dysfunction and increased vascular permeability, which are key features of diabetic retinopathy and nephropathy. Moreover, p38 MAPK activation induces the production of profibrotic factors, such as TGF-β, which drives fibrosis in tissues, including the kidneys and heart [61]. MAPK-related signaling pathways are also involved in modulating insulin signaling, while the activation of these pathways can also impair insulin sensitivity, leading to insulin resistance in skeletal muscle and adipose tissue [62]. Thus, the AGE-induced activation of MAPK pathways plays a crucial role in driving inflammation, fibrosis, and insulin resistance in various tissues, contributing to the progression of diabetic complications.
4.3. NF-κB Pathway in AGE-Mediated Inflammation
The NF-κB pathway is a key regulator of the inflammatory response and is critically involved in the pathogenesis of diabetic complications induced by AGE accumulation [63]. NF-κB is a transcription factor that, upon activation, translocates to the nucleus and regulates the expression of genes involved in inflammation, immune responses, and cellular survival [64]. The NF-κB pathway is activated in response to AGE binding to RAGE, leading to the production of proinflammatory cytokines, chemokines, and adhesion molecules, which promote the recruitment of immune cells to the injury site [65]. NF-κB activation also increases the production of ROS, which further amplifies the inflammatory response and contributes to oxidative stress and tissue damage [66].
For example, in DN, NF-κB activation promotes the expression of cytokines such as TNF-α and IL-6, which effectively contribute to the chronic inflammation observed in the kidneys [67]. This inflammation exacerbates glomerular injury, increases vascular permeability, and promotes the accumulation of extracellular matrix proteins, leading to fibrosis and renal dysfunction [68]. In diabetic retinopathy, NF-κB activation in retinal cells contributes to the breakdown of the blood–retinal barrier, promoting retinal edema and the progression of vision impairment [63]. Furthermore, NF-κB signaling plays a role in the development of insulin resistance by inducing the expression of inflammatory mediators that impair insulin receptor signaling [69]. The role of NF-κB in AGE-induced inflammation underscores the importance of this transcription factor as a therapeutic target for mitigating diabetic complications and slowing the progression of the disease.
4.4. AGEs and the PI3K/Akt Pathway
The PI3K/Akt pathway is a central regulator of cellular metabolism, growth, and survival, playing a crucial role in maintaining insulin sensitivity [70]. In the context of diabetes, AGE accumulation activates PI3K/Akt pathway upon AGEs binding to the RAGE, leading to the phosphorylation of Akt, a protein kinase that regulates numerous cellular processes, including glucose metabolism, cell survival, and protein synthesis [71]. However, in diabetic conditions, excessive activation of this pathway can promote insulin resistance and impaired glucose uptake in peripheral tissues [72].
In muscles, the PI3K/Akt pathway plays a particularly crucial role in regulating endothelial cell function and maintaining vascular homeostasis [73]. AGE-induced activation of PI3K/Akt leads to the disruption of endothelial cell signaling, contributing to endothelial dysfunction, vascular permeability, and the development of atherosclerosis [74]. In pancreatic β-cells, the PI3K/Akt pathway is also involved in regulating insulin secretion and β-cell survival [75]. Further AGE-induced dysregulation of this pathway impairs insulin secretion and promotes β-cell apoptosis, further contributing to the progression of T2D [76].
4.5. NLRP3 Inflammasome Activation
The NLRP3 inflammasomes comprise the NLRP3 sensor, ASC adaptor, and pro-caspase-1, that is a critical mediator of AGE-induced inflammatory signaling [77]. With reference to diabetes, NLRP3 is activated downstream of the AGE–RAGE axis and plays a fundamental role in promotion of chronic low-grade inflammation and tissue injury. Engagement of RAGE by AGEs initiates oxidative stress and NF-κB activation, which prime the transcription of NLRP3 and its downstream cytokines, pro-IL-1β and pro-IL-18 [78]. Subsequent AGE-induced mitochondrial dysfunction, reactive oxygen species (ROS) overproduction, and ion flux imbalances serve as activation signals that promote assembly of the NLRP3 inflammasome complex. The activation of caspase-1 within this complex leads to the maturation and secretion of IL-1β and IL-18, as well as pyroptotic cell death [79], thereby amplifying inflammatory responses and contributing to the progression of diabetic complications.
4.6. PKC Pathway
In diabetes, continued hyperglycemia upsurges the intracellular diacylglycerol (DAG) levels, which unreasonably activate various protein kinase C (PKC) isoforms, particularly PKC-β, PKC-δ, and PKC-θ [80]. The PKC activation alters vascular and metabolic homeostasis by stimulating NADPH oxidase–dependent reactive oxygen species (ROS) production, activating NF-κB and MAPK pathways, and finally spoils the insulin receptor signaling [81]. In vascular tissues, PKC promotes endothelial dysfunction by reducing nitric oxide (NO) bioavailability thereby increasing endothelin-1 expression and enhancing vascular permeability. In renal cells, PKC signaling accelerates mesangial expansion, extracellular matrix deposition, and glomerular sclerosis that finally leads to diabetic nephropathy [82]. Similarly in retina, PKC-β activation drives VEGF overexpression, neovascularization, and capillary leakage, exacerbating diabetic retinopathy [83]. Moreover, PKC cross-talks with the AGE–RAGE pathway by amplifying oxidative stress and inflammation, creating a synergistic loop that accelerates microvascular complications [84].
4.7. Other Signaling Pathways Involved in AGE-Induced Diabetic Complications
In addition to these well-established pathways, the role of several other signaling pathways is also important for consideration. One such pathway is the TGF-β/Smad signaling pathway, which plays a critical role in tissue fibrosis and remodeling [85]. The AGE accumulation activates TGF-β signaling, leading to the production of extracellular matrix components, fibrosis, and renal dysfunction [86]. The activation of TGF-β signaling by AGEs can occur through both direct (AGE binding to RAGE) and indirect mechanisms (activation of oxidative stress and inflammation). TGF-β also promotes the differentiation of fibroblasts into myofibroblasts, which contribute to tissue scarring and organ damage. Additionally, AGEs can modulate autophagy pathways, particularly in pancreatic β-cells, where impaired autophagy contributes to β-cell dysfunction and insulin secretion deficits [87]. AGEs inhibit autophagic processes in pancreatic β-cells, leading to the accumulation of damaged proteins and organelles, further impairing β-cell function. This interference is mainly through RAGE–NF-κB pathway. The AGEs to RAGE attachment activates NF-κB signaling, which in turn suppresses the expression of key autophagy-related genes, including Beclin-1 and LC3 [88]. These additional pathways, along with those already discussed, contribute to the multi-organ complications observed in diabetes and underscore the complexity of AGE-mediated pathophysiology (Table 2).

Table 2.
AGE-RAGE Signaling and Its Role in Diabetic Complications.
5. Phenolic Compounds in Diabetes Management
Phenolic compounds, a diverse group of naturally occurring phytochemicals, have gained significant attention for their potential therapeutic effects in managing diabetes and any associated complications [96]. Phenolic compounds include flavonoids, phenolic acids, stilbenes, and lignans, and each possesses unique biological properties [97]. These compounds have been extensively studied for their antioxidant, anti-inflammatory, anti-glycation, and anti-hyperglycemic effects, all of which play a key role in modulating the underlying mechanisms of diabetes [90] and reducing the associated risks.
5.1. Major Dietary Sources of Phenolic Compounds
Phenolic compounds are widely distributed in plant-based foods, and the consumption of these compounds has been linked to various health benefits, particularly in managing several ailments. These compounds are present in a diverse range of fruits, vegetables, herbs, and spices, which are staples in many diets worldwide [98]. Flavonoids, phenolic acids, stilbenes, and lignans are the primary classes of phenolic compounds found in food sources [99].
As presented earlier, numerous dietary sources of phenolic compounds have been reported, including berries, vegetables, herbs, and fruits. Indeed, berries, such as blueberries, strawberries, and raspberries, are rich in flavonoids, including anthocyanins, which have been shown to promote potent antioxidant, anti-AGE, and anti-inflammatory effects [100]. These food sources are also high in vitamin C, which further enhances their ability to scavenge ROS and reduce oxidative stress [101]. Citrus fruits, such as oranges, lemons, and grapefruits, are another excellent source of flavonoids, particularly flavanones, such as hesperidin and naringenin, which have been linked to improved insulin sensitivity and glucose metabolism [102]. Additionally, the consumption of apples has been associated with reduced inflammation and oxidative stress [103], owing to the rich content of phenolic acids in apples, particularly chlorogenic acid, which is known to inhibit AGE formation and reduce hyperglycemia [104].
Vegetables are another important source of phenolic compounds. Cruciferous vegetables, such as broccoli, brussels sprouts, and kale, contain high levels of phenolic acids, particularly ferulic acid, which has been shown to exhibit strong antioxidant and anti-inflammatory properties [105]. Other vegetables, such as spinach, onions, and tomatoes, are rich in flavonoids and other phenolic compounds that contribute to improved metabolic health and reduced inflammation [106]. Meanwhile, the consumption of herbs and spices, such as turmeric, ginger, cinnamon, and oregano, also provides a significant amount of phenolic compounds with various biological activities [107]. For example, curcumin, the active compound in turmeric, has been extensively studied for its anti-inflammatory and anti-glycation effects, making this spice a valuable dietary supplement in diabetes management [108]. Green tea represents another prominent source of phenolic compounds, particularly catechins, which have been shown to improve glucose metabolism, reduce oxidative stress, and enhance insulin sensitivity [109]. The broad spectrum of phenolic compounds present in these food groups supports their potential role in managing diabetes and its complications through dietary interventions.
5.2. Mechanisms of Action of Phenolic Compounds
The mechanisms through which phenolic compounds exert their effects in diabetes management are multifaceted. The antioxidant properties of phenolic compounds are crucial to their ability to combat oxidative stress, a hallmark of diabetes. By scavenging ROS, phenolic compounds protect cellular components from oxidative damage and limit the formation of AGEs, which are implicated in various diabetic complications [110]. ROS are produced as byproducts of normal metabolic processes, but their levels can become elevated under pathological conditions, such as hyperglycemia. These elevated ROS levels lead to cellular dysfunction by damaging proteins, lipids, and nucleic acids, triggering inflammatory responses, and promoting the formation of AGEs [111]. The antioxidant effects of phenolic compounds neutralize ROS, thereby reducing the cascade of events that cause cellular damage and the progression of diabetic complications [112]. Among these known phenolic compounds, flavonoids such as quercetin, catechins, and resveratrol have been shown to possess potent antioxidant activity [113], making these compounds promising candidates for diabetes management.
In addition to their antioxidant effects, phenolic compounds exhibit strong anti-inflammatory properties that contribute to their therapeutic potential in diabetes. Chronic inflammation is a characteristic feature of diabetes, particularly in adipose tissue, skeletal muscle, and the vasculature [114]. Meanwhile, chronic inflammation is driven by elevated levels of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, which contribute to insulin resistance and endothelial dysfunction [115]. Phenolic compounds modulate the expression of these cytokines by inhibiting the activation of transcription factors such as NF-κB, which regulates the expression of many inflammatory genes [116]. For instance, resveratrol, a stilbene found in grapes and red wine, has been shown to inhibit NF-κB activation and reduce the production of proinflammatory cytokines in vitro and in vivo [117]. Other phenolic compounds, such as curcumin and catechins, also exhibit anti-inflammatory effects by downregulating the expression of inflammatory mediators, contributing to improved insulin sensitivity and reduced risk of diabetic complications. Furthermore, phenolic compounds have been shown to inhibit enzymes involved in carbohydrate digestion, such as α-amylase and α-glucosidase, thereby slowing glucose absorption and improving postprandial glycemic control [118].
5.3. Reducing AGE Formation
One of the most promising therapeutic actions of phenolic compounds in diabetes management is their ability to inhibit the formation of AGEs. As discussed earlier, AGEs play a pivotal role in the pathogenesis of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease. However, several phenolic compounds have been shown to inhibit the Maillard reaction through which AGEs are produced, thereby reducing the formation of AGEs [119]. This effect is particularly important in the context of diabetes, where excessive AGE formation contributes to tissue damage and organ dysfunction [120]. Phenolic compounds, such as chlorogenic acid, curcumin, and resveratrol, have also demonstrated an ability to reduce AGE formation not only by directly inhibiting the glycation of proteins but also through scavenging the reactive intermediates [121]. Chlorogenic acid, a phenolic acid found in coffee and certain fruits, has also been shown to reduce AGE formation in vitro by inhibiting the interaction between glucose and proteins [122]. Similarly, curcumin has been reported to inhibit the formation of AGEs and reduce the accumulation of AGEs in diabetic tissues [123]. Likewise, resveratrol possesses anti-glycation properties and has been shown to reduce the formation of AGEs in both in vitro and in vivo models of diabetes [124].
5.3.1. Inhibition of Free Radical Formation
ROS are generated during the early stages of glycation and can further promote the formation of AGEs. Phenolic compounds possess antioxidant properties that enable the compounds to scavenge these free radicals, thereby reducing oxidative stress and limiting the subsequent formation of AGEs [125,126].
5.3.2. Scavenging of Reactive Dicarbonyl Compounds
MGO and dicarbonyl compounds are extremely reactive intermediates in the glycation pathway in DM [127]. Phenolic compounds can trap these intermediates through nucleophilic attack, forming stable adducts that prevent the progression to AGEs [128].
5.3.3. Metal Ion Chelation
Transition metals, such as iron (Fe2+) and copper (Cu2+), catalyze the oxidation of Amadori products, facilitating the formation of AGEs [129]. Phenolic compounds can chelate these metal ions, reducing their availability and, thus, inhibiting metal-catalyzed oxidative reactions that lead to AGE formation [130].
5.3.4. Modulation of Glyoxalase I Activity
The glyoxalase system, particularly glyoxalase I (GLO I), also plays a crucial role in detoxifying MGO by converting it into less reactive compounds [131]. Certain phenolic compounds, including quercetin, curcumin and resveratrol etc., [132,133] can enhance the activity of GLO I, thereby increasing the clearance of MGO and reducing the formation of AGEs [134].
5.3.5. Inhibition of AGE–Receptor Interactions
AGEs exert their pathological effects by binding to the RAGE, triggering the inflammatory and oxidative pathways. Phenolic compounds can inhibit the expression of the RAGE or block its interaction with AGEs, thereby mitigating the downstream inflammatory responses associated with AGE accumulation [135] (Figure 3 and Table 3).
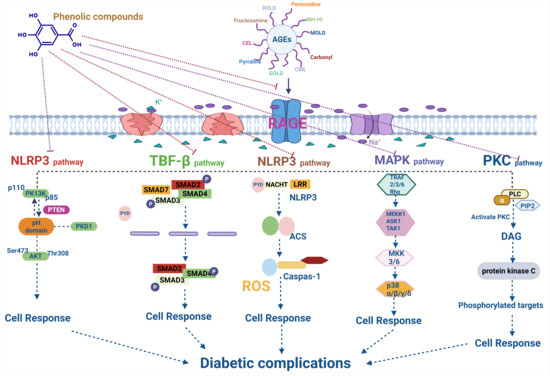
Figure 3.
Mechanistic insights of phenolic compounds in AGEs inhibition and Diabetic complications.

Table 3.
The effect of various phenolic compounds on AGEs inhibition and their mechanistic insights.
5.4. Pharmacokinetics of Phenolic Compounds
Despite the promising therapeutic effects of phenolic compounds, the clinical application of these compounds has remained limited due to their relatively poor bioavailability [148]. Notably, many phenolic compounds are poorly absorbed in the gastrointestinal tract due to their low solubility and rapid metabolism [149]. Therefore, only a small fraction of the phenolic compounds consumed through dietary methods reaches systemic circulation in an active form, thereby limiting their therapeutic efficacy. Several factors influence the bioavailability of phenolic compounds, including their molecular size, solubility, and stability in the digestive system [150]. Additionally, phenolic compounds undergo extensive first-pass metabolism in the liver, where these compounds are converted into metabolites with potentially reduced biological activity [151].
Therefore, various strategies have been employed to enhance the bioavailability of phenolic compounds, including the use of advanced drug delivery systems, such as nanoparticles, liposomes, and micelles [30]. These delivery systems can encapsulate phenolic compounds, improving their solubility, stability, and absorption. Nanoparticles, for example, can protect phenolic compounds from degradation in the gastrointestinal tract and facilitate their targeted delivery to specific tissues, such as the liver, kidneys, or adipose tissue [152]. Another approach to improving the bioavailability of phenolics involves modifying the chemical structure of these compounds to increase solubility and stability [28]. For instance, esterification of phenolic acids can enhance lipophilicity and improve absorption in the intestines [153]. Additionally, combining phenolic compounds with other bioactive agents, such as lipids or essential oils, can enhance their pharmacokinetic properties and therapeutic efficacy [154]. These strategies are critical for maximizing the potential of phenolic compounds as therapeutic agents in the management of diabetes and any associated complications.
6. Evidence from Experimental Studies on Phenolic Compounds in Diabetes
Numerous experimental studies have been conducted to evaluate the therapeutic potential of phenolic compounds in managing diabetes and its complications. Indeed, these in vitro studies, animal models, and human clinical trials have provided valuable insights into the biological mechanisms through which phenolic compounds exert their effects, particularly in reducing oxidative stress, inflammation, and AGE formation. These compounds have shown promising results in preventing and mitigating diabetic complications, including DN, retinopathy, neuropathy, and cardiovascular disease. The accumulation of evidence from these studies suggests that phenolic compounds can play an integral role in diabetes management by targeting multiple pathways involved in the pathophysiology of the disease.
6.1. In Vitro Studies
In vitro studies have played a pivotal role in demonstrating the antioxidant, anti-inflammatory, and anti-glycation effects of phenolic compounds. These studies typically involve the use of cultured cells exposed to diabetic conditions, such as high glucose levels or AGEs, to assess the impact of phenolic compounds on cellular function. One of the key findings from these in vitro studies is that phenolic compounds, such as quercetin, resveratrol, and curcumin, possess potent antioxidant activity that helps neutralize ROS and prevent oxidative stress [155,156,157].
Similarly, in diabetic cell models, phenolic compounds, such as gallic acid, epigallocatechin-3-gallate, resveratrol, oleuropein, and curcumin, have been shown to reduce ROS-mediated oxidative damage to lipids, proteins, and DNA, thereby protecting cells from the deleterious effects of hyperglycemia [112,158]. ROS include both oxygen-centered radicals, such as O2- or hydroxyl (OH•) radicals, and other non-radical oxygen derivatives, including hydrogen peroxide (H2O2) and singlet oxygen (1O2) [159]. Additionally, phenolic compounds, including punicalagin (PC), ellagic acid, naringin, hesperidin, and rutin, have been found to modulate the expression of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which are elevated in diabetes and contribute to insulin resistance [160,161]. Likewise, isorhamnetin, naringenin, and pelargonidin have been shown to inhibit NF-κB activation in macrophages, reducing the production of inflammatory cytokines and improving insulin sensitivity [162].
Phenolic compounds are also known to exert anti-glycation effects that are critical in reducing AGE formation. Several studies have demonstrated that compounds such as chlorogenic acid, curcumin, and catechins can directly inhibit the Maillard reaction, preventing the glycation of proteins and the subsequent formation of AGEs [21,163]. In vitro studies using endothelial cells, for example, have demonstrated that homoprotocatechuic and ferulic acids, as well as quercetin, can inhibit the accumulation of AGEs, thereby reducing AGE-mediated cellular dysfunction and improving endothelial function [164,165]. Thus, by targeting the pathways involved in AGE formation and reducing oxidative stress, phenolic compounds hold significant potential as potential therapeutic agents for managing diabetic complications, as evaluated in vitro
6.2. In Vivo Studies
In vivo studies in animal models of diabetes have provided further evidence supporting the therapeutic efficacy of phenolic compounds in preventing and treating diabetic complications. These studies are particularly valuable in assessing the overall impact of phenolic compounds on glucose metabolism, insulin resistance, and tissue damage in a living organism. Animal studies using diabetic models have shown that phenolic compounds, such as epigallocatechin-3-O-gallate (EGCG) from green tea, can significantly inhibit AGEs and thereby play a role in the management of diabetic complications [166]. Similarly, animal studies have explained the role of resveratrol and curcumin in significantly lowering hyperglycemia, inhibiting AGEs, and playing a role in mitigating the effects of neurodegenerative diseases (NDDs) [121,167].
The effects of phenolic compounds on diabetic complications have also been demonstrated in animal models of DN, retinopathy, and cardiovascular disease. Curcumin has been shown to reduce kidney damage in diabetic rats by decreasing proteinuria, inflammation, and fibrosis [168]. Moreover, the ability of curcumin to modulate the AGE–RAGE signaling pathway and reduce oxidative stress has been suggested as the underlying mechanism of its renoprotective effects [169]. Meanwhile, phenolic compounds such as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, p-coumaric acid, vanillic acid, and quercetin have been shown to protect retinal cells from AGE-induced damage, reduce vascular leakage, and prevent retinal edema in animal models of diabetic retinopathy [170,171]. Furthermore, stilbenes, including trans- and cis-resveratrol, piceatannol, and viniferins, have demonstrated cardioprotective effects by improving endothelial function, reducing arterial stiffness, and preventing the progression of atherosclerosis in diabetic animal models [172]. These studies provide strong evidence that phenolic compounds can prevent or delay the onset of diabetic complications and improve overall metabolic health in vivo.
6.3. Human Clinical Trials and Observational Studies
Human clinical trials and observational studies have provided additional insights into the effectiveness of polyphenolic compounds in managing diabetes and its complications. While in vitro and animal studies provide important mechanistic data, clinical trials are crucial for assessing the real-world applicability and safety of phenolic compounds in humans. Several clinical trials have evaluated the effects of phenolic-rich dietary interventions, such as the consumption of berries, green tea, and red wine, on glycemic control and AGE levels in diabetic patients [173,174,175]. One notable study demonstrated that the intake of polyphenol-rich foods, such as blueberries and strawberries, significantly improved insulin sensitivity and reduced fasting blood glucose levels in individuals with T2D [176]. These findings suggest that dietary phenolic compounds can have a beneficial impact on glycemic control and may complement conventional diabetes treatments.
In addition to improving glucose metabolism, clinical studies have also demonstrated that phenolic compounds can reduce inflammation and oxidative stress biomarkers in patients with DM. For example, resveratrol supplementation was found to decrease serum levels of proinflammatory cytokines and reduce markers of oxidative damage in patients with T2D [177]. Moreover, studies have suggested that diets rich in polyphenols, including hesperidin and resveratrol, are associated with a lower risk of developing diabetic complications, as these compounds can influence various factors related to AGE production [178]. The clinical evidence supports the notion that phenolic compounds can help manage diabetes and its associated complications through their antioxidant, anti-inflammatory, and anti-glycation properties [179]. However, further well-designed large-scale clinical trials are needed to confirm these findings and establish the optimal dosages and long-term effects of phenolic compounds in diabetes management. Similarly, various clinical investigations based on polyphenols from the whortleberry, olive oil, coffee, guava tea, propolis, red wine, grape seed, and cocoa have shown that these are very effective in showing antidiabetic effects in patients with T2D through increasing glucose metabolism and improving vascular function, as well as reducing insulin resistance and HbA1c levels [180].
Though numerous human clinical trials and observational studies have assessed the effects of phenolic-rich dietary interventions, such as the consumption of berries, green tea, and red wine, on glycemic control and metabolic health, it is important to note that these trials do not directly assess pure or combined phenolic compounds. Furthermore, AGEs were not measured as outcomes in these studies, which limits our ability to draw definitive conclusions regarding the direct impact of phenolic compounds on AGE formation in humans. This limitation is acknowledged, and further research that directly evaluates phenolic compounds and quantifies AGEs as an outcome measure is needed to better understand the potential therapeutic role of phenolics in managing diabetic complications.
6.4. The Toxicity of Polyphenolic Compounds and Their Interactions
Despite extensive biological activities of polyphenolic compounds, there exist a great of uncertain and side effects too [181]. It has been reported earlier in several investigations that intake of higher amounts of dietary polyphenols or excessive use of supplements can lead to undesirable consequences, including G.I.T. disturbances, impaired nutrient absorption and metal ion chelation [182,183]. For instance, strong iron-binding activity has been associated with reduced bioavailability of this essential micronutrient, raising concerns for populations with marginal iron status. More importantly, the biological effects of polyphenols are dose- and form-dependent, with purified aglycones or concentrated extracts often produce unusual effects that are not commonly observed with polyphenols consumed naturally within whole foods [184]. Similarly, polyphenol–drug interactions and the occurrence of potential pro-oxidant activities is another important concern to consider [185]. Polyphenols are capable of modifying activity of cytochrome P450 enzymes in liver and various drug transporters, that can alter the pharmacokinetics of co-administered medicines thereby reducing therapeutic efficacy of drugs or enhancing their toxicity [186]. Additionally, although polyphenols are primarily observed as antioxidants, some can exhibit pro-oxidant properties at high concentrations, generating reactive oxygen species that induce genotoxic or mutagenic effects in experimental systems [187]. Polyphenols can also influence hormonal signaling pathways, with implications for endocrine balance. Overall there exists a great need for caution in polyphenol supplement use and the importance of further studies on safety thresholds and interactions.
7. Modern Computational Tools in AGE and Phenolic Compounds Research
Modern computational tools play a crucial role in advancing our understanding of the molecular mechanisms underlying the effects of AGEs and phenolic compounds in diabetes management. These tools enable researchers to study the interactions between AGEs and their receptors, such as the RAGE, as well as the binding affinity and efficacy of phenolic compounds in inhibiting AGE formation and modulating key cellular pathways [188]. By integrating molecular docking, molecular dynamics (MD) simulations, and network pharmacology approaches, researchers can gain insights into the structure–activity relationships of phenolic compounds and their potential therapeutic benefits [189,190].
7.1. Molecular Docking and Virtual Screening
Molecular docking is a computational technique used to predict the binding interactions between small molecules, such as phenolic compounds, and their target proteins, including those involved in AGE formation or the AGE–RAGE signaling pathway [191]. Docking simulations provide valuable information regarding the binding affinity, orientation, and binding sites of compounds with specific receptors or enzymes [192], helping to identify the most promising candidates for therapeutic intervention. This approach has been widely employed to study the interactions between AGEs and RAGE, as well as to explore how phenolic compounds can disrupt these interactions. For instance, docking studies have demonstrated that polyphenolic compounds, such as resveratrol and quercetin, can bind to the RAGE with high affinity, thereby inhibiting the AGE–RAGE interaction and preventing AGE-induced inflammatory responses [193,194]. Such findings support the potential of phenolic compounds as therapeutic agents for mitigating AGE-related complications in diabetes. Similarly, citrus flavonoids, including hesperidin and hesperetin, have been analyzed through in vitro and docking studies to investigate their interaction with the AGE/RAGE/NF-κB pathway, yielding significant results [195].
7.2. Molecular Dynamics Simulations
MD simulations offer a powerful method for studying the time-dependent behavior of molecular systems [196], providing detailed insights into the dynamic interactions between phenolic compounds, AGEs, and their receptors. Unlike static docking studies, MD simulations capture the flexibility of molecules and the conformational changes that may occur during binding [197]. This enables researchers to investigate the stability of the AGE–RAGE complex and to assess the effect of phenolic compounds on the structural integrity and function of the receptor. MD simulations have been used to investigate the interactions between RAGE and AGEs, revealing the mechanisms through which AGE binding induces conformational changes that facilitate the activation of downstream signaling pathways. MD simulations can also provide valuable insights into the binding dynamics of phenolic compounds with enzymes involved in AGE formation, such as GLO I and α-amylase [198]. By simulating the binding of phenolic compounds to these enzymes, researchers can assess the ability of these compounds to inhibit enzyme activity and, in turn, reduce the production of AGEs. For example, MD simulations have shown that certain flavonoids, such as epicatechin and quercetin, can effectively inhibit GLO I, thereby preventing the accumulation of reactive di-carbonyl compounds that contribute to AGE formation [199]. These simulations offer a detailed understanding of the molecular mechanisms through which phenolic compounds exert anti-glycation effects, providing a solid foundation for the design of more effective AGE-targeting therapeutics. In another investigation, MD simulations were performed to cover flavonoids with the RAGE. The MD simulation confirmed that flavonoids, including icariin, kaempferol, luteolin, and quercetin, formed stable complexes with the RAGE. The study identified the RAGE as a novel therapeutic target for epimedium in mitigating VaD through its anti-inflammatory properties [200]. Likewise, MD investigations on glycolipid metabolic disorders such as nonalcoholic fatty liver disease (NAFLD), obesity, and DN have been found to show a significant interaction of dietary polyphenols with their receptors [201].
7.3. Network Pharmacology Approaches
Network pharmacology is an emerging computational approach that integrates systems biology and pharmacology to understand the complex interactions between drugs, targets, and biological pathways [202]. In the context of AGEs and phenolic compounds, network pharmacology approaches can be effectively used to identify the key molecular targets involved in AGE formation, AGE–RAGE signaling, and diabetic complications. This approach allows researchers to construct molecular networks that highlight the interconnected pathways and potential therapeutic targets that phenolic compounds can modulate [203]. Thus, by mapping these interactions between phenolic compounds and the proteins involved in AGE-related pathways, network pharmacology can provide a comprehensive view of the mechanisms through which these compounds exert their therapeutic effects across multiple biological systems [204]. In the context of AGEs and phenolic compounds, one of the primary applications of network pharmacology in the research of AGEs and phenolic compounds is the identification of multi-target effects [205]. Unlike traditional drug discovery methods that focus on single-target molecules, network pharmacology recognizes that diseases, such as diabetes, often involve complex, multifaceted pathways. Several investigators have successfully explored the network pharmacology of phenolic compounds and AGEs. For example, network pharmacology studies have revealed that resveratrol inhibits AGE formation and modulates inflammation, oxidative stress, and insulin resistance, thereby making resveratrol a promising multi-target therapeutic agent [206]. Similarly, network pharmacology analyses have confirmed that resveratrol significantly inhibits intestinal aging by downregulating the ATF4/Chop/Bcl-2/Bax signaling pathway, a finding further confirmed in animal studies [207]. Similarly, the use of network pharmacology in AGE and phenolic compound research can also facilitate the identification of novel targets for drug development and the optimization of existing therapeutic strategies. For instance, an investigation on Gegen Qinlian (GQL) (mainly comprising puerarin, baicalin, and wogonin) decoction confirmed through network pharmacology that the antidiabetic effects of GQL were associated with modulating the TNF and PI3K–AKT–MTOR pathways. These findings were further supported by in vivo experimental data [208].
Presently, strategies to integrate data from molecular docking, MD simulations, network pharmacology, and experimental studies are commonly employed to simulate the effects of compounds on cellular systems and organs [209]. These models are equally valuable in AGE and phenolic interactions analyses [210,211].
7.4. Bioinformatics Tools for Gene Expression and Pathway Analyses
Bioinformatics tools for gene expression and pathway analyses are instrumental in understanding the molecular mechanisms underlying various health complications and the effects of drug moieties on these pathways [212,213]. These tools can also be efficiently used for AGEs. For example, gene expression analysis allows researchers to identify changes in the expression of genes involved in AGE formation, inflammation, oxidative stress, and insulin resistance in response to treatment with phenolic compounds [214,215]. High-throughput technologies, such as RNA sequencing (RNA-seq), enable the identification of differentially expressed genes (DEGs), which can then be mapped to relevant signaling pathways using bioinformatics tools, including Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome, and Gene Ontology (GO) [215] Specifically, several polyphenolic compounds such as resveratrol, quercetin, and curcumin have been analyzed using bioinformatic tools. For instance, resveratrol was evaluated using molecular docking studies to assess its binding affinity to the receptor for advanced glycation end products (RAGE) [193], while quercetin and curcumin were analyzed for their ability to modulate inflammatory pathways through interactions with NF-κB and MAPK signaling [216].
Pathway enrichment analysis is another key tool in bioinformatics that helps researchers understand the biological significance of DEGs and their involvement in specific pathways [217]. By applying pathway analysis tools, researchers can identify the key signaling networks affected by phenolic compounds, such as the AGE–RAGE pathway, NF-κB signaling, and insulin resistance pathways [218]. This approach allows for the identification of novel biomarkers and therapeutic targets, as well as a deeper understanding of how phenolic compounds can modulate complex biological processes to mitigate diabetic complications. Various investigations have previously utilized these tools, and key outcomes have been reported in relation to phenolic compounds and the enrichment analysis of the AGE pathway [218]. Figure 4 provides a comprehensive overview of the various analysis tools that are used to investigate various AGES types, including GOLD, MOLD, CML, carbonyl groups, etc. The AGES research has been broadly divided into in silico analysis, in vitro analysis and in vivo analysis. initially, various predictions regarding bioavailability, ligand-target interaction, and Molecular dynamics can be performed to forecast the potential of the tested molecule for further research. This is further assisted by in silico models to validate the outcomes of in silico analysis. Finally, once both these analysis models are aligned, in vivo analysis is performed to validate all outcomes.
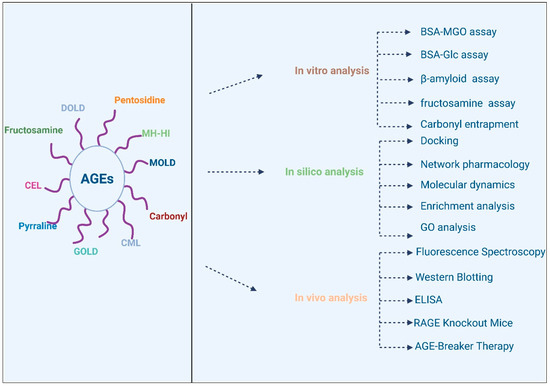
Figure 4.
Analysis of AGEs using various models.
8. Therapeutic Potential and Future Directions
The therapeutic potential of polyphenolic compounds in diabetes management has garnered significant attention due to their multifaceted biological properties, including antioxidant, anti-inflammatory, anti-glycation, and anti-hyperglycemic effects [219]. The ability of the compounds to modulate key pathological processes such as oxidative stress, inflammation, and the formation of AGEs positions them as valuable adjuncts in diabetes therapy [220]. Furthermore, phenolic compounds are known to enhance insulin sensitivity, reduce blood glucose levels, and protect against complications such as DN, retinopathy, and cardiovascular disease [221].
8.1. Future Research
While the therapeutic potential of polyphenolic compounds in diabetes management is well-established, several critical areas require further research to maximize their effectiveness and clinical applicability. One of the main challenges is the limited bioavailability of polyphenolic compounds, which often hinders the ability of these compounds to reach therapeutic concentrations in the bloodstream and target tissues [222]. The development of novel formulation strategies, including nanoparticles, liposomes, or other drug delivery systems, can help enhance the bioavailability of phenolic compounds [223].
In addition to improving bioavailability, more studies are needed to investigate the optimal doses, durations, and combinations of polyphenolic compounds for managing diabetes [224]. While several clinical trials have demonstrated the benefits of phenolic-rich diets or supplements in improving glycemic control and reducing diabetic complications, limited information remains on the long-term effects of these compounds [225]. Therefore, future studies should focus on conducting large-scale, well-designed clinical trials that evaluate the safety and efficacy of phenolic compounds over extended periods, particularly in diverse populations with different forms of diabetes [219,226]. Moreover, the potential for synergistic effects between phenolic compounds and conventional diabetes medications should be explored, as combining natural and pharmaceutical therapies could enhance treatment outcomes and provide a more holistic approach to managing diabetes [227].
8.2. Potential Synergism with Antidiabetic Drugs
The combination of polyphenolic compounds with conventional antidiabetic drugs has shown great promise in improving diabetes management. The polyphenolic compounds, such as resveratrol, quercetin, and curcumin, possess antioxidant, anti-inflammatory, and anti-glycation properties that can complement the action of conventional antidiabetic drugs. For instance, metformin, the first-line therapy for T2D, functions by improving insulin sensitivity and reducing hepatic glucose production [228]. The polyphenolic compound resveratrol has been shown to enhance insulin sensitivity by modulating pathways such as AMP-activated protein kinase (AMPK) [229], which is also activated by metformin. The combined action of metformin and resveratrol can lead to enhanced glucose uptake in muscle cells and improved glucose homeostasis. Similarly, polyphenolic compounds can complement sulfonylureas, which stimulate insulin secretion from pancreatic β-cells [230]. Hence, by reducing oxidative stress and inflammation, polyphenolic compounds improve β-cell function and insulin secretion, enhancing the effectiveness of sulfonylureas and potentially decreasing the required doses.
9. Conclusions
The polyphenolic compounds derived from numerous plant-based foods offer significant therapeutic potential in managing diabetes and any subsequent complications. polyphenolic compounds possess potent antioxidant, anti-inflammatory, anti-glycation, and anti-hyperglycemic properties, which provide a multifaceted approach to addressing the pathophysiology of diabetes. By modulating key biological processes such as oxidative stress, inflammation, and the formation of AGEs, polyphenolic compounds play a crucial role in reducing the risk and severity of diabetic complications, including nephropathy, retinopathy, and cardiovascular disease. Additionally, the ability of these compounds to enhance insulin sensitivity, regulate blood glucose levels, and improve overall metabolic health further underscores the promising role of phenolic compounds in diabetes therapy. The accumulation of evidence from in vitro, in vivo, and clinical studies highlights the effectiveness of phenolic compounds in enhancing glucose metabolism, mitigating oxidative stress, and counteracting the detrimental effects of AGEs. These compounds, such as resveratrol, curcumin, and quercetin, have demonstrated beneficial effects on insulin signaling, AGE–RAGE signaling, and efficient glycemic control, making phenolics valuable adjuncts to conventional diabetes treatments. Moreover, the anti-inflammatory effects of polyphenolic compounds help alleviate the chronic low-grade inflammation that contributes to the progression of diabetes and any subsequent complications. However, despite the promising biological activities of these compounds, the clinical application of polyphenolic compounds is hindered by challenges related to their bioavailability. The low solubility, rapid metabolism, and limited absorption of these compounds in the gastrointestinal tract reduce their efficacy at therapeutic doses. Therefore, addressing these bioavailability challenges through innovative formulation strategies, such as nanoparticle delivery systems, is essential for enhancing the therapeutic potential of polyphenolic compounds. Furthermore, additional research, particularly large-scale clinical trials, is necessary to establish optimal dosages, long-term safety, and efficacy in diverse diabetic populations.
As the body of research continues to grow, polyphenolic compounds hold great promise not only as therapeutic agents in the management of diabetes but also as preventative measures to reduce the risk of disease progression in high-risk individuals. The natural origin of these compounds, combined with their potential to target multiple pathways involved in diabetes pathogenesis, makes them an attractive option for inclusion in diabetes care strategies. Ongoing advancements in formulation techniques and clinical research may make polyphenolic compounds an integral part of diabetes management, providing patients with a complementary and effective treatment option.
Author Contributions
Data collections, data analysis, writing, original draft preparation, resources, software, validation, visualization, Writing, review, and editing W.Z. and A.A.; conceptualization, supervision, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maity, M.; Kumari, A. Understanding Diabetes Mellitus: A Descriptive Study of Its Types, Causes and Health Impacts. Bharati Int. J. Multidiscip. Res. Dev. 2025, 3, 34–39. [Google Scholar]
- Panjiyev, J. Etiological Factors and Treatment Principles of Diabetes Mellitus. Mod. Sci. Res. 2025, 4, 1788–1794. [Google Scholar]
- Vashishth, D.; Dhaliwal, R.; Rubin, M. AGEs (Advanced Glycation End-products) in bone come of age. Bone 2025, 190, 117301. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Kim, Y.S. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z. Advanced glycation end products: Potential mechanism and therapeutic target in cardiovascular complications under diabetes. Oxidative Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Ribeiro, P.V.M.; Tavares, J.F.; Costa, M.A.C.; Mattar, J.B.; Alfenas, R.C.G. Effect of reducing dietary advanced glycation end products on obesity-associated complications: A systematic review. Nutr. Rev. 2019, 77, 725–734. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Alam, M.R.; Kamal, M.A.; Seo, K.J.; Singh, L.R. AGE-RAGE axis culminates into multiple pathogenic processes: A central road to neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1155175. [Google Scholar] [CrossRef]
- Rezatabar, S.; Karimian, A.; Rameshknia, V.; Parsian, H.; Majidinia, M.; Kopi, T.A.; Bishayee, A.; Sadeghinia, A.; Yousefi, M.; Monirialamdari, M.; et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell. Physiol. 2019, 234, 14951–14965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Gong, N. Role of the PI3K/Akt signaling pathway in liver ischemia reperfusion injury: A narrative review. Ann. Palliat. Med. 2022, 11, 806–817. [Google Scholar]
- Hu, H.; Jiang, H.; Zhu, L.; Wu, X.; Han, C. Accumulation of advanced glycation endproducts and subclinical inflammation in deep tissues of adult patients with and without diabetes. Can. J. Diabetes 2018, 42, 525–532.E4. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A.M. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.F.; Justino, G.C. Structural analysis of flavonoids and related compounds-a review of spectroscopic applications. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; InTech: Vienna, Austria, 2012; p. 33. [Google Scholar]
- Santos-Buelga, C.; Feliciano, A.S. Flavonoids: From structure to health issues. 2017, 22, 477. Molecules 2017, 22(3), 477. [CrossRef]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids a. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [PubMed]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar]
- Han, Z.; Zhu, M.; Wan, X.; Zhai, X.; Ho, C.-T.; Zhang, L. Food polyphenols and Maillard reaction: Regulation effect and chemical mechanism. Crit. Rev. Food Sci. Nutr. 2024, 64, 4904–4920. [Google Scholar]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. [Google Scholar]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—A review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Caro-Ordieres, T.; Marín-Royo, G.; Opazo-Ríos, L.; Jiménez-Castilla, L.; Moreno, J.A.; Gómez-Guerrero, C.; Egido, J. The coming age of flavonoids in the treatment of diabetic complications. J. Clin. Med. 2020, 9, 346. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Gambardella, L.; Straface, E.; Cafeo, G.; Russo, M.; Caruso, D.; Dugo, P.; Dossena, S.; Marino, A.; et al. Anion exchanger1 (AE1/SLC4A1) function is impaired in red blood cells from prediabetic subjects: Potential benefits of finger lime (Citrus australasica, Faustrime cultivar) juice extract. Cell Biochem. Funct. 2024, 42, e4105. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxidative Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Novel delivery systems of polyphenols and their potential health benefits. Pharmaceuticals 2021, 14, 946. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Cell signaling and receptors in toxicity of advanced glycation end products (AGEs): α-dicarbonyls, radicals, oxidative stress and antioxidants. J. Recept. Signal Transduct. 2011, 31, 332–339. [Google Scholar] [CrossRef]
- Provost, J. The Maillard Reaction. In Food Aroma Evolution; CRC Press: Boca Raton, FL, USA, 2019; pp. 281–291. [Google Scholar]
- Fallavena, L.P.; Rodrigues, N.P.; Marczak, L.D.F.; Mercali, G.D. Formation of advanced glycation end products by novel food processing technologies: A review. Food Chem. 2022, 393, 133338. [Google Scholar] [CrossRef]
- Yamagishi, S.-i.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced glycation end products: A molecular target for vascular complications in diabetes. Mol. Med. 2015, 21, S32–S40. [Google Scholar] [CrossRef]
- Yamagishi, S.-i. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp. Gerontol. 2011, 46, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, P.; Rabbani, G.; Khan, R. The role of advanced glycation end products in various types of neurodegenerative disease: A therapeutic approach. Cell. Mol. Biol. Lett. 2014, 19, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Zhang, J. Mechanistic and Biological Insights into Carbohydrate and Lipid Modifications of Proteins. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2011. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=case1308315383 (accessed on 12 October 2025).
- Wrobel, K.; Wrobel, K.; Ortiz, S.J.; Escobosa, A.R.C. What are AGEs, their chemical structure, and how can they be measured. In Dietary AGEs and Their Role in Health and Disease; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–18. [Google Scholar]
- Khan, M.I.; Ashfaq, F.; Alsayegh, A.A.; Hamouda, A.; Khatoon, F.; Altamimi, T.N.; Alhodieb, F.S.; Beg, M.M.A. Advanced glycation end product signaling and metabolic complications: Dietary approach. World J. Diabetes 2023, 14, 995. [Google Scholar] [CrossRef]
- Marshall, C.B. Rethinking glomerular basement membrane thickening in diabetic nephropathy: Adaptive or pathogenic? Am. J. Physiol.-Ren. Physiol. 2016, 311, F831–F843. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Del Turco, S.; Basta, G. An update on advanced glycation endproducts and atherosclerosis. Biofactors 2012, 38, 266–274. [Google Scholar] [CrossRef]
- Mota, K.O.; de Vasconcelos, C.M.L.; Kirshenbaum, L.A.; Dhalla, N.S. The Role of Advanced Glycation End-Products in the Pathophysiology and Pharmacotherapy of Cardiovascular Disease. Int. J. Mol. Sci. 2025, 26, 7311. [Google Scholar] [CrossRef]
- Steens, I.L.M.; Schram, M.T.; Houben, A.J.H.M.; Berendschot, T.T.J.M.; Koster, A.; Bosma, H.; Eussen, S.J.P.M.; de Galan, B.E.; van Sloten, T.T. Type 2 diabetes and depression via microvascular dysfunction, neurodegeneration, inflammation, advanced glycation end products (AGEs), arterial stiffness. Diabetes Obes. Metab. 2025, 27, 4847–4858. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Tian, N.; Xie, J. Exploring the protective effects of Spinosin against advanced glycation end product-induced cellular damage through modulation RAGE/MAPK/NF-κB pathway. Food Biosci. 2025, 64, 105951. [Google Scholar] [CrossRef]
- Vianello, E.; Beltrami, A.P.; Aleksova, A.; Janjusevic, M.; Fluca, A.L.; Corsi Romanelli, M.M.; La Sala, L.; Dozio, E. The advanced glycation end-products (AGE)–receptor for AGE system (RAGE): An inflammatory pathway linking obesity and cardiovascular diseases. Int. J. Mol. Sci. 2025, 26, 3707. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, T.; Wang, Y.; Zhang, C.; Chu, Y.; Guo, Y.; Xi, J.; Jiao, D.; Li, B.; Xie, C.; et al. Paeoniflorin modulates AGEs/RAGE/P38MAPK/ERK/mTOR autophagy pathway to improve cognitive dysfunction in MRL/lpr mice. Int. J. Biol. Macromol. 2025, 307, 141765. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Pathological role of RAGE underlying progression of various diseases: Its potential as biomarker and therapeutic target. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 3467–3487. [Google Scholar] [CrossRef]
- Ayoub, S.; Arabi, M.; Al-Najjar, Y.; Laswi, I.; Outeiro, T.F.; Chaari, A. Glycation in Alzheimer’s Disease and Type 2 Diabetes: The Prospect of Dual Drug Approaches for Therapeutic Interventions. Mol. Neurobiol. 2025, 62, 14859–14882. [Google Scholar] [CrossRef]
- Flores-Estrada, J.; Cano-Martínez, A.; Ibarra-Lara, L.; Jiménez, A.; Palacios-Reyes, C.; García, L.J.P.; Ortiz-López, M.G.; Rodríguez-Peña, O.N.; Hernández-Portilla, L.B. Spinach Extract Reduces Kidney Damage in Diabetic Rats by Impairing the AGEs/RAGE Axis. Int. J. Mol. Sci. 2025, 26, 4730. [Google Scholar] [CrossRef]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in synaptic function and dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Anshu, T.; Maharana, K.C.; Sinha, S. Molecular insights into diabetic wound healing: Focus on Wnt/β-catenin and MAPK/ERK signaling pathways. Cytokine 2025, 191, 156957. [Google Scholar] [CrossRef]
- Kavitha, S.A.; Zainab, S.; Muthyalaiah, Y.S.; John, C.M.; Arockiasamy, S. Mechanism and implications of advanced glycation end products (AGE) and its receptor RAGE axis as crucial mediators linking inflammation and obesity. Mol. Biol. Rep. 2025, 52, 556. [Google Scholar] [CrossRef]
- Wang, Z.-j.; Wang, W.; Jia, K.-x.; Zhang, H.-b.; Wang, J.-a.; Chen, C. The influence of PPARγ mediated MAPK and NF-κB activation in AGEs stimulated apoptosis and autophagy in human chondrocytes. J. Orthop. 2025, 66, 204–212. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.; Su, Y.; Lian, X.; Jiang, L.; Li, Z.; Zhang, M.; Gao, Y.; Zhang, H.; Zhu, X.; et al. Screening and identification of host signaling pathways for alleviating influenza-induced production of pro-inflammatory cytokines, IP-10, IL-8, and MCP-1, using a U937 cell-based influenza model. Front. Microbiol. 2025, 16, 1535002. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Liu, L.; Huang, X.; Cai, Y.; Liao, L.; Min, X.; Gu, Y. The mechanistic study of quercetin in the treatment of alcoholic brain injury via the JNK/P38 MAPK signaling pathway. Apoptosis 2025, 30, 1875–1892. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, T.; Wang, K.; Liang, X.; Zong, X.; Yang, H.; Li, Z. Effect of imatinib on lipopolysaccharide-induced acute lung injury and endothelial dysfunction through the P38 MAPK and NF-κB signaling pathways in vivo and in vitro. Respir. Physiol. Neurobiol. 2025, 333, 104388. [Google Scholar] [CrossRef] [PubMed]
- Hota, A.; Bennett, A.M. MAP kinase phosphatases in metabolic diseases. Trends Endocrinol. Metab. 2025. In press. [Google Scholar] [CrossRef]
- Ritu; Goyal, R.K.; Narwal, S.; Bhatt, A.; Sehrawat, R.; Devi, P.; Gulati, M.; Singla, R.K. Role of MMP-9 and NF-κB in diabetic retinopathy: Progression and potential role of bioflavonoids in the mitigation of diabetic retinopathy. Curr. Med. Chem. 2025, 32, 4992–5007. [Google Scholar] [CrossRef] [PubMed]
- Serasanambati, M.; Chilakapati, S.R. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. S. Indian J. Biol. Sci. 2016, 2, 368–387. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Sindhughosa, D.A.; Pranamartha, A. The involvement of proinflammatory cytokines in diabetic nephropathy: Focus on interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α) signaling mechanism. Bali Med. J. 2017, 6, 44–51. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef] [PubMed]
- Zand, H.; Morshedzadeh, N.; Naghashian, F. Signaling pathways linking inflammation to insulin resistance. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S307–S309. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, L.; Jin, C.; Lin, J.; Bi, X.; Shen, Z.; Yu, L.; Fu, G.; Zhu, J. Advanced glycation end products upregulates the expression of connexin43 via ERK MAPK and PI3K/Akt pathways in human endothelial cells. Int. J. Clin. Exp. Med. 2019, 12, 1545–1553. [Google Scholar]
- Zhang, Z.; Liu, H.; Liu, J. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Res. Clin. Pract. 2019, 156, 107092. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Liang, F.-Q.; Lin, S.-Z.; Zeng, K.; Cai, H.-D.; Liang, M. Advanced glycation end products induce autophagy in endothelial cells through oxidative stress and regulation of the RhoA-mTOR signaling pathway. Immunobiology 2025, 230, 153088. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef]
- Passarelli, M.; Machado, U.F. AGEs-induced and endoplasmic reticulum stress/inflammation-mediated regulation of GLUT4 expression and atherogenesis in diabetes mellitus. Cells 2021, 11, 104. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Zhang, Y.; Geng, W.; Liu, W.; Gao, Y.; Li, S.; Wang, K.; Wu, X.; Kang, L.; et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell. Mol. Med. 2017, 21, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; You, H.; Li, X.-M.; Liu, T.-H.; Wang, P.; Wang, B.-E. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 1365–1370. [Google Scholar] [CrossRef]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.-D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef]
- Idris, I.; Gray, S.; Donnelly, R. Protein kinase C activation: Isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia 2001, 44, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.W.; Pula, G.; Hers, I.; Crosby, D.; Jones, M.L. PKC-interacting proteins: From function to pharmacology. Trends Pharmacol. Sci. 2004, 25, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; King, G.L. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. 2007, 72, S49–S53. [Google Scholar] [CrossRef]
- Aiello, L.P. The potential role of PKC β in diabetic retinopathy and macular edema. Surv. Ophthalmol. 2002, 47, S263–S269. [Google Scholar] [CrossRef]
- Tang, Q.; Buonfiglio, F.; Böhm, E.W.; Zhang, L.; Pfeiffer, N.; Korb, C.A.; Gericke, A. Diabetic retinopathy: New treatment approaches targeting redox and immune mechanisms. Antioxidants 2024, 13, 594. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem.-Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.-L.; Liu, T.-T.; Lan, H.-Y. TGF-beta as a master regulator of diabetic nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef]
- Hela, F.; Aguayo-Mazzucato, C. Interaction between autophagy and senescence in pancreatic beta cells. Biology 2023, 12, 1205. [Google Scholar] [CrossRef]
- Sruthi, C.R.; Raghu, K.G. Advanced glycation end products and their adverse effects: The role of autophagy. J. Biochem. Mol. Toxicol. 2021, 35, e22710. [Google Scholar] [CrossRef]
- Piperi, C.; Goumenos, A.; Adamopoulos, C.; Papavassiliou, A.G. AGE/RAGE signalling regulation by miRNAs: Associations with diabetic complications and therapeutic potential. Int. J. Biochem. Cell Biol. 2015, 60, 197–201. [Google Scholar]
- Parveen, A.; Sultana, R.; Lee, S.M.; Kim, T.H.; Kim, S.Y. Phytochemicals against anti-diabetic complications: Targeting the advanced glycation end product signaling pathway. Arch. Pharmacal Res. 2021, 44, 378–401. [Google Scholar] [CrossRef]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A., Jr. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Nedić, O.; Rattan, S.I.S.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic. Res. 2013, 47, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-i.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 663–671. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- Nie, T.; Cooper, G.J.S. Mechanisms underlying the antidiabetic activities of polyphenolic compounds: A review. Front. Pharmacol. 2021, 12, 798329. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventós, R.M. Phenolic compounds: Chemistry and occurrence in fruits and vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; Wiley: Hoboken, NJ, USA, 2009; pp. 53–88. [Google Scholar]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Golovinskaia, O.; Wang, C.-K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- de Ancos, B.; González, E.M.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; bin Asad, M.H.H. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef]
- Mohammadi, N.; Guo, Y.; Wang, K.; Granato, D. Macroporous resin purification of phenolics from Irish apple pomace: Chemical characterization, and cellular antioxidant and anti-inflammatory activities. Food Chem. 2024, 437, 137815. [Google Scholar] [CrossRef] [PubMed]
- Cano-Lou, J.; Millán-Laleona, A.; Candrea, R.; Les, F.; Pina, A.; Caprioli, G.; López, V. Apple peels as an edible source of phenolic bioactive compounds with antidiabetic and antiglycation properties. Food Funct. 2025, 16, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.; Armstrong, E.; Moses, A.; Fahlman, R.; Koosha, H.; Yager, J.Y. Broccoli, Kale, and radish sprouts: Key phytochemical constituents and DPPH free radical scavenging activity. Molecules 2023, 28, 4266. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.-M.; Kammiovirta, K.; Oksman-Caldentey, K.M. Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Delgado, Y.; Cassé, C.; Ferrer-Acosta, Y.; Suárez-Arroyo, I.J.; Rodríguez-Zayas, J.; Torres, A.; Torres-Martínez, Z.; Pérez, D.; González, M.J.; Velázquez-Aponte, R.A.; et al. Biomedical effects of the phytonutrients turmeric, garlic, cinnamon, graviola, and oregano: A comprehensive review. Appl. Sci. 2021, 11, 8477. [Google Scholar] [CrossRef]
- Ho, S.C.; Chang, P.W. Inhibitory effects of several spices on inflammation caused by advanced glycation endproducts. Am. J. Plant Sci. 2012, 3(7A), 998–1002. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kruk, I.; Kładna, A.; Lichszteld, K.; Michalska, T. Scavenging effects of phenolic compounds on reactive oxygen species. Orig. Res. Biomol. 2007, 86, 222–230. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The triple defensive barrier of phenolic compounds against the lipid oxidation-induced damage in food products. Trends Food Sci. Technol. 2016, 54, 165–174. [Google Scholar] [CrossRef]
- Pannucci, E.; Spagnuolo, L.; De Gara, L.; Santi, L.; Dugo, L. Phenolic compounds as preventive and therapeutic agents in Diabetes-Related oxidative stress, inflammation, advanced glycation End-Products production and insulin sensitivity. Discov. Med. 2023, 35, 715–732. [Google Scholar] [CrossRef]
- Shah, S.; Narang, R.; Singh, V.J.; Pilli, G.; Nayak, S.K. A review on anticancer profile of flavonoids: Sources, chemistry, mechanisms, structure-activity relationship and anticancer activity. Curr. Drug Res. Rev. 2023, 15, 122–148. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Wieser, V.; Moschen, A.R.; Tilg, H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Et Ther. Exp. 2013, 61, 119–125. [Google Scholar] [CrossRef]
- Pal, P.P.; Begum, A.S.; Basha, S.A.; Araya, H.; Fujimoto, Y. New natural pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) and iNOS inhibitors identified from Penicillium polonicum through in vitro and in vivo studies. Int. Immunopharmacol. 2023, 117, 109940. [Google Scholar]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Hu, X.; Tao, N.; Wang, M. Impact and inhibitory mechanism of phenolic compounds on the formation of toxic Maillard reaction products in food. Front. Agric. Sci. Eng. 2018, 5, 321–329. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial effects of epigallocatechin-3-O-gallate, chlorogenic acid, resveratrol, and curcumin on neurodegenerative diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, I.-H.; Kim, C.-S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharmacal Res. 2011, 34, 495–500. [Google Scholar] [CrossRef]
- Alizadeh, M.; Kheirouri, S. Curcumin against advanced glycation end products (AGEs) and AGEs-induced detrimental agents. Crit. Rev. Food Sci. Nutr. 2019, 59, 1169–1177. [Google Scholar] [CrossRef]
- Markiewicz, E.; Jerome, J.; Mammone, T.; Idowu, O.C. Anti-glycation and anti-aging properties of resveratrol derivatives in the in-vitro 3D models of human skin. Clin. Cosmet. Investig. Dermatol. 2022, ume 15, 911–927. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Bednarska, K.; Kowalczyk, A. In vitro antiglycation and methylglyoxal trapping effect of peppermint leaf (Mentha × piperita L.) and its polyphenols. Molecules 2023, 28, 2865. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, X.; Xie, T.; Li, W.; Xie, D.; Zhang, G.; Xiao, Y.; Zhang, L. Amadori compounds (N-(1-Deoxy-D-fructos-1-yl)-amino acid): The natural transition metal ions (Cu2+, Fe2+, Zn2+) chelators formed during food processing. LWT 2024, 191, 115600. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Kausar, M.A.; Singh, R.; Siddiqui, A.J.; Akhter, A. The role of glyoxalase in glycation and carbonyl stress induced metabolic disorders. Curr. Protein Pept. Sci. 2020, 21, 846–859. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, Y.-q.; Lu, Q.; Du, L.; Yin, X.-x.; Liu, Y.-w. Enhancement of glyoxalase 1, a polyfunctional defense enzyme, by quercetin in the brain in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Ferreira, C.; Alves Passos, C.L.; Silva, J.L.; Fialho, E. Resveratrol, curcumin and piperine alter human glyoxalase 1 in MCF-7 breast cancer cells. Int. J. Mol. Sci. 2020, 21, 5244. [Google Scholar] [CrossRef]
- Laus, M.N.; Blando, F.; Soccio, M. Glyoxalase I assay as a possible tool for evaluation of biological activity of antioxidant-rich plant extracts. Plants 2023, 12, 1150. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Bek, Z.A.; Sarıbaş, G.S.; Özoğul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. TLR4, Rage, and p-JNK/JNK mediated inflammatory aggression in osteoathritic human chondrocytes are counteracted by redox-sensitive phenolic olive compounds: Comparison with ibuprofen. J. Tissue Eng. Regen. Med. 2020, 14, 1841–1857. [Google Scholar]
- Endale, M.; Park, S.-C.; Kim, S.; Kim, S.-H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar]
- Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Front. Endocrinol. 2022, 13, 990299. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, R.; Shi, L.; Wang, S.; Liang, Y.; Zhang, Z. Anti-glycation and anti-inflammatory activities of anthocyanins from purple vegetables. Food Funct. 2023, 14, 2034–2044. [Google Scholar] [PubMed]
- Jeong, J.-W.; Lee, W.S.; Shin, S.C.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar]
- Peng, X.; Wu, G.; Zhao, A.; Huang, K.; Chai, L.; Natarajan, B.; Yang, S.; Chen, H.; Lin, C. Synthesis of novel caffeic acid derivatives and their protective effect against hydrogen peroxide induced oxidative stress via Nrf2 pathway. Life Sci. 2020, 247, 117439. [Google Scholar] [CrossRef]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Ferulic acid prevents methylglyoxal-induced protein glycation, DNA damage, and apoptosis in pancreatic β-cells. J. Physiol. Biochem. 2017, 73, 121–131. [Google Scholar]
- Li, L.; Liu, R.; Meng, X.; Xu, Z.; Liu, S.; Liu, J.; Zhang, Y.; Wang, H. Higher inhibition of caffeic acid than chlorogenic acid against methylglyoxal and advanced glycosylation end products induced oxidative damage. Food Sci. Hum. Wellness 2024. [Google Scholar] [CrossRef]
- Pilar, B.; Güllich, A.; Oliveira, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective role of flaxseed oil and flaxseed lignan secoisolariciresinol diglucoside against oxidative stress in rats with metabolic syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef]
- Wang, Y.; Fofana, B.; Roy, M.; Ghose, K.; Yao, X.-H.; Nixon, M.-S.; Nair, S.; Nyomba, G.B.L. Flaxseed lignan secoisolariciresinol diglucoside improves insulin sensitivity through upregulation of GLUT4 expression in diet-induced obese mice. J. Funct. Foods 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Sun, C.; McIntyre, K.; Saleem, A.; Haddad, P.S.; Arnason, J.T. The relationship between antiglycation activity and procyanidin and phenolic content in commercial grape seed products. Can. J. Physiol. Pharmacol. 2012, 90, 167–174. [Google Scholar] [CrossRef]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In vitro antioxidant and anti-glycation activity of resveratrol and its novel triester with trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.E.; Casanova, L.M.; Costa, S.S. Bioavailability of phenolic compounds: A major challenge for drug development? Rev. Fitos 2015, 9, 55–67. [Google Scholar]
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: Vienna, Austria, 2019. [Google Scholar]
- Chen, L.; Cao, H.; Xiao, J. metabolomics. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–67. [Google Scholar]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A.; Pintado, M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Marathe, S.J.; Dedhia, N.; Singhal, R.S. Esterification of sugars and polyphenols with fatty acids: Techniques, bioactivities, and applications. Curr. Opin. Food Sci. 2022, 43, 163–173. [Google Scholar] [CrossRef]
- Murugesan, N.; Damodaran, C.P. Antioxidant activity of synergistic quercetin resveratrol. J. Mol. Chem. 2023, 3, 581. [Google Scholar]
- Nishimoto-Sauceda, D.; Romero-Robles, L.E.; Antunes-Ricardo, M. Biopolymer nanoparticles: A strategy to enhance stability, bioavailability, and biological effects of phenolic compounds as functional ingredients. J. Sci. Food Agric. 2022, 102, 41–52. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; Volume 595, pp. 105–125. [Google Scholar]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Chaudhuri, A.; Ghanim, H.; Mohanty, P. Anti-inflammatory effects of insulin and the pro-inflammatory effects of glucose. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 293–301. [Google Scholar] [CrossRef]
- Chojnacka, K.; Lewandowska, U. Inhibition of pro-inflammatory cytokine secretion by polyphenol-rich extracts in macrophages via NF-κB pathway. Food Rev. Int. 2023, 39, 5459–5478. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Jiang, J.-G.; Huang, C.-L.; Zhu, W.; Zheng, C.-Y. Polyphenols from blossoms of Citrus aurantium L. var. amara Engl. show significant anti-complement and anti-inflammatory effects. J. Agric. Food Chem. 2017, 65, 9061–9068. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Del Turco, S.; Basta, G. Can dietary polyphenols prevent the formation of toxic compounds from Maillard reaction? Curr. Drug Metab. 2016, 17, 598–607. [Google Scholar] [CrossRef]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The antiglycoxidative ability of selected phenolic compounds—An in vitro study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Ouyang, J.; Li, M.; Rengasamy, K.R.R.; Liu, Z. Effects of green tea polyphenol extract and epigallocatechin-3-O-gallate on diabetes mellitus and diabetic complications: Recent advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 5719–5747. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a potential adjuvant to alleviates diabetic retinal injury via reducing oxidative stress and maintaining Nrf2 pathway homeostasis. Front. Pharmacol. 2021, 12, 796565. [Google Scholar] [CrossRef]
- Chethan, S.; Dharmesh, S.M.; Malleshi, N.G. Inhibition of aldose reductase from cataracted eye lenses by finger millet (Eleusine coracana) polyphenols. Bioorganic Med. Chem. 2008, 16, 10085–10090. [Google Scholar] [CrossRef]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Parvu, A.E.; Pop, N.; Marín, F.Z.; Parvu, M. Polyphenolic compounds, antioxidant, and cardioprotective effects of pomace extracts from Fetească neagră cultivar. Oxidative Med. Cell. Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wedick, N.M.; Tworoger, S.S.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; Van Dam, R.M. Urinary excretion of select dietary polyphenol metabolites is associated with a lower risk of type 2 diabetes in proximate but not remote follow-up in a prospective investigation in 2 cohorts of US women. J. Nutr. 2015, 145, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Tapola, N.; Törrönen, R.; Sarkkinen, E. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br. J. Nutr. 2009, 103, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Wang, W.-Y.; Fan, W.-Y.; Deng, Q.; Wang, X. Tea consumption and risk of type 2 diabetes: A dose–response meta-analysis of cohort studies. Br. J. Nutr. 2014, 111, 1329–1339. [Google Scholar] [CrossRef]
- Paquette, M.; Larqué, A.S.M.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Brudzynski, K.; Maldonado-Alvarez, L. Polyphenol-protein complexes and their consequences for the redox activity, structure and function of honey. A current view and new hypothesis-a review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Ham, S.-K.; Shigenaga, M.K.; Han, O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of natural polyphenols on skin and hair health: A review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as antioxidant/pro-oxidant compounds and donors of reducing species: Relationship with human antioxidant metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and dietary polyphenols. Oxidative Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. 1994, 54, 1895s–1898s. [Google Scholar]
- Alaparthi, M.D.; Gopinath, G.; Bandaru, S.; Sankeshi, V.; Mangalarapu, M.; Nagamalla, S.S.; Sudhakar, K.; Rani, A.R.; Sagurthi, S.R. Virtual screening of RAGE inhibitors using molecular docking. Bioinformation 2016, 12, 124. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Dong, A.; Li, N.; Ma, Y.; Zhang, S.; Deng, Y.; Chen, S.; Zhang, M. Mechanistic study of Schisandra chinensis fruit mixture based on network pharmacology, molecular docking and experimental validation to improve the inflammatory response of DKD through AGEs/RAGE signaling pathway. Drug Des. Dev. Ther. 2023, ume 17, 613–632. [Google Scholar] [CrossRef]
- Manchalkar, M.; Patil, C.C. Computational Approaches To Phytochemical Drug Discovery: Bridging Plant Genomics And Pharmaceutical Chemistry. In Botanical Insights: From Traditional Knowledge to Modern Science; Nature Light Publication: New York, NY, USA, 2025; p. 19. [Google Scholar]
- Wang, W.; Zhao, W.; Song, X.; Wang, H.; Gu, L. Zhongfeng decoction attenuates cerebral ischemia-reperfusion injury by inhibiting autophagy via regulating the AGE-RAGE signaling pathway. J. Ethnopharmacol. 2025, 336, 118718. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Muthyalaiah, Y.S.; Arockiasamy, S.; Abhinand, P.A. Exploring the molecular interactions and binding affinity of resveratrol and calcitriol with RAGE and its intracellular proteins and kinases involved in colorectal cancer. J. Biomol. Struct. Dyn. 2024, 42, 10800–10823. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Xian, W.; Li, X.; Chen, Y.; Geng, J.; Wang, Q.; Gao, Q.; Tang, B.; Wang, H.; Kang, P. Mechanisms of Quercetin against atrial fibrillation explored by network pharmacology combined with molecular docking and experimental validation. Sci. Rep. 2022, 12, 9777. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shen, J.; Zhan, J.; Guo, L.; Ho, C.-T.; Li, S. Antiglycating effects of citrus flavonoids and associated mechanisms. Food Sci. Hum. Wellness 2024, 13, 2363–2372. [Google Scholar] [CrossRef]
- Badar, M.S.; Shamsi, S.; Ahmed, J.; Alam, M.A. Molecular dynamics simulations: Concept, methods, and applications. In Transdisciplinarity; Springer: Berlin/Heidelberg, Germany, 2022; pp. 131–151. [Google Scholar]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of molecular dynamics and related methods in drug discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef]
- Liao, S.-M.; Shen, N.-K.; Liang, G.; Lu, B.; Lu, Z.-L.; Peng, L.-X.; Zhou, F.; Du, L.-Q.; Wei, Y.-T.; Zhou, G.-P.; et al. Inhibition of α-amylase activity by Zn2+: Insights from spectroscopy and molecular dynamics simulations. Med. Chem. 2019, 15, 510–520. [Google Scholar] [CrossRef]
- Nageswari, P.; Swathi, K. In silico docking and Molecular Dynamic (MD) simulations studies of selected phytochemicals against Human Glycolate Oxidase (hGOX) and Oxalate oxidase (OxO). Drug Res. 2023, 73, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Chen, W.; Wang, X.; Li, L.; Peng, Z.; Mu, S.; You, M.; Xu, H. RAGE: A potential target for Epimedium’s anti-neuroinflammation role in vascular dementia—Insights from network pharmacology and molecular simulation. J. Biomol. Struct. Dyn. 2024, 42, 10856–10875. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Li, W.; Zhao, X.; Zhang, M.; Wang, M.; Xie, J. Effects and mechanisms of dietary polyphenols in ameliorating glycolipid metabolic disorders: Inhibition of advanced glycation end products. Fitoterapia 2025, 184, 106668. [Google Scholar] [CrossRef]
- Leung, E.L.; Cao, Z.-W.; Jiang, Z.-H.; Zhou, H.; Liu, L. Network-based drug discovery by integrating systems biology and computational technologies. Brief. Bioinform. 2013, 14, 491–505. [Google Scholar] [CrossRef]
- Arrell, D.K.; Terzic, A. Network systems biology for drug discovery. Clin. Pharmacol. Ther. 2010, 88, 120–125. [Google Scholar] [CrossRef]
- Zhao, S.; Iyengar, R. Systems pharmacology: Network analysis to identify multiscale mechanisms of drug action. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 505–521. [Google Scholar] [CrossRef]
- Berger, S.I.; Iyengar, R. Network analyses in systems pharmacology. Bioinformatics 2009, 25, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Jia, N.; Sun, Q. A network pharmacology study on mechanism of resveratrol in treating preeclampsia via regulation of AGE-RAGE and HIF-1 signalling pathways. Front. Endocrinol. 2023, 13, 1044775. [Google Scholar] [CrossRef]
- Liu, T.-h.; Tu, W.-q.; Tao, W.-c.; Liang, Q.-e.; Xiao, Y.; Chen, L.-g. Verification of Resveratrol Inhibits Intestinal Aging by Downregulating ATF4/Chop/Bcl-2/Bax Signaling Pathway: Based on Network Pharmacology and Animal Experiment. Front. Pharmacol. 2020, 11, 2020. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Wang, N.; Tan, H.-Y.; Zhang, C.; Li, S.; Tang, G.; Feng, Y. Network pharmacology–based analysis and experimental exploration of antidiabetic mechanisms of gegen Qinlian decoction. Front. Pharmacol. 2021, 12, 649606. [Google Scholar] [CrossRef]
- Peng, W.; Yang, Y.; Lu, H.; Shi, H.; Jiang, L.; Liao, X.; Zhao, H.; Wang, W.; Liu, J. Network pharmacology combines machine learning, molecular simulation dynamics and experimental validation to explore the mechanism of acetylbinankadsurin A in the treatment of liver fibrosis. J. Ethnopharmacol. 2024, 323, 117682. [Google Scholar] [CrossRef] [PubMed]
- Ekowati, J.; Tejo, B.A.; Maulana, S.; Kusuma, W.A.; Fatriani, R.; Ramadhanti, N.S.; Norhayati, N.; Nofianti, K.A.; Sulistyowaty, M.I.; Zubair, M.S.; et al. Potential utilization of phenolic acid compounds as anti-inflammatory agents through TNF-α convertase inhibition mechanisms: A network pharmacology, docking, and molecular dynamics approach. Acs Omega 2023, 8, 46851–46868. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, Z.; Wang, Z.; Gao, C.; Liang, Y.; Zeng, W.; Sun, W. Profiling of Potential Anti-Diabetic Active Compounds in White Tea: An Integrated Study of Polyphenol-Targeted Metabolomics, Network Pharmacology, and Computer Simulation. Foods 2024, 13, 3354. [Google Scholar] [CrossRef]
- Karnovsky, A.; Weymouth, T.; Hull, T.; Tarcea, V.G.; Scardoni, G.; Laudanna, C.; Sartor, M.A.; Stringer, K.A.; Jagadish, H.V.; Burant, C.; et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics 2012, 28, 373–380. [Google Scholar] [CrossRef]
- Koumakis, L.; Mizzi, C.; Potamias, G. Bioinformatics tools for data analysis. In Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 339–351. [Google Scholar]
- Wang, J.; Liu, H.; Xie, G.; Cai, W.; Xu, J. Identification of hub genes and key pathways of dietary advanced glycation end products-induced non-alcoholic fatty liver disease by bioinformatics analysis and animal experiments. Mol. Med. Rep. 2020, 21, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, Z.; Bai, W.; Wang, S.; Huang, C.; Li, N.; Yan, L.; Gu, Y.; Shao, F. Integrating bioinformatics and machine learning to elucidate the role of protein glycosylation-related genes in the pathogenesis of diabetic kidney disease. PLoS ONE 2025, 20, e0329640. [Google Scholar] [CrossRef] [PubMed]
- Cheemanapalli, S.; Chinthakunta, N.; Shaikh, N.M.; Shivaranjani, V.; Pamuru, R.R.; Chitta, S.K. Comparative binding studies of curcumin and tangeretin on up-stream elements of NF-kB cascade: A combined molecular docking approach. Netw. Model. Anal. Health Inform. Bioinform. 2019, 8, 15. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Identification of differentially expressed genes and enriched pathways in lung cancer using bioinformatics analysis. Mol. Med. Rep. 2019, 19, 2029–2040. [Google Scholar] [CrossRef]
- Cho, S.; Duong, V.-A.; Mok, J.-H.; Joo, M.; Park, J.-M.; Lee, H. Enrichment and analysis of glycated proteins. Rev. Anal. Chem. 2022, 41, 83–97. [Google Scholar] [CrossRef]
- Aryal, D.; Joshi, S.; Thapa, N.K.; Chaudhary, P.; Basaula, S.; Joshi, U.; Bhandari, D.; Rogers, H.M.; Bhattarai, S.; Sharma, K.R.; et al. Dietary phenolic compounds as promising therapeutic agents for diabetes and its complications: A comprehensive review. Food Sci. Nutr. 2024, 12, 3025–3045. [Google Scholar] [CrossRef]
- Ouamnina, A.; Alahyane, A.; Elateri, I.; Ouhammou, M.; Abderrazik, M. In vitro and molecular docking studies of Antiglycation potential of phenolic compounds in date palm (Phoenix dactylifera L.) fruit: Exploring local varieties in the food industry. Horticulturae 2024, 10, 657. [Google Scholar] [CrossRef]
- Akpoveso, O.-O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant phytochemicals as potential therapy for diabetic complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef]
- Aalim, H.; Shishir, M.R.I.; Yosri, N.; Arslan, M.; Tahir, H.E.; Hashim, S.B.H.; Karim, N.; Zhai, X.; Li, Z.; Zhou, C.; et al. Systematic review of the digestive fate of rice phenolic compounds: Insights into bioavailability, influencing factors, encapsulation strategies, and health implications. Trends Food Sci. Technol. 2025, 156, 104833. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Savadkouhi, N.; Ebrahimzadeh, M.A. Drug design strategies that aim to improve the low solubility and poor bioavailability conundrum in quercetin derivatives. Expert Opin. Drug Discov. 2023, 18, 1117–1132. [Google Scholar] [CrossRef]
- Kannan, K.; George, J.A.; Sahadevan, R.; Kothari, M.; Sadhukhan, S. Insights into one drug, multi-target aspects of polyphenols for diabetes management: In vitro, in vivo, and clinical evidence. Phytochem. Rev. 2024, 1–49, In press. [Google Scholar] [CrossRef]
- Baptista, F.; Paié-Ribeiro, J.; Almeida, M.; Barros, A.N. Exploring the role of phenolic compounds in chronic kidney disease: A systematic review. Molecules 2024, 29, 2576. [Google Scholar] [CrossRef]
- Kozłowska, A.; Nitsch-Osuch, A. Anthocyanins and type 2 diabetes: An update of human study and clinical trial. Nutrients 2024, 16, 1674. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Grammatiki, M.; Sagar, R.; Ajjan, R.A. Metformin: Is it still the first line in type 2 diabetes management algorithm? Curr. Pharm. Des. 2021, 27, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of high-insulin-induced skeletal muscle cell insulin resistance by resveratrol is linked to activation of AMPK and restoration of GLUT4 translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef]
- Lv, W.; Wang, X.; Xu, Q.; Lu, W. Mechanisms and characteristics of sulfonylureas and glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).