The Role of MALDI-TOF Mass Spectrometry in Photodynamic Therapy: From Photosensitizer Design to Clinical Applications
Abstract
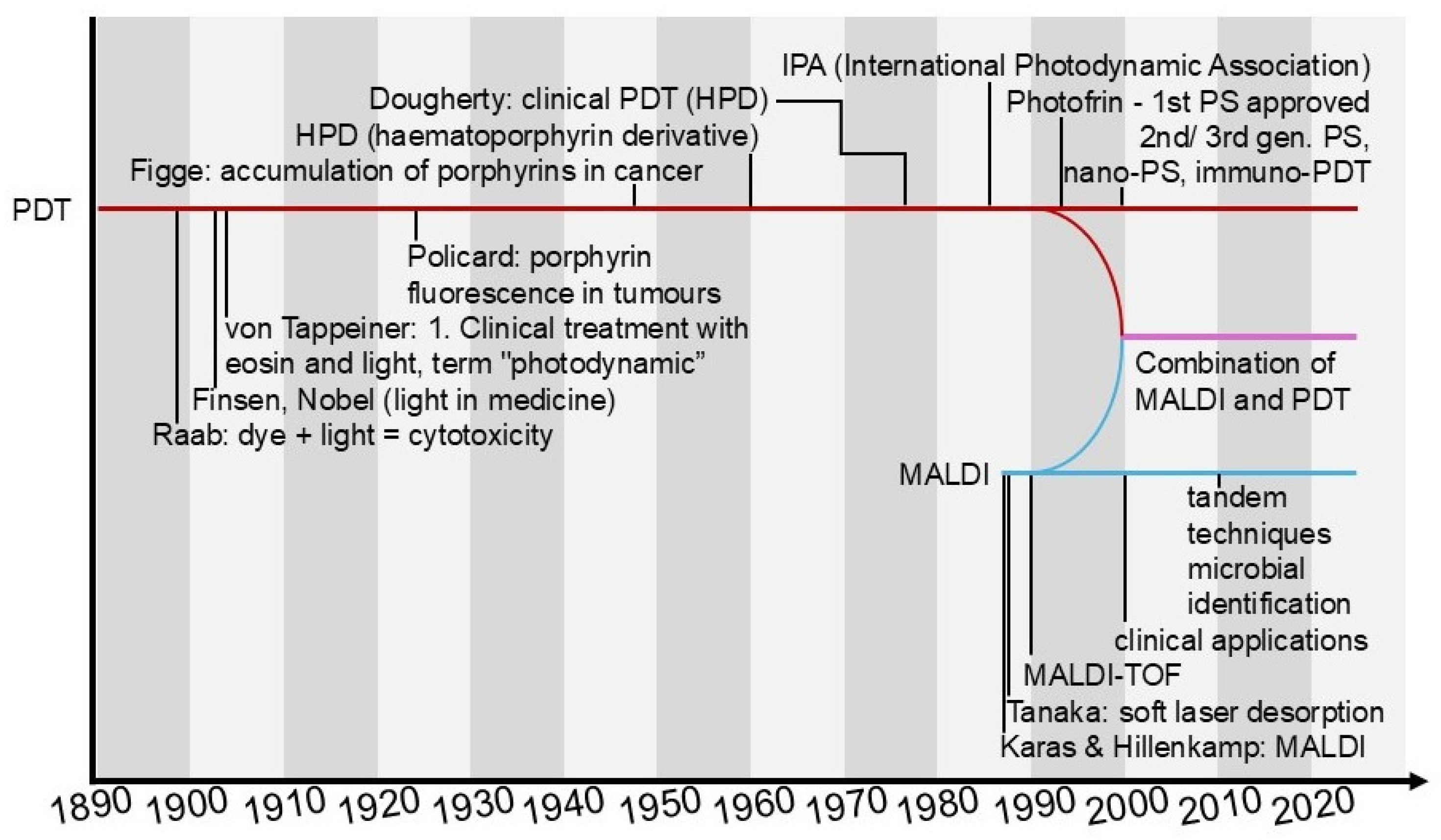
1. Introduction
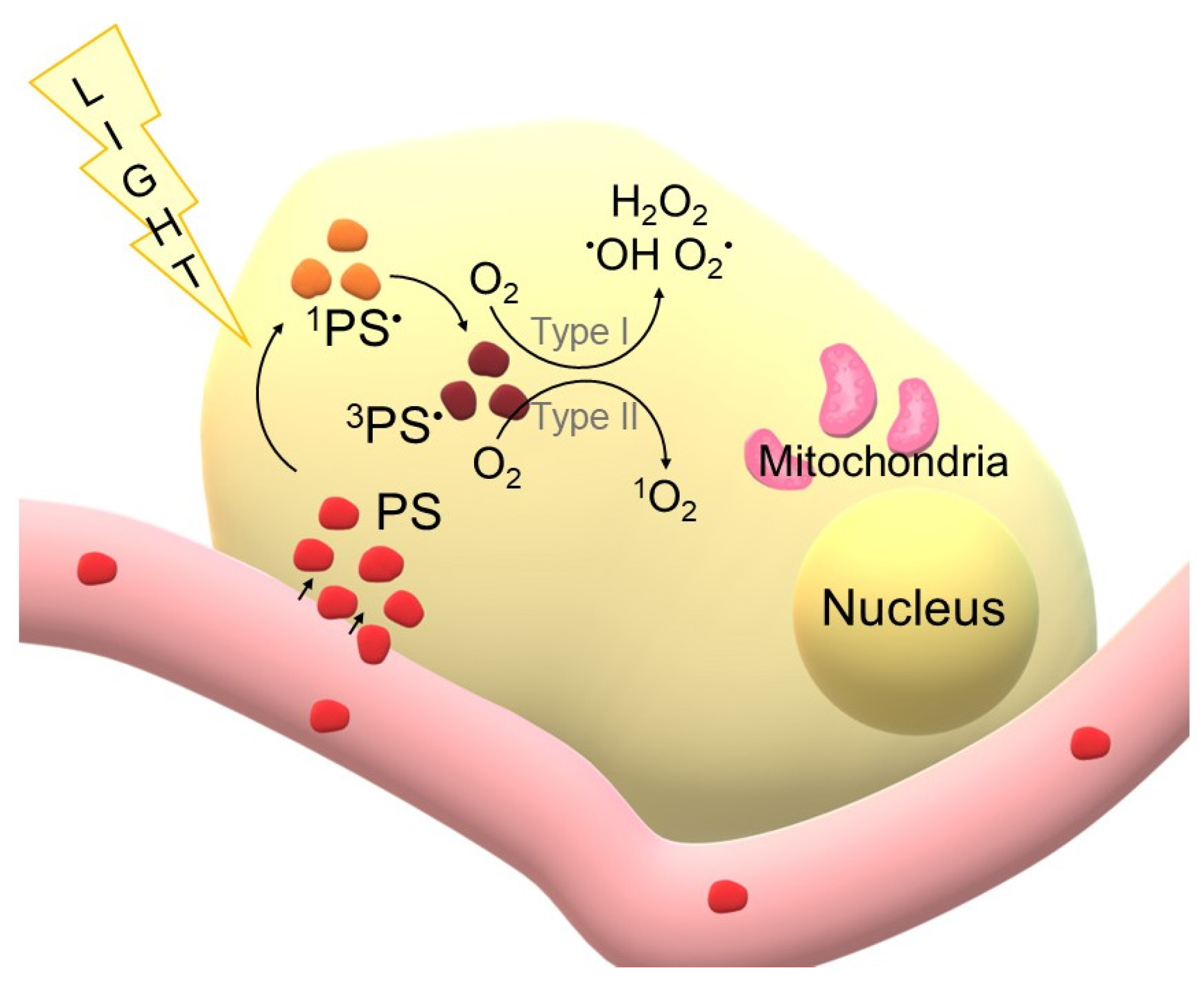
1.1. Photodynamic Therapy (PDT)
1.1.1. Principles of PDT
1.1.2. PDT Components
1.1.3. Models Undergoing PDT
1.1.4. Latest Developments in PDT
1.2. Matrix-Assisted Laser Desorption/Ionisation (MALDI)
1.2.1. Principles of MALDI
1.2.2. MALDI System Components and Research Materials
1.2.3. Recent Applications of MALDI
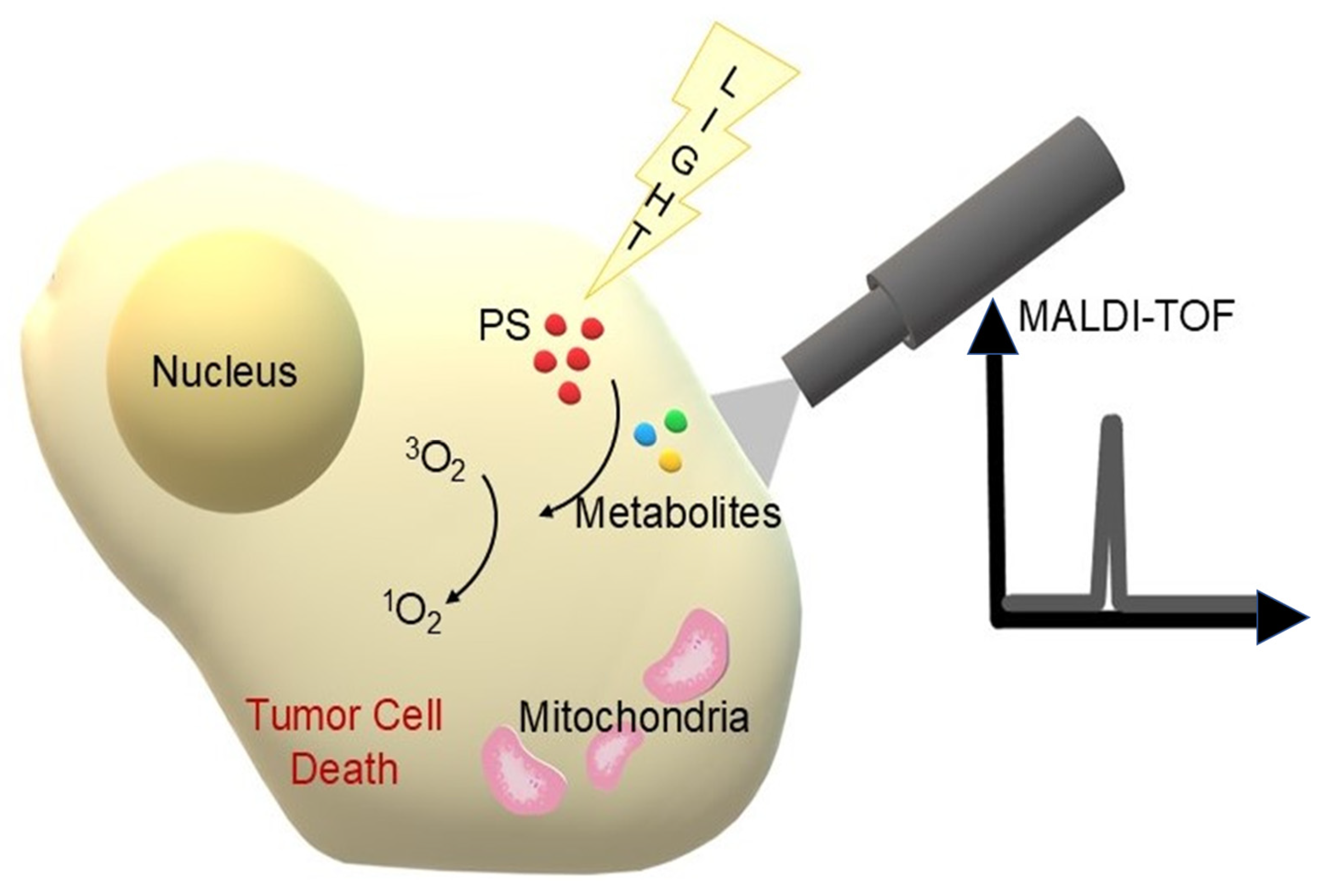
1.3. Common Point of PDT and MALDI
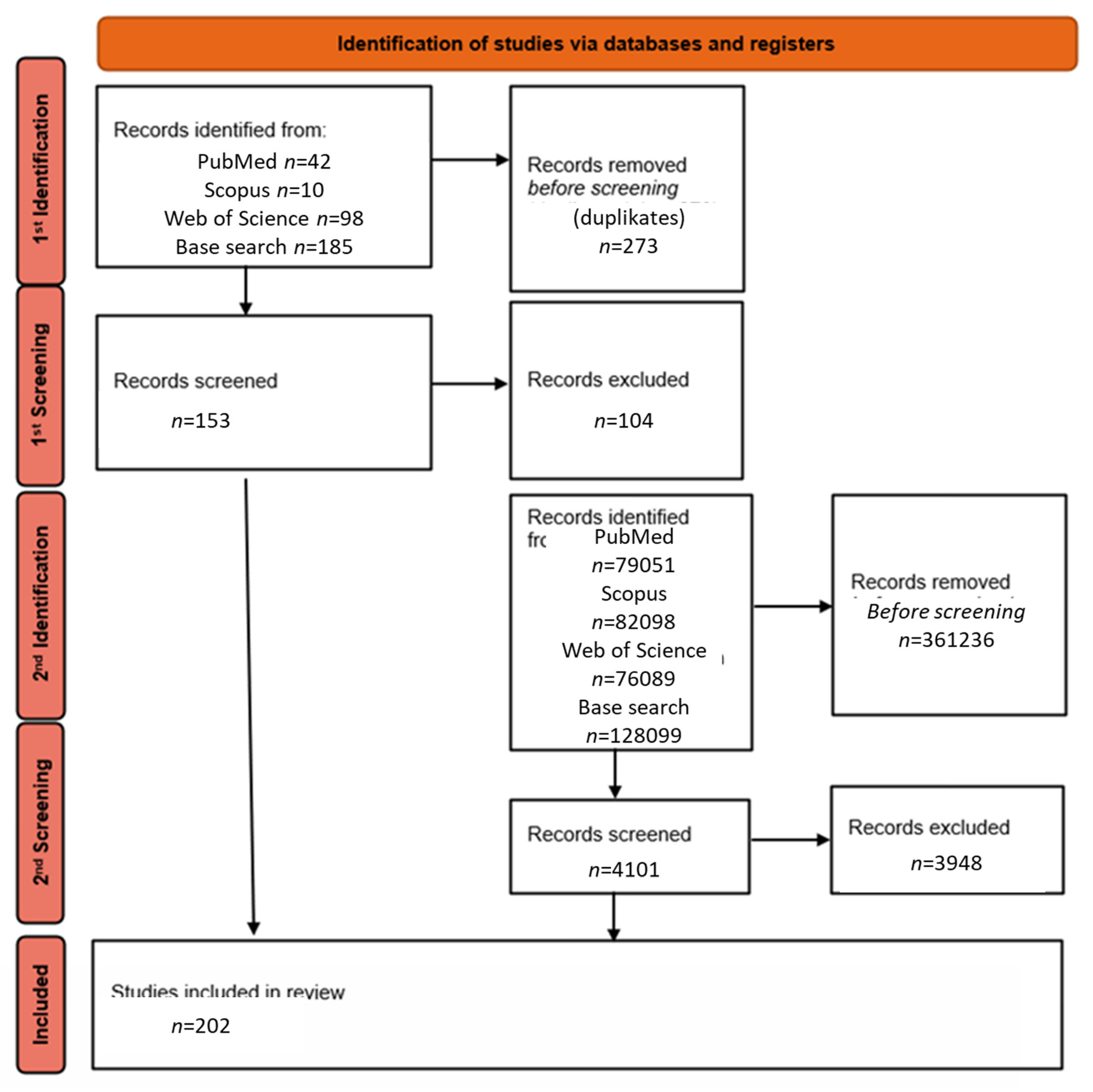
2. Materials and Methods
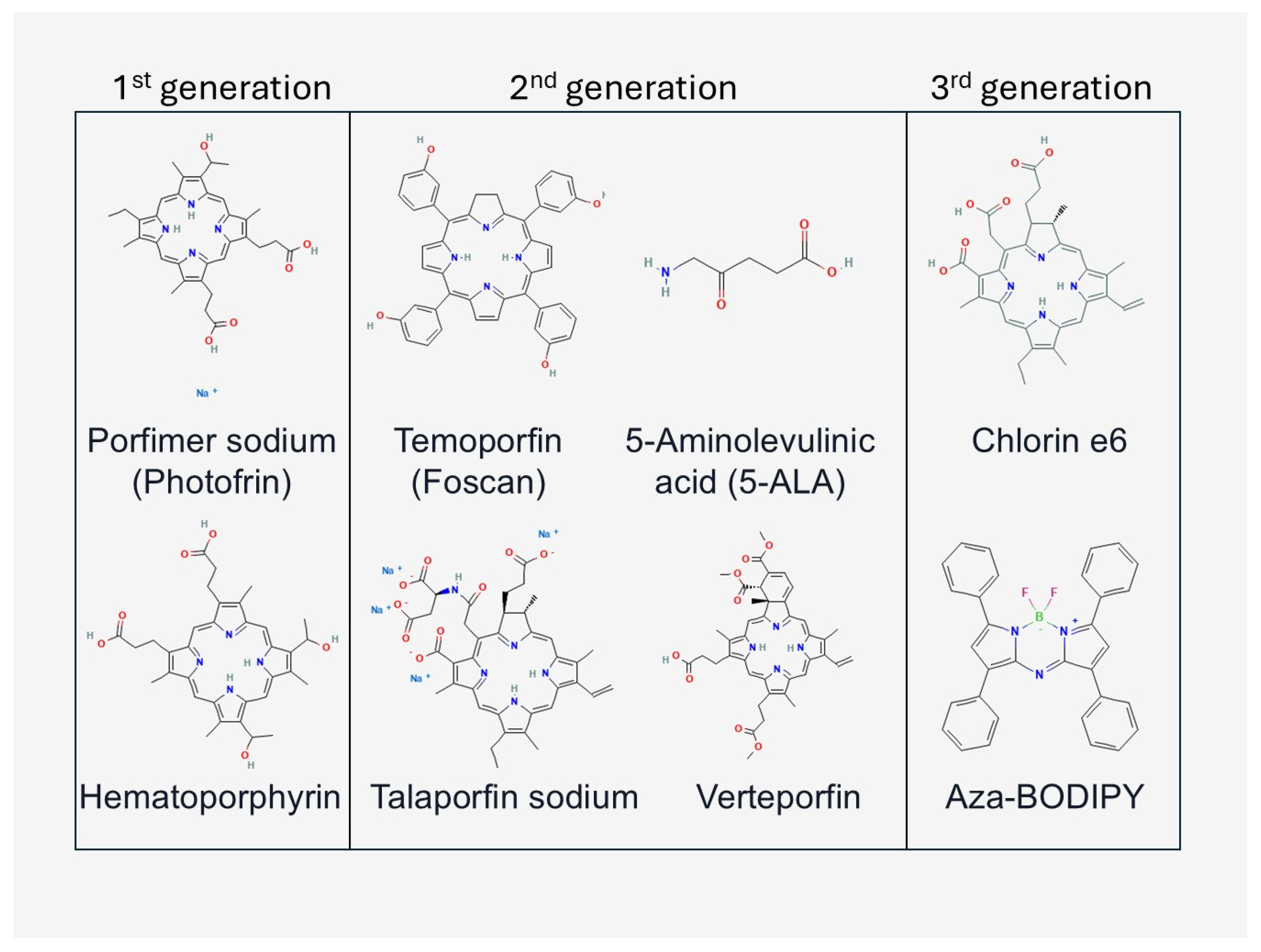
3. Photosensitizers Checked with MALDI
4. MALDI at the Administration of the Compound and Its Metabolism In Vivo During and After PDT
5. MALDI-TOF Detects Infection and Allows Rapid Treatment of PDT
6. Future Perspectives
7. Limitations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Abdel-kader, M.H. The Journey of PDT Throughout History: PDT from Pharos to Present. In Photodynamic Medicine: From Bench to Clinic; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Roelandts, R. The History of Phototherapy: Something New under the Sun? J. Am. Acad. Dermatol. 2002, 46, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, H.; Wang, B.; Wang, X. History of Photodynamic Therapy. In Photodynamic Therapy in Dermatology; Wang, X., Wang, B., Eds.; Springer Nature: Singapore, 2025; pp. 1–8. ISBN 978-981-96-8915-6. [Google Scholar]
- Calzavara-Pinton, P.; Szeimies, R.-M.; Ortel, B. Photodynamic Therapy and Fluorescence Diagnosis in Dermatology; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 978-0-08-053884-6. [Google Scholar]
- Sharma, S.K.; Mroz, P.; Dai, T.; Huang, Y.-Y.; St Denis, T.G.; Hamblin, M.R. Photodynamic Therapy for Cancer and for Infections: What Is the Difference? Isr. J. Chem. 2012, 52, 691–705. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The Use of Photodynamic Therapy in Medical Practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Czech, S.; Dynarowicz, K.; Misiołek, M.; Komosińska-Vassev, K.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy: Past, Current, and Future. Int. J. Mol. Sci. 2024, 25, 11325. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met.-Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef] [PubMed]
- Wickens, J.; Blinder, K.J. A Preliminary Benefit-Risk Assessment of Verteporfin in Age-Related Macular Degeneration. Drug Saf. 2006, 29, 189–199. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, J.; Li, Z.; Chen, H.; Gao, Y. Recent Progress in Sono-Photodynamic Cancer Therapy: From Developed New Sensitizers to Nanotechnology-Based Efficacy-Enhancing Strategies. Acta Pharm. Sin. B 2021, 11, 2197–2219. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Osuchowski, M.; Adamczyk, M.; Stopa, J.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Advancements in Photodynamic Therapy of Esophageal Cancer. Front. Oncol. 2022, 12, 1024576. [Google Scholar] [CrossRef]
- Mata, A.I.; Pereira, N.A.M.; Cardoso, A.L.; Nascimento, B.F.O.; Pineiro, M.; Schaberle, F.A.; Gomes-da-Silva, L.C.; Brito, R.M.M.; Pinho e Melo, T.M.V.D. Novel Foscan®-Derived Ring-Fused Chlorins for Photodynamic Therapy of Cancer. Bioorg. Med. Chem. 2023, 93, 117443. [Google Scholar] [CrossRef]
- Jones, H.J.; Vernon, D.I.; Brown, S.B. Photodynamic Therapy Effect of M-THPC (Foscan®) in Vivo: Correlation with Pharmacokinetics. Br. J. Cancer 2003, 89, 398–404. [Google Scholar] [CrossRef]
- Carobeli, L.R.; Meirelles, L.E.d.F.; Damke, G.M.Z.F.; Damke, E.; Souza, M.V.F.d.; Mari, N.L.; Mashiba, K.H.; Shinobu-Mesquita, C.S.; Souza, R.P.; Silva, V.R.S.d.; et al. Phthalocyanine and Its Formulations: A Promising Photosensitizer for Cervical Cancer Phototherapy. Pharmaceutics 2021, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Hosik, J.; Hosikova, B.; Binder, S.; Lenobel, R.; Kolarikova, M.; Malina, L.; Dilenko, H.; Langova, K.; Bajgar, R.; Kolarova, H. Effects of Zinc Phthalocyanine Photodynamic Therapy on Vital Structures and Processes in Hela Cells. Int. J. Mol. Sci. 2024, 25, 10650. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Alexiades-Armenakas, M. Laser-Mediated Photodynamic Therapy. Clin. Dermatol. 2006, 24, 16–25. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef]
- Zhu, T.C.; Finlay, J.C. The Role of Photodynamic Therapy (PDT) Physics. Med. Phys. 2008, 35, 3127–3136. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic Therapy in Endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic Therapy (PDT) for Lung Cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- Lim, H.S. Development and Optimization of a Diode Laser for Photodynamic Therapy. Laser Ther. 2011, 20, 195–203. [Google Scholar] [CrossRef]
- Yüksek, M.N.; Eroğlu, C.N. Clinical Evaluation of Single and Repeated Sessions of Photobiomodulation with Two Different Therapeutic Wavelengths for Reducing Postoperative Sequelae after Impacted Mandibular Third Molar Surgery: A Randomized, Double-Blind Clinical Study. J. Appl. Oral. Sci. 2021, 29, e20210383. [Google Scholar] [CrossRef] [PubMed]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-Infrared Photothermal/Photodynamic Therapy with Indocyanine Green Induces Apoptosis of Hepatocellular Carcinoma Cells through Oxidative Stress. Sci. Rep. 2017, 7, 13958. [Google Scholar] [CrossRef] [PubMed]
- Sorbellini, E.; Rucco, M.; Rinaldi, F. Photodynamic and Photobiological Effects of Light-Emitting Diode (LED) Therapy in Dermatological Disease: An Update. Lasers Med. Sci. 2018, 33, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Nguyen, J.K.; Jagdeo, J. Light-Emitting Diode-Based Photodynamic Therapy for Photoaging, Scars, and Dyspigmentation: A Systematic Review. Dermatol. Surg. 2020, 46, 1388–1394. [Google Scholar] [CrossRef]
- Quishida, C.C.C.; Carmello, J.C.; Mima, E.G.d.O.; Bagnato, V.S.; Machado, A.L.; Pavarina, A.C. Susceptibility of Multispecies Biofilm to Photodynamic Therapy Using Photodithazine. Lasers Med. Sci. 2015, 30, 685–694. [Google Scholar] [CrossRef]
- Opel, D.R.; Hagstrom, E.; Pace, A.K.; Sisto, K.; Hirano-Ali, S.A.; Desai, S.; Swan, J. Light-Emitting Diodes. J. Clin. Aesthet. Dermatol. 2015, 8, 36–44. [Google Scholar] [PubMed] [PubMed Central]
- Hu, Y.; Masamune, K. Flexible Laser Endoscope for Minimally Invasive Photodynamic Diagnosis (PDD) and Therapy (PDT) toward Efficient Tumor Removal. Opt. Express 2017, 25, 16795–16812. [Google Scholar] [CrossRef]
- Hu, Y.; Minzioni, P.; Hui, J.; Yun, S.-H.; Yetisen, A.K. Fiber Optic Devices for Diagnostics and Therapy in Photomedicine. Adv. Opt. Mater. 2024, 12, 2400478. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Jin, B.; Sun, T.; Sun, K.; Wang, S.; Fan, Z. Advances in Smart Nanotechnology-Supported Photodynamic Therapy for Cancer. Cell Death Discov. 2024, 10, 466. [Google Scholar] [CrossRef]
- Lee, C.-N.; Hsu, R.; Chen, H.; Wong, T.-W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Sotiriou, E.; Kiritsi, D.; Chaitidis, N.; Arabatzis, M.; Lallas, A.; Vakirlis, E. Daylight Photodynamic Therapy for Actinic Keratosis and Field Cancerization: A Narrative Review. Cancers 2025, 17, 1050. [Google Scholar] [CrossRef]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Caputo, E.N.; Rivero, M.N.; Gutiérrez, M.L.; Romero, E.L. Photodynamic Therapy with Nebulized Nanocurcumin on A549 Cells, Model Vessels, Macrophages and Beyond. Pharmaceutics 2022, 14, 2637. [Google Scholar] [CrossRef] [PubMed]
- Aniogo, E.C.; George, B.P.; Abrahamse, H. Characterization of Resistant MCF-7 Breast Cancer Cells Developed by Repeated Cycles of Photodynamic Therapy. Front. Pharmacol. 2022, 13, 964141. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, L.; Gries, M.; Chateau, A.; Daouk, J.; Lux, F.; Rocchi, P.; Cedervall, J.; Olsson, A.-K.; Tillement, O.; Frochot, C.; et al. Targeting Glioblastoma-Associated Macrophages for Photodynamic Therapy Using AGuIX®-Design Nanoparticles. Pharmaceutics 2023, 15, 997. [Google Scholar] [CrossRef]
- Aebisher, D.; Rogóż, K.; Yakub, Z.A.; Dynarowicz, K.; Myśliwiec, A.; Mytych, W.; Komosińska-Vassev, K.; Misiołek, M.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Photodynamic Therapy in Glioma Cell Culture. Oncologie 2024, 26, 885–897. [Google Scholar] [CrossRef]
- Nkune, N.W.; Simelane, N.W.N.; Montaseri, H.; Abrahamse, H. Photodynamic Therapy-Mediated Immune Responses in Three-Dimensional Tumor Models. Int. J. Mol. Sci. 2021, 22, 12618. [Google Scholar] [CrossRef]
- Silva, Z.S.; Bussadori, S.K.; Fernandes, K.P.S.; Huang, Y.-Y.; Hamblin, M.R. Animal Models for Photodynamic Therapy (PDT). Biosci. Rep. 2015, 35, e00265. [Google Scholar] [CrossRef]
- Yu, R.; Maswikiti, E.P.; Yu, Y.; Gao, L.; Ma, C.; Ma, H.; Deng, X.; Wang, N.; Wang, B.; Chen, H. Advances in the Application of Preclinical Models in Photodynamic Therapy for Tumor: A Narrative Review. Pharmaceutics 2023, 15, 197. [Google Scholar] [CrossRef]
- Zhou, H.-Z.; Wang, D.-X.; Qian, Y.-Q.; Wei, J.-Q.; Ma, S.; Feng, Y.-J.; Hao, Y. Large Animal Models for Investigating the Applications of Photodynamic Therapy. Zool. Res. 2025, 46, 551–575. [Google Scholar] [CrossRef]
- Guimarães, T.G.; Cardoso, K.M.; Marto, C.M.; Teixo, R.; Serambeque, B.; Silva, F.C.e.; Alexandre, N.; Botelho, M.F.; Laranjo, M. Oncological Applications of Photodynamic Therapy in Dogs and Cats. Appl. Sci. 2022, 12, 12276. [Google Scholar] [CrossRef]
- Williams, S.T.; Wells, G.; Conroy, S.; Gagg, H.; Allen, R.; Rominiyi, O.; Helleday, T.; Hullock, K.; Pennington, C.E.W.; Rantala, J.; et al. Precision Oncology Using Ex Vivo Technology: A Step towards Individualised Cancer Care? Expert Rev. Mol. Med. 2022, 24, e39. [Google Scholar] [CrossRef]
- Mendoza-Garcia, J.; Sebastian, A.; Alonso-Rasgado, T.; Bayat, A. Optimization of an Ex Vivo Wound Healing Model in the Adult Human Skin: Functional Evaluation Using Photodynamic Therapy. Wound Repair. Regen. 2015, 23, 685–702. [Google Scholar] [CrossRef]
- Hollander, C.D.; Visser, J.; De Haas, E.; Incrocci, L.; Smijs, T. Effective Single Photodynamic Treatment of Ex Vivo Onychomycosis Using a Multifunctional Porphyrin Photosensitizer and Green Light. J. Fungi 2015, 1, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Schastak, S.I.; Enzmann, V.; Loebel, S.; Zhavrid, E.A.; Voropai, E.S.; Alexandrova, E.N.; Samtsov, M.P.; Wiedemann, P. PDT of Melanoma in vivo and ex vivo with new Tricarbocyanin Sensitizer. In Laser in der Medizin Laser in Medicine; Waidelich, W., Waidelich, R., Waldschmidt, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 285–289. [Google Scholar]
- Azzouzi, A.-R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin Vascular-Targeted Photodynamic Therapy versus Active Surveillance in Men with Low-Risk Prostate Cancer (CLIN1001 PCM301): An Open-Label, Phase 3, Randomised Controlled Trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J. Low-risk prostate cancer: To treat or not to treat. Lancet Oncol. 2017, 18, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. The Latest Look at PDT and Immune Checkpoints. Curr. Issues Mol. Biol. 2024, 46, 7239–7257. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. STING-Activating Nanoparticles Combined with PD-1/PD-L1 Blockade: A Synergistic Approach in Cancer Immunotherapy. Biomedicines 2025, 13, 2160. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Krishnaswami, V.; Natarajan, B.; Sethuraman, V.; Natesan, S.; RajSelvaraj, B. Nano Based Photodynamic Therapy to Target Tumor Microenvironment. Nano Trends 2023, 1, 100003. [Google Scholar] [CrossRef]
- Menilli, L.; Milani, C.; Reddi, E.; Moret, F. Overview of Nanoparticle-Based Approaches for the Combination of Photodynamic Therapy (PDT) and Chemotherapy at the Preclinical Stage. Cancers 2022, 14, 4462. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion Nanoparticles for Photodynamic Therapy and Other Cancer Therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zang, Y.; Lu, Y.; Han, J.; Xiong, Q.; Xiong, J. Photodynamic Therapy of Up-Conversion Nanomaterial Doped with Gold Nanoparticles. Int. J. Mol. Sci. 2022, 23, 4279. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, A.; Claeson, M.; Paoli, J.; Heckemann, B. Exploring Patient Pain Experiences during and after Conventional Red Light and Simulated Daylight Photodynamic Therapy for Actinic Keratosis: A Qualitative Interview Study. Acta Derm. Venereol. 2024, 104, 19459. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, S.; Ma, S.; Lu, J.; Jiang, G. Clinical Trial of Two-Step Photodynamic Therapy for Reduced Pain in the Treatment of Precancerous Squamous Lesions (Actinic Keratoses). Photodiagn. Photodyn. Ther. 2024, 49, 104294. [Google Scholar] [CrossRef]
- Walch, A.; Rauser, S.; Deininger, S.-O.; Höfler, H. MALDI Imaging Mass Spectrometry for Direct Tissue Analysis: A New Frontier for Molecular Histology. Histochem. Cell Biol. 2008, 130, 421–434. [Google Scholar] [CrossRef]
- Calligaris, D.; Caragacianu, D.; Liu, X.; Norton, I.; Thompson, C.J.; Richardson, A.L.; Golshan, M.; Easterling, M.L.; Santagata, S.; Dillon, D.A.; et al. Application of Desorption Electrospray Ionization Mass Spectrometry Imaging in Breast Cancer Margin Analysis. Proc. Natl. Acad. Sci. USA 2014, 111, 15184–15189. [Google Scholar] [CrossRef]
- Kasselouri, A.; Bourdon, O.; Demore, D.; Blais, J.C.; Prognon, P.; Bourg-Heckly, G.; Blais, J. Fluorescence and Mass Spectrometry Studies of Meta-Tetra(Hydroxyphenyl)Chlorin Photoproducts. Photochem. Photobiol. 1999, 70, 275–279. [Google Scholar] [PubMed]
- Li, T.; Xu, Z.; Chen, H.; Zhen, S.; Gu, H.; Zhao, Z.; Tang, B.Z. Constructing Efficient and Photostable Photosensitizer with Aggregation-Induced Emission by Introducing Highly Electronegative Nitrogen Atom for Photodynamic Therapy. Chem. Eng. J. 2023, 468, 143829. [Google Scholar] [CrossRef]
- Aichler, M.; Walch, A. MALDI Imaging Mass Spectrometry: Current Frontiers and Perspectives in Pathology Research and Practice. Lab. Investig. 2015, 95, 422–431. [Google Scholar] [CrossRef]
- Karas, M.; Krüger, R. Ion Formation in MALDI: The Cluster Ionization Mechanism. Chem. Rev. 2003, 103, 427–440. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wang, Y.-S. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry: Mechanistic Studies and Methods for Improving the Structural Identification of Carbohydrates. Mass. Spectrom. 2017, 6, S0072. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yi, J.; Han, G.; Qiao, L. MALDI-TOF Mass Spectrometry in Clinical Analysis and Research. ACS Meas. Sci. Au 2022, 2, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Hillenkamp, F. Laser Desorption Ionization of Proteins with Molecular Masses Exceeding 10,000 Daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.E.; Trost, M. MALDI-TOF Mass Spectrometry in the 21st Century. Biochemist 2022, 44, 2–4. [Google Scholar] [CrossRef]
- Tanaka, K. The Origin of Macromolecule Ionization by Laser Irradiation (Nobel Lecture). Angew. Chem. Int. Ed. 2003, 42, 3860–3870. [Google Scholar] [CrossRef]
- Kaufmann, R. Matrix-Assisted Laser Desorption Ionization (MALDI) Mass Spectrometry: A Novel Analytical Tool in Molecular Biology and Biotechnology. J. Biotechnol. 1995, 41, 155–175. [Google Scholar] [CrossRef]
- Nicolardi, S.; Joseph, A.A.; Zhu, Q.; Shen, Z.; Pardo-Vargas, A.; Chiodo, F.; Molinaro, A.; Silipo, A.; van der Burgt, Y.E.M.; Yu, B.; et al. Analysis of Synthetic Monodisperse Polysaccharides by Wide Mass Range Ultrahigh-Resolution MALDI Mass Spectrometry. Anal. Chem. 2021, 93, 4666–4675. [Google Scholar] [CrossRef]
- Schiller, J.; Süß, R.; Arnhold, J.; Fuchs, B.; Leßig, J.; Müller, M.; Petković, M.; Spalteholz, H.; Zschörnig, O.; Arnold, K. Matrix-Assisted Laser Desorption and Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry in Lipid and Phospholipid Research. Prog. Lipid Res. 2004, 43, 449–488. [Google Scholar] [CrossRef]
- Butler, J.M.; Jiang-Baucom, P.; Huang, M.; Belgrader, P.; Girard, J. Peptide Nucleic Acid Characterization by MALDI-TOF Mass Spectrometry. Anal. Chem. 1996, 68, 3283–3287. [Google Scholar] [CrossRef]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry: A Fundamental Shift in the Routine Practice of Clinical Microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- Dixon, P.; Davies, P.; Hollingworth, W.; Stoddart, M.; MacGowan, A. A Systematic Review of Matrix-Assisted Laser Desorption/Ionisation Time-of-Flight Mass Spectrometry Compared to Routine Microbiological Methods for the Time Taken to Identify Microbial Organisms from Positive Blood Cultures. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 863–876. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef]
- Sharman, K.; Patterson, N.H.; Migas, L.G.; Neumann, E.K.; Allen, J.; Gibson-Corley, K.N.; Spraggins, J.M.; Van de Plas, R.; Skaar, E.P.; Caprioli, R.M. MALDI IMS-Derived Molecular Contour Maps: Augmenting Histology Whole-Slide Images. J. Am. Soc. Mass. Spectrom. 2023, 34, 905–912. [Google Scholar] [CrossRef]
- Gustafsson, J.O.R.; Oehler, M.K.; Ruszkiewicz, A.; McColl, S.R.; Hoffmann, P. MALDI Imaging Mass Spectrometry (MALDI-IMS)-Application of Spatial Proteomics for Ovarian Cancer Classification and Diagnosis. Int. J. Mol. Sci. 2011, 12, 773–794. [Google Scholar] [CrossRef]
- Gogichaeva, N.V.; Williams, T.; Alterman, M.A. MALDI TOF/TOF Tandem Mass Spectrometry as a New Tool for Amino Acid Analysis. J. Am. Soc. Mass. Spectrom. 2007, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Falkner, J.A.; Kachman, M.; Veine, D.M.; Walker, A.; Strahler, J.R.; Andrews, P.C. Validated MALDI-TOF/TOF Mass Spectra for Protein Standards. J. Am. Soc. Mass. Spectrom. 2007, 18, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, G.; de Puit, M.; Bleay, S.; Bradshaw, R.; Francese, S. Detection and Mapping of Illicit Drugs and Their Metabolites in Fingermarks by MALDI MS and Compatibility with Forensic Techniques. Sci. Rep. 2015, 5, 11716. [Google Scholar] [CrossRef] [PubMed]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.-N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-Based Proteomics in Biomedical Research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef]
- Greco, V.; Piras, C.; Pieroni, L.; Ronci, M.; Putignani, L.; Roncada, P.; Urbani, A. Applications of MALDI-TOF Mass Spectrometry in Clinical Proteomics. Expert. Rev. Proteom. 2018, 15, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Zaima, N.; Hayasaka, T.; Goto-Inoue, N.; Setou, M. Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry. Int. J. Mol. Sci. 2010, 11, 5040–5055. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, M.; Schnapp, A.; Koch, A.; Soltwisch, J.; Dreisewerd, K. New Insights into the Wavelength Dependence of MALDI Mass Spectrometry. Anal. Chem. 2017, 89, 7734–7741. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.K. Lasers for Matrix-Assisted Laser Desorption Ionization. J. Mass. Spectrom. 2021, 56, e4664. [Google Scholar] [CrossRef]
- Chen, L.C.; Asakawa, D.; Hori, H.; Hiraoka, K. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Using a Visible Laser. Rapid Commun. Mass. Spectrom. 2007, 21, 4129–4134. [Google Scholar] [CrossRef]
- Perry, W.J.; Patterson, N.H.; Prentice, B.M.; Neumann, E.K.; Caprioli, R.M.; Spraggins, J.M. Uncovering Matrix Effects on Lipid Analyses in MALDI Imaging Mass Spectrometry Experiments. J. Mass. Spectrom. 2020, 55, e4491. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.; Kim, Y.; Nguyen, H.-Q.; Han, S.; Kim, J. Effects of Matrices and Additives on Multiple Charge Formation of Proteins in MALDI-MS Analysis. J. Am. Soc. Mass. Spectrom. 2019, 30, 1174–1178. [Google Scholar] [CrossRef]
- Frecklington, D. General Method for MALDI-MS Analysis of Proteins and Peptides. Cold Spring Harb. Protoc. 2007, 2007, pdb.prot4679. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Zhang, J.Y. Ionization Mechanism of Oligonucleotides in Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2001, 15, 57–64. [Google Scholar] [CrossRef]
- Leopold, J.; Prabutzki, P.; Engel, K.M.; Schiller, J. A Five-Year Update on Matrix Compounds for MALDI-MS Analysis of Lipids. Biomolecules 2023, 13, 546. [Google Scholar] [CrossRef]
- Peterson, D.S. Matrix-Free Methods for Laser Desorption/Ionization Mass Spectrometry. Mass. Spectrom. Rev. 2007, 26, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hennemann, A.L.; Nogueira, H.P.; Ramos, M.D., Jr.; Correra, T.C.; Hennemann, B.L.; Araki, K. Amorphous Titanium Dioxide Nanoparticles and Their Unexpected Fragmentation in MALDI-TOF/MS. ACS Omega 2024, 9, 47831–47841. [Google Scholar] [CrossRef] [PubMed]
- Gladchuk, A.S.; Gorbunov, A.Y.; Keltsieva, O.A.; Ilyushonok, S.K.; Babakov, V.N.; Shilovskikh, V.V.; Kolonitskii, P.D.; Stepashkin, N.A.; Soboleva, A.; Muradymov, M.Z.; et al. Coating of a MALDI Target with Metal Oxide Nanoparticles by Droplet-Free Electrospraying—A Versatile Tool for in Situ Enrichment of Human Globin Adducts of Halogen-Containing Drug Metabolites. Microchem. J. 2023, 191, 108708. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, J.; Li, J.; Wang, Y. Graphene as a Novel Matrix for the Analysis of Small Molecules by MALDI-TOF MS. Anal. Chem. 2010, 82, 6208–6214. [Google Scholar] [CrossRef]
- Dietrich, S.; Dollinger, A.; Wieser, A.; Haisch, C. Optimization of MALDI Matrices and Their Preparation for the MALDI-TOF MS Analysis of Oligonucleotides. Rapid Commun. Mass. Spectrom. 2025, 39, e10061. [Google Scholar] [CrossRef]
- Leopold, J.; Popkova, Y.; Engel, K.M.; Schiller, J. Recent Developments of Useful MALDI Matrices for the Mass Spectrometric Characterization of Lipids. Biomolecules 2018, 8, 173. [Google Scholar] [CrossRef]
- Qiao, Z.; Lissel, F. MALDI Matrices for the Analysis of Low Molecular Weight Compounds: Rational Design, Challenges and Perspectives. Chem. Asian J. 2021, 16, 868–878. [Google Scholar] [CrossRef]
- Liu, X.; Hummon, A.B. Mass Spectrometry Imaging of Therapeutics from Animal Models to Three-Dimensional Cell Cultures. Anal. Chem. 2015, 87, 9508–9519. [Google Scholar] [CrossRef]
- Hess, R.A.; Linton, J.; Kanigan, T.S.; Brenan, C.; Ozbal, C. Apparatus for Assay, Synthesis and Storage, and Methods of Manufacture, Use, and Manipulation Thereof. US Patent 6,716,629, 2004. [Google Scholar]
- Zhou, Q.; Fülöp, A.; Hopf, C. Recent Developments of Novel Matrices and On-Tissue Chemical Derivatization Reagents for MALDI-MSI. Anal. Bioanal. Chem. 2021, 413, 2599–2617. [Google Scholar] [CrossRef]
- Ding, J.; Xiao, H.M.; Liu, S.; Wang, C.; Liu, X.; Feng, Y.Q. A matrix-assisted laser desorption/ionization mass spectroscopy method for the analysis of small molecules by integrating chemical labeling with the supramolecular chemistry of cucurbituril. Anal. Chim. Acta. 2018, 1026, 77–86. [Google Scholar] [CrossRef]
- Neagu, A.N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef]
- Gessel, M.M.; Norris, J.L.; Caprioli, R.M. MALDI Imaging Mass Spectrometry: Spatial Molecular Analysis to Enable a New Age of Discovery. J. Proteom. 2014, 107, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, Y.; Xiong, C.; Han, J.; Wang, X.; Chen, J.; Nie, Z. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging (MALDI MSI) for in Situ Analysis of Endogenous Small Molecules in Biological Samples. TrAC Trends Anal. Chem. 2022, 157, 116809. [Google Scholar] [CrossRef]
- Zhang, X.; Scalf, M.; Berggren, T.W.; Westphall, M.S.; Smith, L.M. Identification of mammalian cell lines using MALDI-TOF and LC-ESI-MS/MS mass spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Bonnel, D.; Legouffe, R.; Willand, N.; Baulard, A.; Hamm, G.; Deprez, B.; Stauber, J. MALDI Imaging Techniques Dedicated to Drug-Distribution Studies. Bioanalysis 2011, 3, 1399–1406. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Leal Rodriguez, C.; García-Riestra, C. Application and Perspectives of MALDI-TOF Mass Spectrometry in Clinical Microbiology Laboratories. Microorganisms 2021, 9, 1539. [Google Scholar] [CrossRef]
- Montaudo, G.; Samperi, F.; Montaudo, M.S. Characterization of Synthetic Polymers by MALDI-MS. Prog. Polym. Sci. 2006, 31, 277–357. [Google Scholar] [CrossRef]
- Calderaro, A.; Chezzi, C. MALDI-TOF MS: A Reliable Tool in the Real Life of the Clinical Microbiology Laboratory. Microorganisms 2024, 12, 322. [Google Scholar] [CrossRef]
- Becker, K.; Lupetti, A. Editorial: MALDI-TOF MS in Microbiological Diagnostics: Future Applications beyond Identification. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef]
- Gant, M.S.; Chamot-Rooke, J. Present and Future Perspectives on Mass Spectrometry for Clinical Microbiology. Microbes Infect. 2024, 26, 105296. [Google Scholar] [CrossRef]
- Kluz, M.I.; Waszkiewicz-Robak, B.; Kačániová, M. The Applications of MALDI-TOF MS in the Diagnosis of Microbiological Food Contamination. Appl. Sci. 2025, 15, 7863. [Google Scholar] [CrossRef]
- Shariatgorji, M.; Nilsson, A.; Fridjonsdottir, E.; Vallianatou, T.; Källback, P.; Katan, L.; Sävmarker, J.; Mantas, I.; Zhang, X.; Bezard, E.; et al. Comprehensive Mapping of Neurotransmitter Networks by MALDI-MS Imaging. Nat. Methods 2019, 16, 1021–1028. [Google Scholar] [CrossRef]
- Dowling, P.; Trollet, C.; Negroni, E.; Swandulla, D.; Ohlendieck, K. How Can Proteomics Help to Elucidate the Pathophysiological Crosstalk in Muscular Dystrophy and Associated Multi-System Dysfunction? Proteomes 2024, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Berezin, D.B.; Zorin, V.P.; Morshnev, P.K.; Kukushkina, N.V.; Krestyaninov, M.A.; Kustova, T.V.; Strelnikov, A.I.; Lyalyakina, E.V.; Zorina, T.E.; et al. Monocationic Chlorin as a Promising Photosensitizer for Antitumor and Antimicrobial Photodynamic Therapy. Pharmaceutics 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Z.; Wang, R.; Wang, J.; Liu, B.; Wang, Y.; Qin, S.; Yang, J.; Liu, J. A Novel Photosensitizer DTPP-Mediated Photodynamic Therapy Induces Oxidative Stress and Apoptosis through Mitochondrial Pathways in LA795 Cells. Photodiagn. Photodyn. Ther. 2024, 45, 103894. [Google Scholar] [CrossRef] [PubMed]
- Laville, I.; Pigaglio, S.; Blais, J.-C.; Loock, B.; Maillard, P.; Grierson, D.S.; Blais, J. A Study of the Stability of Tri(Glucosyloxyphenyl)Chlorin, a Sensitizer for Photodynamic Therapy, in Human Colon Tumoural Cells: A Liquid Chromatography and MALDI-TOF Mass Spectrometry Analysis. Bioorg. Med. Chem. 2004, 12, 3673–3682. [Google Scholar] [CrossRef]
- Özdemir, M.; Abliatipova, A.; Benian, S.; Yalçin, B.; Salan, Ü.; Durmus, M.; Bulut, M. 1,2,3-Triazole Incorporated Coumarin Carrying Metal-Free, Zn(II), Mg(II) Phthalocyanines: Synthesis, Characterization, Theoretical Studies, Photophysical and Photochemical Properties. J. Photochem. Photobiol. A Chem. 2020, 403, 112845. [Google Scholar] [CrossRef]
- Yalazan, H.; Ömeroğlu, İ.; Durmuş, M.; Kantekin, H. 4-Aminoantipyrine-Derived Schiff Base Substituted Novel Symmetrical Zinc(II) Phthalocyanine Photosensitizers: Design, Synthesis, Photophysical, and Photochemical Properties for Photodynamic Therapy of Cancer. Inorg. Chem. Commun. 2025, 181, 115125. [Google Scholar] [CrossRef]
- Karapinar, B.; Özdemir, M.; Salan, Ü.; Durmus, M.; Yalçin, B.; Bulut, M. 7-Oxy-3,4-Cyclohexenecoumarin Carrying Novel Zinc(II) and Indium(III) Acetate Phthalocyanines: Synthesis, Characterization, Photophysical and Photochemical Properties. Chemistryselect 2019, 4, 9632–9639. [Google Scholar] [CrossRef]
- Göl, C.; Malkoç, M.; Yeşilot, S.; Durmuş, M. A First Archetype of Boron Dipyrromethene-Phthalocyanine Pentad Dye: Design, Synthesis, and Photophysical and Photochemical Properties. Dalton Trans. 2014, 43, 7561–7569. [Google Scholar] [CrossRef]
- Köse, G. A Novel Diaxially Silicon Phthalocyanine Sensitizer for the Generation of High Efficiency Singlet Oxygen in Photochemical and Sono-Photochemical Studies. J. Organomet. Chem. 2023, 998, 122814. [Google Scholar] [CrossRef]
- Ghazal, B.; Kaya, E.; Husain, A.; Ganesan, A.; Durmus, M.; Makhseed, S. Biotinylated-Cationic Zinc(II) Phthalocyanine towards Photodynamic Therapy. J. Porphyr. Phthalocyanines 2019, 23, 46–55. [Google Scholar] [CrossRef]
- Cabir, B.; Cetindere, S. BODIPY-Based Iridium and Ruthenium Complexes: Synthesis, Photophysical, and Photochemical Properties. J. Chin. Chem. Soc. 2024, 71, 1402–1409. [Google Scholar] [CrossRef]
- Nam, G.; Rangasamy, S.; Ju, H.; Samson, A.A.S.; Song, J.M. Cell Death Mechanistic Study of Photodynamic Therapy against Breast Cancer Cells Utilizing Liposomal Delivery of 5,10,15,20-Tetrakis(Benzo[b]Thiophene) Porphyrin. J. Photochem. Photobiol. B 2017, 166, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kücük, T.; Harmandar, K.; Aydogdu, B.; Göksel, M.; Balcik-Ercin, P.; Ibisoglu, H.; Atilla, D. Chalcone-Containing Phthalocyanines: Determination of Photophysical and Photochemical Properties and Cytotoxicity on Breast Cancer Cell Models. J. Porphyr. Phthalocyanines 2025, 29, 191–199. [Google Scholar] [CrossRef]
- Yalazan, H.; Barut, B.; Ertem, B.; Yalçin, C.; Ünver, Y.; Özel, A.; Ömeroglu, I.; Durmus, M.; Kantekin, H. DNA Interaction and Anticancer Properties of New Peripheral Phthalocyanines Carrying Tosylated 4-Morpholinoaniline Units. Polyhedron 2020, 177, 114319. [Google Scholar] [CrossRef]
- Köse, G.G.; Erdoğmuş, A. Dual Effect of Light and Ultrasound for Efficient Singlet Oxygen Generation with Novel Diaxial Silicon Phthalocyanine Sensitizer. Photochem. Photobiol. 2024, 100, 52–66. [Google Scholar] [CrossRef]
- Gupta, I.; Bishnoi, R.; Manav, N.; Jain, A.; Chavda, J. Endoplasmic Reticulum Targeting Tin Porphyrins with Oligoethyleneglycol Chains: Synthesis, DFT Studies and Anti-Cancer Activities. J. Mol. Struct. 2025, 1345, 143121. [Google Scholar] [CrossRef]
- Karimi, A.; Khodadadi, A.; Azadikhah, F.; Hadizadeh, M. In Vitro Photodynamic Activities of Amphiphilic Phthalocyanine-Amino Appended β-Cyclodextrin Conjugates as Efficient Schiff Base Photosensitizer. Chemistryselect 2023, 8, e202203378. [Google Scholar] [CrossRef]
- Zeki, K.; Yabas, E.; Erden, F.; Salih, B.; Canlica, M. Lu, Sm, and Y-Based Double-Decker Phthalocyanines with Enhanced Photodynamic Therapy Performance. Polyhedron 2024, 261, 117138. [Google Scholar] [CrossRef]
- Hisir, A.; Köse, G.; Atmaca, G.; Erdogmus, A.; Karaoglan, G. Novel Carboxylic Acid Terminated Silicon(IV) and Zinc(II) Phthalocyanine Photosensitizers: Synthesis, Photophysical and Photochemical Studies. J. Porphyr. Phthalocyanines 2018, 22, 1010–1021. [Google Scholar] [CrossRef]
- Kamiloglu, A. Photochemical Properties of Fluoro-Chalcone Substituted Peripherally Tetra Zn(II)Pc and Mg(II)Pc. J. Incl. Phenom. Macrocycl. Chem. 2021, 99, 185–196. [Google Scholar] [CrossRef]
- Yalazan, H.; Kantekin, H.; Durmus, M. Photophysicochemical Properties of Pyrazoline Substituted ZnII-Phthalocyanine-Based Photosensitizers. J. Organomet. Chem. 2024, 1020, 123326. [Google Scholar] [CrossRef]
- Köse, G.; Karaoglan, G. Synthesis of a Novel Axially Substituted Silicon Phthalocyanine Sensitizer for Efficient Singlet Oxygen Generation by Comparing PDT and SPDT Studies. Chem. Phys. 2023, 565, 111737. [Google Scholar] [CrossRef]
- Akkus, N.; Eksin, E.; Sahin, G.; Yildiz, E.; Bagda, E.; Altun, A.; Bagda, E.; Durmus, M.; Erdem, A. The Targeted Photodynamic Therapy of Breast Cancer with Novel AS1411-Indium(III) Phthalocyanine Conjugates. J. Mol. Struct. 2024, 1305, 137718. [Google Scholar] [CrossRef]
- Picard, N.; Ali, H.; van Lier, J.E.; Klarskov, K.; Paquette, B. Bromines on N-Allyl Position of Cationic Porphyrins Affect Both Radio- and Photosensitizing Properties. Photochem. Photobiol. Sci. 2009, 8, 224–232. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, S.; Monisha, M.; Bhowmick, S.; Roy, A.; Das, N.; Das, P. Hierarchical Coassembly of DNA-Triptycene Hybrid Molecular Building Blocks and Zinc Protoporphyrin IX. Beilstein J. Nanotechnol. 2016, 7, 697–707. [Google Scholar] [CrossRef]
- Yurttas, A.; Gokduman, K.; Hekim, N. Liposomes Loaded with Activatable Disulfide Bridged Photosensitizer: Towards Targeted and Effective Photodynamic Therapy on Breast Cancer Cells. Biointerface Res. Appl. Chem. 2022, 12, 304–325. [Google Scholar] [CrossRef]
- Kara, M.; Kocaaga, N.; Akgul, B.; Abamor, E.S.; Erdogmus, A.; Topuzogullari, M.; Acar, S. Micelles of Poly[Oligo(Ethylene Glycol) Methacrylate] as Delivery Vehicles for Zinc Phthalocyanine Photosensitizers. Nanotechnology 2024, 35, 475602. [Google Scholar] [CrossRef]
- Martínez-Alonso, M.; Gandioso, A.; Thibaudeau, C.; Qin, X.; Arnoux, P.; Demeubayeva, N.; Guérineau, V.; Frochot, C.; Jung, A.C.; Gaiddon, C.; et al. A Novel Near-IR Absorbing Ruthenium(II) Complex as Photosensitizer for Photodynamic Therapy and Its Cetuximab Bioconjugates. Chembiochem 2023, 24, e202300203. [Google Scholar] [CrossRef]
- Günsel, A. Comparative Studies of Photophysicochemical Properties of Non-Peripherally Anisole/Thioanisole-Tetrasubstituted Gallium(III) Phthalocyanines Containing Oxygen/Sulfur Bridge. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 269–282. [Google Scholar] [CrossRef]
- Schneider, R.; Schmitt, F.; Frochot, C.; Fort, Y.; Lourette, N.; Guillemin, F.; Müller, J.-F.; Barberi-Heyob, M. Design, Synthesis, and Biological Evaluation of Folic Acid Targeted Tetraphenylporphyrin as Novel Photosensitizers for Selective Photodynamic Therapy. Bioorg. Med. Chem. 2005, 13, 2799–2808. [Google Scholar] [CrossRef]
- Kantekin, H.; Yalazan, H.; Barut, B.; Gungör, Ö.; Ünlüer, D.; Demirbas, Ü.; Özel, A.; Durmus, M. Dual-Purpose Both Peripheral and Non-Peripheral Triazole Substituted ZnII, MgII and PbII Phthalocyanines: Synthesis, Characterization, Photophysicochemical and Acetylcholinesterase Inhibitory Properties. Polyhedron 2021, 208, 115416. [Google Scholar] [CrossRef]
- Laville, I.; Pigaglio, S.; Blais, J.-C.; Doz, F.; Loock, B.; Maillard, P.; Grierson, D.S.; Blais, J. Photodynamic Efficiency of Diethylene Glycol-Linked Glycoconjugated Porphyrins in Human Retinoblastoma Cells. J. Med. Chem. 2006, 49, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Kannen, H.; Nomura, S.; Hazama, H.; Kaneda, Y.; Fujino, T.; Awazu, K. Enhancement of Ionization Efficiency Using Zeolite in Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Multiple Drugs in Cancer Cells (Mass Spectrometry of Multiple Drugs in Cells Using Zeolite). Mass. Spectrom. 2020, 9, A0091. [Google Scholar] [CrossRef] [PubMed]
- Kannen, H.; Hazama, H.; Kaneda, Y.; Fujino, T.; Awazu, K. Development of Laser Ionization Techniques for Evaluation of the Effect of Cancer Drugs Using Imaging Mass Spectrometry. Int. J. Mol. Sci. 2014, 15, 11234–11244. [Google Scholar] [CrossRef]
- Yoshida, T.; Kohno, E.; Dodeller, M.; Sakurai, T.; Yamamoto, S.; Terakawa, S. Novel PDD-PDT System Based on Spectrophotometric Real-Time Fluorescence Monitoring and MALDI-TOF-MS Analysis of Tumors. In Proceedings of the 12th World Congress of the International Photodynamic Association, Seattle, WA, USA, 11–15 June 2009; Volume 7380. [Google Scholar]
- Dodeller, M. Analyse Par Spectrométrie de Masse de l’oxygène Moléculaire Singulet et de Protéines Potentiellement Ciblées Au Sein de Cellules Tumorales Lors de La Thérapie Photodynamique. Ph.D. Thesis, Université Paul Verlaine, Metz, France, 2007. [Google Scholar]
- Zhou, Z.; Liu, Y.; Qin, M.; Sheng, W.; Wang, X.; Li, Z.; Zhong, R. Depletion of PKM2 Leads to Impaired Glycolysis and Cell Death in 2-Demethoxy-2,3-Ethylenediamino Hypocrellin B-Photoinduced A549 Cells. J. Photochem. Photobiol. B 2014, 134, 1–8. [Google Scholar] [CrossRef]
- Halada, P.; Man, P.; Grebenová, D.; Hrkal, Z.; Havlícek, V. Identification of HL60 Proteins Affected by 5-Aminolevulinic Acid-Based Photodynamic Therapy Using Mass Spectrometric Approach. Collect. Czechoslov. Chem. Commun. 2001, 66, 1720–1728. [Google Scholar] [CrossRef]
- Baglo, Y.; Sousa, M.M.L.; Slupphaug, G.; Hagen, L.; Håvåg, S.; Helander, L.; Zub, K.A.; Krokan, H.E.; Gederaas, O.A. Photodynamic Therapy with Hexyl Aminolevulinate Induces Carbonylation, Posttranslational Modifications and Changed Expression of Proteins in Cell Survival and Cell Death Pathways. Photochem. Photobiol. Sci. 2011, 10, 1137–1145. [Google Scholar] [CrossRef]
- Xu, D.D.; Xu, C.B.; Lam, H.M.; Wong, F.-L.; Leung, A.W.N.; Leong, M.M.L.; Cho, W.C.S.; Hoeven, R.; Lv, Q.; Rong, R. Proteomic Analysis Reveals That Pheophorbide A-Mediated Photodynamic Treatment Inhibits Prostate Cancer Growth by Hampering GDP-GTP Exchange of Ras-Family Proteins. Photodiagn. Photodyn. Ther. 2018, 23, 35–39. [Google Scholar] [CrossRef]
- Fugmann, T.; Neri, D.; Roesli, C. DeepQuanTR: MALDI-MS-based Label-free Quantification of Proteins in Complex Biological Samples. Proteomics 2010, 10, 2631–2643. [Google Scholar] [CrossRef]
- Kriska, T.; Korytowski, W.; Girotti, A.W. Role of Mitochondrial Cardiolipin Peroxidation in Apoptotic Photokilling of 5-Aminolevulinate-Treated Tumor Cells. Arch. Biochem. Biophys. 2005, 433, 435–446. [Google Scholar] [CrossRef]
- Serain, A.F.; Morosi, L.; Ceruti, T.; Matteo, C.; Meroni, M.; Minatel, E.; Zucchetti, M.; Salvador, M.J. Betulinic Acid and Its Spray Dried Microparticle Formulation: In Vitro PDT Effect against Ovarian Carcinoma Cell Line and in Vivo Plasma and Tumor Disposition. J. Photochem. Photobiol. B 2021, 224, 112328. [Google Scholar] [CrossRef]
- Zhong, Y.; Xing, Y. Diagnosis of Mycobacterium Marinum Infection by Metagenomic Next-Generation Sequencing. Clin. Lab. 2024, 70, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, A.; Prod’hom, G.; Greub, G. Applications of MALDI-TOF Mass Spectrometry in Clinical Diagnostic Microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, X.A.; Manríquez-Troncoso, J.M.; Sepúlveda, A.Y.; Soto, P.S. Integrating Machine Learning with MALDI-TOF Mass Spectrometry for Rapid and Accurate Antimicrobial Resistance Detection in Clinical Pathogens. Int. J. Mol. Sci. 2025, 26, 1140. [Google Scholar] [CrossRef]
- Mai, B.; Gao, Y.; Li, M.; Wang, X.; Zhang, K.; Liu, Q.; Xu, C.; Wang, P. Photodynamic Antimicrobial Chemotherapy for Staphylococcus Aureus and Multidrug-Resistant Bacterial Burn Infection in Vitro and in Vivo. Int. J. Nanomed. 2017, 12, 5915–5931. [Google Scholar] [CrossRef]
- Czeszewska-Rosiak, G.; Adamczyk, I.; Ludwiczak, A.; Fijałkowski, P.; Fijałkowski, P.; Twarużek, M.; Złoch, M.; Gabryś, D.; Miśta, W.; Tretyn, A.; et al. Analysis of the Efficacy of MALDI-TOF MS Technology in Identifying Microorganisms in Cancer Patients and Oncology Hospital Environment. Heliyon 2025, 11, e42015. [Google Scholar] [CrossRef]
- Xing, Y.; Li, M.; Jiang, Y.; Zhong, Q. Three Cases of Non-Tuberculosis Mycobacterium Skin Infection Outbreak in Beauty Institutions. Clin Lab 2024, 70, 1192–1195. [Google Scholar] [CrossRef]
- Dragana, R.; Jelena, M.; Jovan, M.; Biljana, N.; Dejan, M. Antibacterial Efficiency of Adjuvant Photodynamic Therapy and High-Power Diode Laser in the Treatment of Young Permanent Teeth with Chronic Periapical Periodontitis. A Prospective Clinical Study. Photodiagn. Photodyn. Ther. 2023, 41, 103129. [Google Scholar] [CrossRef]
- Arciola, C.R.; Montanaro, L.; Costerton, J.W. New Trends in Diagnosis and Control Strategies for Implant Infections. Int. J. Artif. Organs 2011, 34, 727–736. [Google Scholar] [CrossRef]
- Burchard, T.; Karygianni, L.; Hellwig, E.; Wittmer, A.; Al-Ahmad, A. Microbial Composition of Oral Biofilms after Visible Light and Water-Filtered Infrared a Radiation (VIS wIRA) in Combination with Indocyanine Green (ICG) as Photosensitizer. Antibiotics 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Bucher, M.; Anderson, A.C.; Tennert, C.; Hellwig, E.; Wittmer, A.; Vach, K.; Karygianni, L. Antimicrobial Photoinactivation Using Visible Light Plus Water-Filtered Infrared-A (VIS wIRA) Alters In Situ Oral Biofilms. Sci. Rep. 2015, 9, 20325. [Google Scholar] [CrossRef] [PubMed]
- Cazares, L.H.; Troyer, D.A.; Wang, B.; Drake, R.R.; Semmes, O.J. MALDI Tissue Imaging: From Biomarker Discovery to Clinical Applications. Anal. Bioanal. Chem. 2011, 401, 17. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Wang, S.-L.; Qi, L.-W.; Zang, H.; Huang, F.-Q. MALDI Mass Spectrometry Imaging in Targeted Drug Discovery and Development: The Pros, the Cons, and Prospects in Global Omics Techniques. TrAC Trends Anal. Chem. 2024, 178, 117860. [Google Scholar] [CrossRef]
- Gruber, L.; Schmidt, S.; Enzlein, T.; Vo, H.G.; Bausbacher, T.; Cairns, J.L.; Ucal, Y.; Keller, F.; Kerndl, M.; Abu Sammour, D.; et al. Deep MALDI-MS Spatial Omics Guided by Quantum Cascade Laser Mid-Infrared Imaging Microscopy. Nat. Commun. 2025, 16, 4759. [Google Scholar] [CrossRef]
- Tuck, M.; Grélard, F.; Blanc, L.; Desbenoit, N. MALDI-MSI Towards Multimodal Imaging: Challenges and Perspectives. Front. Chem. 2022, 10, 904688. [Google Scholar] [CrossRef]
- Zhang, W.; Patterson, N.H.; Verbeeck, N.; Moore, J.L.; Ly, A.; Caprioli, R.M.; Moor, B.D.; Norris, J.L.; Claesen, M. Multimodal MALDI Imaging Mass Spectrometry for Improved Diagnosis of Melanoma. PLoS ONE 2024, 19, e0304709. [Google Scholar] [CrossRef]
- Dong, J.; Wang, F.; Xu, Y.; Gao, X.; Zhao, H.; Zhang, J.; Wang, N.; Liu, Z.; Yan, X.; Jin, J.; et al. Using Mixed Reality Technique Combines Multimodal Imaging Signatures to Adjuvant Glioma Photodynamic Therapy. Front. Med. 2023, 10, 1171819. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Zhu, L.; Zhou, X.; Chen, S.; Jiang, Z.-X. Enabling 19F MRI and Boosting Phototherapy Through Facile Counterion Pairing of Photosensitizers. Small 2025, 21, e2505497. [Google Scholar] [CrossRef]
- Thomas, A.M.; Yang, E.; Smith, M.D.; Chu, C.; Calabresi, P.A.; Glunde, K.; van Zijl, P.C.M.; Bulte, J.W.M. CEST MRI and MALDI Imaging Reveal Metabolic Alterations in the Cervical Lymph Nodes of EAE Mice. J. Neuroinflamm. 2022, 19, 130. [Google Scholar] [CrossRef]
- Chen, C.-J.; Lai, C.-C.; Tseng, M.-C.; Liu, Y.-C.; Lin, S.-Y.; Tsai, F.-J. Simple Fabrication of Hydrophobic Surface Target for Increased Sensitivity and Homogeneity in Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry Analysis of Peptides, Phosphopeptides, Carbohydrates and Proteins. Anal. Chim. Acta 2013, 783, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tressler, C.M.; Ayyappan, V.; Nakuchima, S.; Yang, E.; Sonkar, K.; Tan, Z.; Glunde, K. A Multimodal Pipeline Using NMR Spectroscopy and MALDI-TOF Mass Spectrometry Imaging from the Same Tissue Sample. NMR Biomed. 2023, 36, e4770. [Google Scholar] [CrossRef] [PubMed]
- Chaurand, P.; Schwartz, S.A.; Billheimer, D.; Xu, B.J.; Crecelius, A.; Caprioli, R.M. Integrating Histology and Imaging Mass Spectrometry. Anal. Chem. 2004, 76, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters That Affect the Photodegradation of Dyes and Pigments in Solution and on Substrate—An Overview. Dye. Pigment. 2023, 210, 110999. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A. Spectral Precision: Recent Advances in Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for Pathogen Detection and Resistance Profiling. Microorganisms 2025, 13, 1473. [Google Scholar] [CrossRef]
- Zhang, J.; Tavares de Sousa Júnior, W.; Mello da Silva, V.C.; Rodrigues, M.C.; Vasconcelos Morais, J.A.; Song, J.-L.; Cheng, Z.-Q.; Longo, J.P.F.; Bentes Azevedo, R.; Jiang, C.-S.; et al. Synthesis and Evaluation of New Potential Benzo[a]Phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy. Molecules 2018, 23, 1436. [Google Scholar] [CrossRef]
- Samat, N.; Tan, P.J.; Shaari, K.; Abas, F.; Lee, H.B. Prioritization of Natural Extracts by LC–MS-PCA for the Identification of New Photosensitizers for Photodynamic Therapy. Anal. Chem. 2014, 86, 1324–1331. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Bilea, F.; Avram, S.; Staicu, A. Laser-Induced Dimeric Photoproducts of Chlorpromazine: LC-MS Identification and Molecular Docking Evidence of Enhanced Anticancer Potential. Int. J. Mol. Sci. 2025, 26, 6668. [Google Scholar] [CrossRef]
- Ucal, Y.; Coskun, A.; Ozpinar, A. Quality Will Determine the Future of Mass Spectrometry Imaging in Clinical Laboratories: The Need for Standardization. Expert. Rev. Proteom. 2019, 16, 521–532. [Google Scholar] [CrossRef]
- Moore, J.L.; Patterson, N.H.; Norris, J.L.; Caprioli, R.M. Prospective on Imaging Mass Spectrometry in Clinical Diagnostics. Mol. Cell Proteom. 2023, 22, 100576. [Google Scholar] [CrossRef]
- Son, A.; Kim, W.; Park, J.; Park, Y.; Lee, W.; Lee, S.; Kim, H. Mass Spectrometry Advancements and Applications for Biomarker Discovery, Diagnostic Innovations, and Personalized Medicine. Int. J. Mol. Sci. 2024, 25, 9880. [Google Scholar] [CrossRef]
- Djambazova, K.V.; Van Ardenne, J.M.; Spraggins, J.M. Advances in Imaging Mass Spectrometry for Biomedical and Clinical Research. TrAC Trends Anal. Chem. 2023, 169, 117344. [Google Scholar] [CrossRef]
- Ksenofontova, K.V.; Ksenofontov, A.A.; Kerner, A.A.; Molchanov, E.E.; Gessel, T.V.; Galembikova, A.R.; Krestova, A.N.; Borisovskaya, E.P.; Khodov, I.A.; Boichuk, S.V. Spectral Properties and Anticancer Activity of Novel Cisplatin-BODIPY Conjugates. Opt. Mater. 2025, 159, 116680. [Google Scholar] [CrossRef]


| Name of PS | Theoretical Mass/Proven Mass | MALDI Matrix | Type of Biological Validation | Main Conclusion of the Analysis/Significance of Potential Use | Source |
|---|---|---|---|---|---|
| ANTS-ZnPcp (peripheral Zn-phthalocyanine with Schiff base substituents) | Calc.:1799.23/Found: 1800.24 [M+H]+ | - | Not reported | MALDI-TOF confirmed synthesis and structure; PS with high singlet oxygen yield (ΦΔ = 0.53), potential candidate for PDT | [133] |
| ANTS-ZnPcnp (non-peripheral Zn-phthalocyanine with Schiff base substituents) | Calc.: 1799.23/Found:1800.16 [M+H]+ | - | Not reported | MALDI-TOF confirmed synthesis and structure; PS with moderate singlet oxygen yield (ΦΔ = 0.27), potential candidate for PDT | |
| Zn(II)-phthalocyanine (4,6) with coumarin substituents | Found: ~1434 m/z [M]+ | - | Not reported | MALDI-TOF confirmed structure; compounds well soluble, generate singlet oxygen at acceptable levels, potential PS for PDT | [134] |
| In(III) acetate-phthalocyanine (5,7) with coumarin substituents | Found: ~1543 m/z [M]+ | -. | Not reported | MALDI-TOF confirmed structure; show good photophysical/photochemical properties, potential PS for PDT | |
| Bis(4-(6—bromo-2-naphtoxy)-phthalocyaninato- silicon (IV) (SiPc-BrNph) | Calc.: 984.74 g/mol/Found: m/z 984.52 [M]+ | - | Not reported | Novel diaxially substituted SiPc complex with high singlet oxygen generation efficiency (ΦΔ = 0.78 in DMSO, 0.69 in DMF in PDT; ΦΔ = 0.94 in DMSO, 0.81 in DMF in SPDT). Candidate for photosensitiser/photosensitiser in PDT, SDT and SPDT, especially for cancer treatment. | [136] |
| Ru-Mal-CTX (Ru-NH2 conjugate with cetuximab via maleimide) | Found: m/z 157,661 (conjugate mass); the difference corresponds to ~3 units of Ru per 1 CTX | Sinapic Acid (SA) | In vitro | First described Ru-CTX conjugate in the literature; successful coupling of approximately 3 Ru fragments per 1 Ab. Less photoactive than free Ru-NH2, but allows targeted delivery of PS. | [155] |
| Ru-BAA-CTX (Ru-NH2 conjugate with cetuximab via benzoylacrylic acid) | Found: m/z 158,503 (conjugate mass); the difference corresponds to ~4 units of Ru per 1 CTX | Sinapic Acid (SA) | In vitro | More stable conjugation method; conjugation of approximately 4 Ru fragments per 1 Ab. Similarly to Ru-Mal-CTX, less photoactive than free Ru-NH2, but important as a selective PS delivery system. | |
| Ir-BODIPY | Calc.: m/z 902.84/Found: m/z 902.866 [M–PF6−] | -. | Not reported | MALDI-TOF confirmed the structure of the complexes. The complexes show high singlet oxygen generation efficiency (1O2) and stronger photosensitising properties than typical BODIPY-PS. The authors indicate potential applications in photodynamic therapy (PDT) and other areas requiring efficient PS. | [138] |
| Ru-BODIPY | Calc.: m/z 815.69/Found: m/z 815.091 [M–2PF6−] | -. | Not reported | ||
| N-allyl bromoporphyrin (non-metalated) | Calc.: 1414.1415/Found: 1419.8168 [M+] | Dithranol or without matrix | In vitro | Highest photocytotoxicity and radiosensitization in the comparisons studied; potential PS for PDT and radiation therapy | [151] |
| 5,10,15,20-Tetrakis(benzo[b]thiophene) porphyrin (BTP) | Calc.: 839.08/Found: 839.2 [M+] | - | In vitro | Confirmed formation of target PS in high purity; effective in PDT against MCF-7 cells, generates ROS, induces DNA fragmentation, controlled subcellular localization affects cell death mechanism | [139] |
| Zn(II) phthalocyanine derivative (Pc1) | Calc.: 1667.10/Found: 1666.11 [M-H]+ | - | In vitro | Structure and formation of target PS confirmed; shows phototoxicity to breast cancer cells, potential PS for PDT | [140] |
| Zn(II) phthalocyanine derivative (Pc2) | Calc.: 1667.10/Found: 1664.64 [M-3H]+ | -. | In vitro | Structure and formation of target PS confirmed; shows phototoxicity to breast cancer cells, potential PS for PDT | |
| Si(IV) phthalocyanine derivative (Pc3) | Calc.: 1311.64/Found: 1339.96 [M+Na+5H]+ | - | In vitro | The structure and formation of the target PS was confirmed; intense NMR signals confirm the presence of a Pc ring and chalcone groups, a potential PS for PDT. | |
| 1(4),8(11),15(18),22(25)-Tetrakis(3-(4-methoxy-phenoxy)phthalocyaninato)gallium(III) chloride | Calc.: 1106.18/Found: 1071.68 [M-Cl+H]+ | 2,5-dihydroxybenzoic acid | In vitro | Structure of target PS confirmed; high solubility in many solvents; potential PS for PDT (singlet oxygen ΦΔ = 0.64) | [156] |
| 1(4),8(11),15(18),22(25)-Tetrakis(3-(4-(methylthio)phenoxy)phthalocyaninato)gallium(III) chloride | Calc.: 1170.45/Found: 1135.88 [M-Cl+H]+ | 2,5-dihydroxybenzoic acid | In vitro | Structure of target PS confirmed; high solubility in many solvents; potential PS for PDT (singlet oxygen ΦΔ = 0.56) | |
| Folic acid/hexane-1,6-diamine/4-carboxyphenylporphyrin | Calc.: 1180.494/Found: 1180.47 | α-cyano-4-hydroxy-trans-cinnamic acid (CHCA) | In vitro | Conjugate structure confirmed; high selectivity to cells overexpressing the folate receptor; effective PS in PDT (LD50 = 22.6 J/cm2) | [157] |
| Folic acid/2,2′-(ethylenedioxy)-bis-ethylamine/4-carboxyphenylporphyrin | Calc.: 1212.484/Found: 1212.49 | α-cyano-4-hydroxy-trans-cinnamic acid (CHCA) | In vitro | Confirmed conjugate structure; increased solubility and biocompatibility; high cellular uptake and effective PS in PDT (LD50 = 6.7 J/cm2) | |
| bis{4-[2-(2H-1,2,3-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenoxy]} phthalocyaninato silicon(IV) (SiPc) | Calc.: 1185.49 g/mol/Found: 1185.66 m/z | -_ | Not reported | Potential sono-photosensitizer in SPDTs; high singlet oxygen generation quantum yields (ΦΔ) in different solvents; correct molecular structure confirmed | [142] |
| 2(3),9(10),16(17),23(24)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato zinc(II) (p-ZnPc) | Calc.: 1679.13 g/mol/Found: 1679.44 m/z | - | In vitro | All PS have a similar structural formula, differing in central metal (Zn, Mg, Pb) and location of substituents (peripheral ‘p’ or non-peripheral ‘np’). Structure confirmed; well soluble; potential PS in PDT | [158] |
| 1(4),8(11),15(18),22(25)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato zinc(II) (np-ZnPc) | Calc.: 1679.13 g/mol/Found: 1679.21 m/z | -. | In vitro | ||
| 2(3),9(10),16(17),23(24)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato magnesium(II) (p-MgPc) | Calc.: 1638.06 g/mol/Found: 1638.39 m/z | - | In vitro | ||
| 1(4),8(11),15(18),22(25)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato magnesium(II) (np-MgPc) | Calc.: 1638.06 g/mol/Found: 1638.54 m/z | - | In vitro | ||
| 2(3),9(10),16(17),23(24)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato lead(II) (p-PbPc) | Calc.: 1820.95 g/mol/Found: 1820.11 m/z | -. | In vitro | ||
| 1(4),8(11),15(18),22(25)-Tetrakis-4-(3,4-dimethoxyphenethyl)-5-ethyl-2H-1,2,4-triazol-3(4H)-one phthalocyaninato lead(II) (np-PbPc) | Calc.: 1820.95 g/mol/Found: 1820.19 m/z | - | In vitro |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. The Role of MALDI-TOF Mass Spectrometry in Photodynamic Therapy: From Photosensitizer Design to Clinical Applications. Curr. Issues Mol. Biol. 2025, 47, 834. https://doi.org/10.3390/cimb47100834
Bartusik-Aebisher D, Rogóż K, Aebisher D. The Role of MALDI-TOF Mass Spectrometry in Photodynamic Therapy: From Photosensitizer Design to Clinical Applications. Current Issues in Molecular Biology. 2025; 47(10):834. https://doi.org/10.3390/cimb47100834
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Kacper Rogóż, and David Aebisher. 2025. "The Role of MALDI-TOF Mass Spectrometry in Photodynamic Therapy: From Photosensitizer Design to Clinical Applications" Current Issues in Molecular Biology 47, no. 10: 834. https://doi.org/10.3390/cimb47100834
APA StyleBartusik-Aebisher, D., Rogóż, K., & Aebisher, D. (2025). The Role of MALDI-TOF Mass Spectrometry in Photodynamic Therapy: From Photosensitizer Design to Clinical Applications. Current Issues in Molecular Biology, 47(10), 834. https://doi.org/10.3390/cimb47100834








