Quinoa and Colonic Health: A Review of Bioactive Components and Mechanistic Insights
Abstract
1. Introduction
1.1. The Importance of Colonic Health
1.2. Introduction of Quinoa
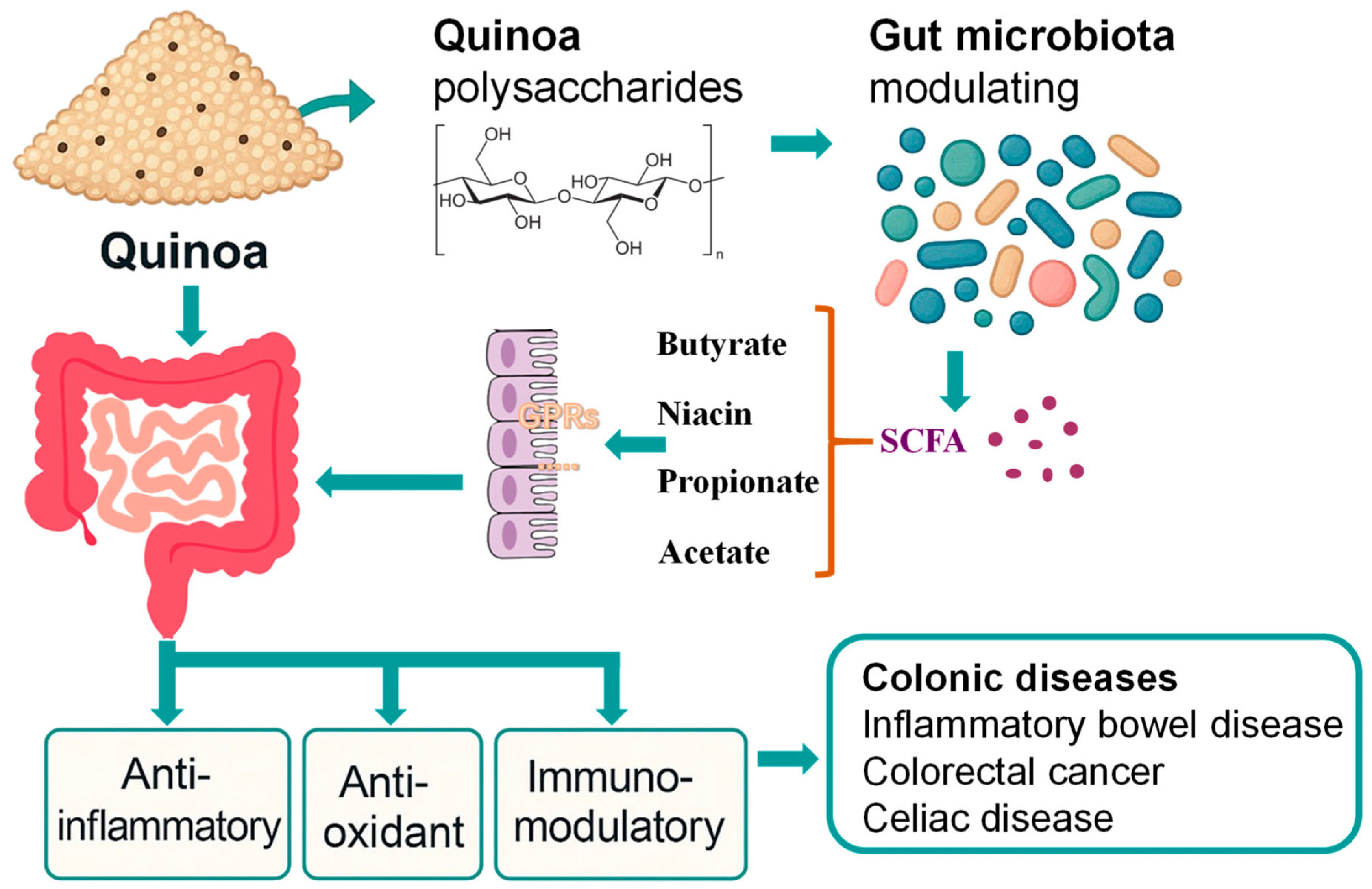
2. Effects of Quinoa Polysaccharides on Colon Health
2.1. Structural and Functional Analysis of Quinoa Polysaccharides
2.2. Quinoa Polysaccharides and the Gut Microbiota
2.3. Challenges in Development of Quinoa Polysaccharides
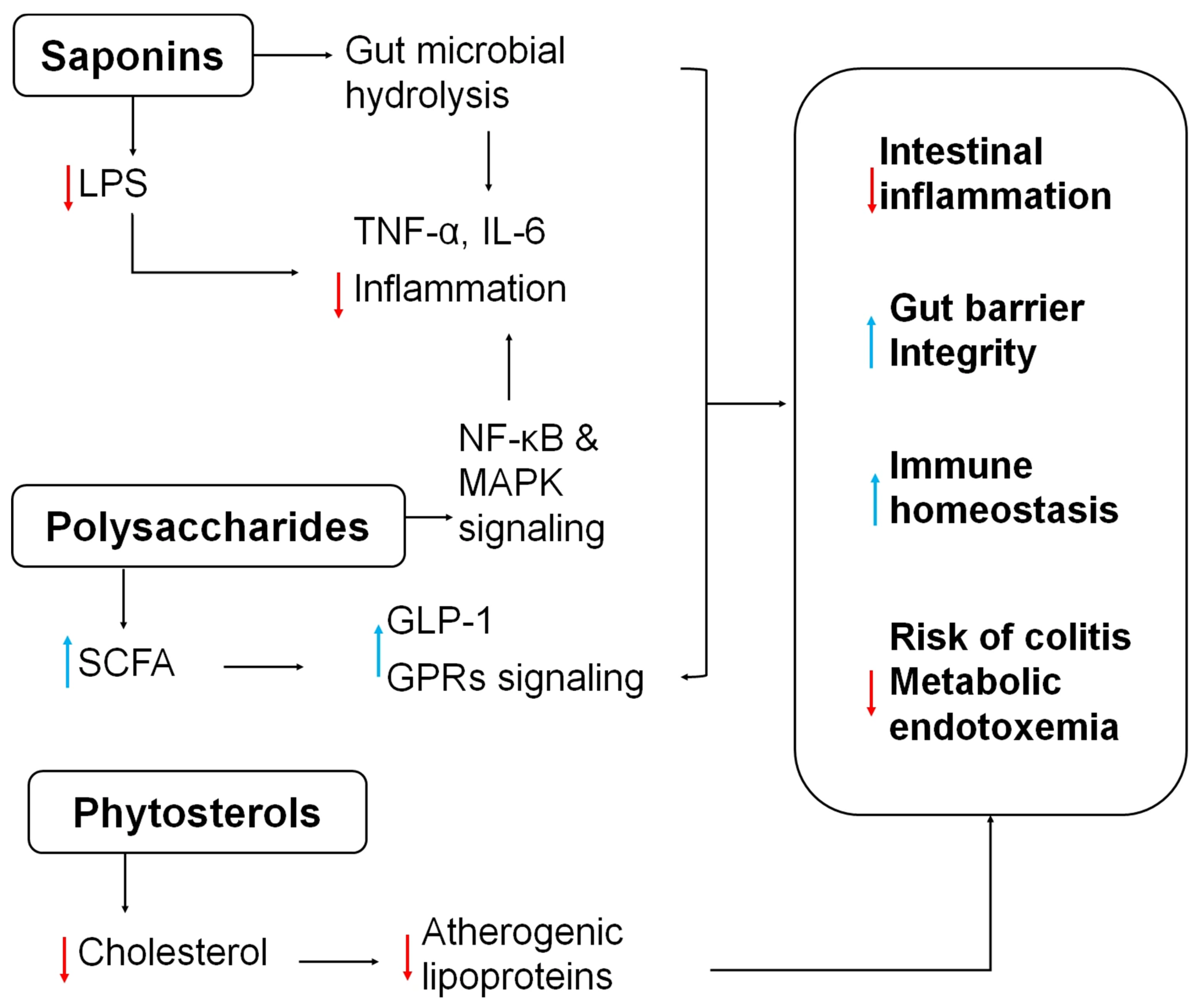
3. The Auxiliary Role of Quinoa in Alleviating Intestinal Inflammation
4. Quinoa’s Supportive Role in the Management of Celiac Disease
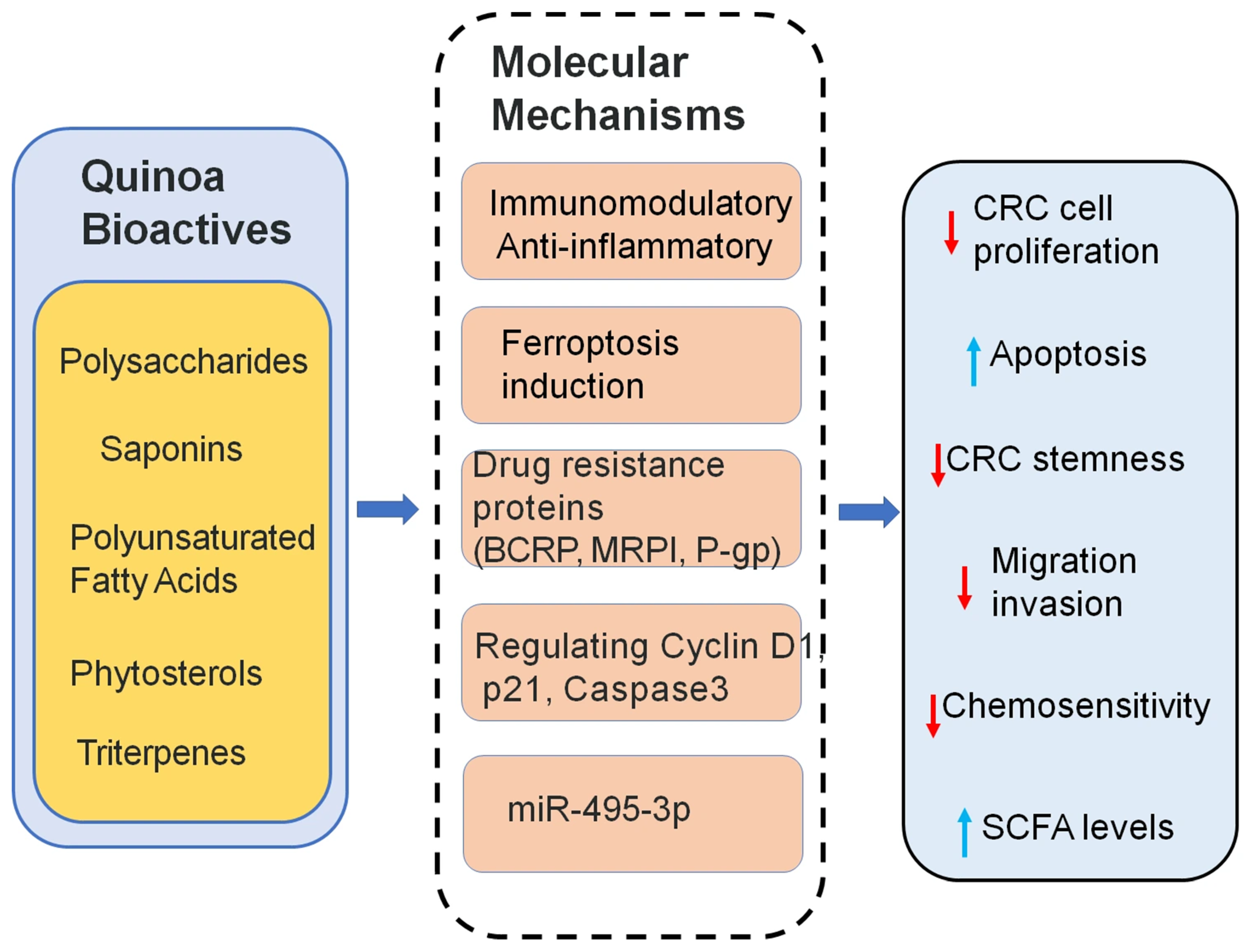
5. Quinoa’s Supportive Role in Colorectal Cancer Treatment
5.1. Overview of Colorectal Cancer
5.2. The Role of Quinoa Fatty Acids in CRC Treatment
5.3. Quinoa Saponins in CRC Therapy
5.4. Quinoa Triterpenes in CRC Therapy
5.5. Quinoa Polysaccharides in CRC Therapy
6. Conclusions
| Component | Key Pathway | Biological Function | Target Outcome |
|---|---|---|---|
| Polysaccharides | SCFA-GPRs activation HDAC inhibition TLR4/NF-κB | Immunomodulation, gut microbiota modulation, SCFA production | Alleviate colitis Enhance gut integrity Improved barrier integrity |
| Saponins | LPS suppression Regulating Cyclin D1, p21, Caspase3 | Anti-inflammatory, induce apoptosis and autophagy in CRC cells | Suppress CRC cell growth and migration |
| Polyunsaturated Fatty Acids (PUFAs) | Ferroptosis induction via SLC7A11 inhibition | Ferroptosis induction, downregulate drug-resistance protein | Reverse chemoresistance in CRC |
| Phytosterols | Cholesterol-lowering | Suppressing atherogenic lipoproteins | Anti-inflammatory cardiometabolic support |
| Triterpenes | miR-495-3p demethylation | miRNA modulation, apoptosis induction | Inhibit CRC progression |
| Natural gluten-free | Gluten-free Remain serum lipid levels | Intestinal homeostasis improves histological and serological profiles | Gluten-free diet in celiac patients |
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CRC | Colorectal cancer |
| QPs | Quinoa polysaccharides |
| FAO | Food and Agriculture Organization |
| NMR | Nuclear magnetic resonance |
| MS | Mass spectrometry |
| NK | Natural killer |
| SCFAs | Short-chain fatty acids |
| GLP-1 | Glucagon-like peptide-1 |
| LPS | Lipopolysaccharide |
| HDAC | Histone deacetylase |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| DSS | Dextran sulfate sodium |
| PUFAs | Polyunsaturated fatty acids |
| P-gp | P-glycoprotein |
| MRP1 | Multidrug Resistance-associated Protein 1 |
| BCRP | Breast Cancer Resistance Protein |
| QBT | Quinoa-derived terpenoid compounds |
References and Note
- Yang, K.Q.; Zhang, C.H.; Gong, R.; Jiang, W.; Ding, Y.C.; Yu, Y.; Chen, J.L.; Zhu, M.; Zuo, J.X.; Huang, X.P.; et al. From west to east: Dissecting the global shift in inflammatory bowel disease burden and projecting future scenarios. BMC Public Health 2025, 25, 2696. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P.F. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.W.K.; Segelov, E.; Deng, Y.; Ho, G.F.; Wang, W.; Han, S.; Sharma, A.; Ding, K.; Chen, G.; Jeffery, M.G.; et al. Aspirin after completion of standard adjuvant therapy for colorectal cancer (ASCOLT): An international, multicentre, phase 3, randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2025, 10, 198–209. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastro Hepat. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Xu, D.C.; Hu, J.L.; Zhong, Y.D.; Zhang, Y.L.; Liu, W.T.; Nie, S.P.; Xie, M.Y. Effects of Rosa roxburghii & edible fungus fermentation broth on immune response and gut microbiota in immunosuppressed mice. Food Sci. Hum. Well 2024, 13, 154–165. [Google Scholar]
- Jancurova, M.; Minarovicová, L.; Dandár, A. Quinoa—A Review. Czech J. Food Sci. 2009, 27, 71–79. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agr. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Maliro, M.F.A.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary Studies of the Performance of Quinoa (Chenopodium quinoa Willd.) Genotypes under Irrigated and Rainfed Conditions of Central Malawi. Front. Plant Sci. 2017, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.P.; Liu, S.X. Effects of separation and purification on structural characteristics of polysaccharide from quinoa (Chenopodium quinoa willd). Biochem. Biophys. Res. Commun. 2020, 522, 286–291. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind. Crop Prod. 2010, 32, 258–263. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Maradini, A.M.; Pirozi, M.R.; Borges, J.T.D.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. 2017, 57, 1618–1630. [Google Scholar]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Martínez, E.A.; Veas, E.; Jorquera, C.; San Martín, R.; Jara, P. Re-Introduction of Quinoa into Arid Chile: Cultivation of Two Lowland Races under Extremely Low Irrigation. J. Agron. Crop Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Brady, K.; Ho, C.T.; Rosen, R.T.; Sang, S.M.; Karwe, M.V. Effects of processing on the nutraceutical profile of quinoa. Food Chem. 2007, 100, 1209–1216. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeon, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Shi, R.Y.; Dan, B.; Lü, L.J. Bioactive effects advances of natural polysaccharides. J. Future Foods 2023, 3, 234–239. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, Z.X.; Ren, G.X. Antioxidant and Immunoregulatory Activity of Polysaccharides from Quinoa (Chenopodium quinoa Willd.). Int. J. Mol. Sci. 2014, 15, 19307–19318. [Google Scholar] [CrossRef]
- Zhu, X.C.; Yang, G.Y.; Shen, Y.B.; Niu, L.Q.; Peng, Y.; Chen, H.T.; Li, H.M.; Yang, X.Q. Physicochemical Properties and Biological Activities of Quinoa Polysaccharides. Molecules 2024, 29, 1576. [Google Scholar] [CrossRef]
- Hu, Y.C.; Zhang, J.M.; Zou, L.; Fu, C.M.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Bai, J.; Buccato, D.G.; Zhang, J.Y.; He, Y.F.; Zhu, Y.; Yang, Z.H.; Xiao, X.; Daglia, M. Cereal-Derived Water-Unextractable Arabinoxylans: Structure Feature, Effects on Baking Products and Human Health. Foods 2024, 13, 2369. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Jia, C.X.; Chen, X.Y.; Zhang, L.; Jiang, Y.; Meng, X.L.; Liu, X.J. Progress in research on the effects of quinoa (Chenopodium quinoa) bioactive compounds and products on intestinal flora. Front. Nutr. 2024, 11, 1308384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, J.; Luo, X.; Mo, M.M.Q.; Li, W.B.; Huang, J.W.; Wang, S.P.; Hu, Y.C.; Zou, L.; Wu, D.T. Potential structure-function relationships of pectic polysaccharides from quinoa microgreens: Impact of various esterification degrees. Food Res. Int. 2024, 187, 114395. [Google Scholar] [CrossRef]
- Fan, S.H.; Li, J.N.; Bai, B.Q. Purification, structural elucidation and in vivo immunity-enhancing activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2019, 83, 2334–2344. [Google Scholar] [CrossRef]
- Li, S.P.; Wu, D.T.; Lv, G.P.; Zhao, J. Carbohydrates analysis in herbal glycomics. TrAC Trends Anal. Chem. 2013, 52, 155–169. [Google Scholar] [CrossRef]
- Teng, C.; Qin, P.Y.; Shi, Z.X.; Zhang, W.Y.; Yang, X.S.; Yao, Y.; Ren, G.X. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocolloid 2021, 113, 106392. [Google Scholar] [CrossRef]
- Tan, M.H.; Zhao, Q.S.; Zhao, B. Physicochemical properties, structural characterization and biological activities of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Int. J. Biol. Macromol. 2021, 193, 1635–1644. [Google Scholar] [CrossRef]
- Huo, Z.Q.; Li, J.X.; Li, X.F.; Xiao, H.; Lin, Y.; Ma, Y.C.; Li, J.R.; Yang, H.; Zhang, C.J. Functional fractions of Astragalus polysaccharides as a potential prebiotic to alleviate ulcerative colitis. Int. J. Biol. Macromol. 2024, 271, 132580. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Huang, H.L.; Huang, G.L. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019, 140, 1047–1053. [Google Scholar] [CrossRef]
- Zhu, R.G.; Zhang, X.Y.; Wang, Y.; Zhang, L.J.; Zhao, J.; Chen, G.; Fan, J.G.; Jia, Y.F.; Yan, F.W.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohyd Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Lung, M.Y.; Chang, Y.C. antioxidant properties of polysaccharides from Armillaria mellea in batch fermentation. Afr. J. Biotechnol. 2011, 10, 7048–7057. [Google Scholar]
- Lei, T.; Li, H.F.; Fang, Z.; Lin, J.B.; Wang, S.S.; Xiao, L.Y.; Yang, F.; Liu, X.; Zhang, J.J.; Huang, Z.B.; et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen. Res. 2014, 9, 260–267. [Google Scholar] [CrossRef]
- Xiong, S.L.; Li, A.L.; Huang, N.; Lu, F.; Hou, D.B. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohyd. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Luis, A.S.; Baslé, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.Y.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70, Correction in Nature 2017, 548, 612. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Lai, W.J.; Wang, C.Y.; Lai, R.F.; Peng, X.C.; Luo, J.M. Lycium barbarum polysaccharide modulates gut microbiota to alleviate rheumatoid arthritis in a rat model. Npj Sci. Food 2022, 6, 34. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.P.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. Ebiomedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wang, Y.B.; Yang, G.; Zhang, Q.H.; Meng, L.B.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ge, F.; Yao, X.X.; Guo, X.; Bao, P.J.; Ma, X.M.; Wu, X.Y.; Chu, M.; Yan, P.; Liang, C.N. Microbiome and Metabolomics Reveal the Effects of Different Feeding Systems on the Growth and Ruminal Development of Yaks. Front. Microbiol. 2021, 12, 682989. [Google Scholar] [CrossRef]
- Lei, Y.Y.; Tang, L.; Liu, S.; Hu, S.P.; Wu, L.Y.; Liu, Y.J.; Yang, M.; Huang, S.J.; Tang, X.F.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef] [PubMed]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Hickey, J.W.; Becker, W.R.; Nevins, S.A.; Horning, A.; Perez, A.E.; Zhu, C.C.; Zhu, B.K.; Wei, B.; Chiu, R.; Chen, D.C.; et al. Organization of the human intestine at single-cell resolution. Nature 2023, 619, 572–584. [Google Scholar] [CrossRef]
- Cheng, A.G.; Ho, P.Y.; Aranda-Díaz, A.; Jain, S.; Yu, F.Q.B.; Meng, X.D.; Wang, M.; Iakiviak, M.; Nagashima, K.; Zhao, A.S.; et al. Design, construction, and in vivo augmentation of a complex gut microbiome. Cell 2022, 185, 3617–3636.e19. [Google Scholar] [CrossRef]
- Davey, L.E.; Malkus, P.N.; Villa, M.; Dolat, L.; Holmes, Z.C.; Letourneau, J.; Ansaldo, E.; David, L.A.; Barton, G.M.; Valdivia, R.H. A genetic system for Akkermansia muciniphila reveals a role for mucin foraging in gut colonization and host sterol biosynthesis gene expression. Nat. Microbiol. 2023, 8, 1450–1467. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Ioannou, A.; Berkhout, M.D.; Geerlings, S.Y.; Belzer, C. Akkermansia muciniphila: Biology, microbial ecology, host interactions and therapeutic potential. Nat. Rev. Microbiol. 2024, 23, 162–177. [Google Scholar] [CrossRef]
- Hou, Q.H.; Jia, J.P.; Lin, J.; Zhu, L.D.; Xie, S.; Yu, Q.H.; Li, Y.C. programs the differentiation of intestinal secretory lineages to inhibit Salmonella infection. Cell Rep. 2022, 40, 111416. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Fuentes, F.F.; Paredes-Gonzalez, X. Nutraceutical Perspectives of Quinoa: Biological Properties and Functional Applications. In The State of the World’s Quinoa; Regional Office for Latin America and Caribbean at Food and Agriculture Organization (FAO): Santiago, Chile, 2015; pp. 286–299. [Google Scholar]
- Roubille, C.; Martel-Pelletier, J.; Davy, J.-M.; Haraoui, B.; Pelletier, J.-P. Cardiovascular Adverse Effects of Anti-Inflammatory Drugs. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2013, 12, 55–67. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, Y.; Cao, Y.N.; Zhang, L.L.; Zhao, G.; Wu, D.T.; Zou, L. Total saponins from quinoa bran alleviate high-fat diet-induced obesity and systemic inflammation via regulation of gut microbiota in rats. Food Sci. Nutr. 2022, 10, 3876–3889. [Google Scholar] [CrossRef]
- Del Hierro, J.N.; Cueva, C.; Tamargo, A.; Núñez-Gómez, E.; Moreno-Arribas, M.V.; Reglero, G.; Martin, D. In Vitro Colonic Fermentation of Saponin-Rich Extracts from Quinoa, Lentil, and Fenugreek. Effect on Sapogenins Yield and Human Gut Microbiota. J. Agr. Food Chem. 2020, 68, 106–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, R.J.; Chen, J.D.; Huang, K.; Li, S.; Cao, H.W.; Guan, X. Gut-Derived Ursodeoxycholic Acid from Saponins of Quinoa Regulated Colitis via Inhibiting the TLR4/NF-κB Pathway. J. Agr. Food Chem. 2025, 73, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Food Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef]
- Graf, B.L.; Cheng, D.M.; Esposito, D.; Shertel, T.; Poulev, A.; Plundrich, N.; Itenberg, D.; Dayan, N.; Lila, M.A.; Raskin, I. Compounds leached from quinoa seeds inhibit matrix metalloproteinase activity and intracellular reactive oxygen species. Int. J. Cosmet. Sci. 2015, 37, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Pal, S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells. Atherosclerosis 2005, 182, 29–36. [Google Scholar] [CrossRef]
- Cao, R.A.; Ma, N.; Subramanian, P.; Talapphet, N.; Zhang, J.M.; Wang, C.Y.; You, S.G. Structural Elucidation and Immunostimulatory Activities of Quinoa Non-starch Polysaccharide Before and After Deproteinization. J. Polym. Environ. 2022, 30, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Zeyneb, H.; Pei, H.R.; Cao, X.L.; Wang, Y.X.; Win, Y.; Gong, L.X. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745, 2540. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y.C. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.W.; Mai, P.S.; Hao, Y.M.; Wang, Z.Y.; Wang, J. Quinoa bran soluble dietary fiber ameliorates dextran sodium sulfate induced ulcerative colitis in BALB/c mice by maintaining intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa: An Indian perspective. Ind. Crop Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Peñas, E.; Uberti, F.; di Lorenzo, C.; Ballabio, C.; Brandolini, A.; Restani, P. Biochemical and Immunochemical Evidences Supporting the Inclusion of Quinoa (Chenopodium quinoa Willd.) as a Gluten-free Ingredient. Plant Food Hum. Nutr. 2014, 69, 297–303. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Herencia, L.I.; Chang, F.J.; Donnelly, S.; Ellis, H.J.; Ciclitira, P.J. Gastrointestinal Effects of Eating Quinoa (Chenopodium quinoa Willd.) in Celiac Patients. Am. J. Gastroenterol. 2014, 109, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A.; Hanahan, D. The hallmarks of cancer. Cell Press. 2000, 100, 57–70. [Google Scholar]
- Li, L.C.; Carroll, P.R.; Dahiya, R. Epigenetic changes in prostate cancer: Implication for diagnosis and treatment. J. Natl. Cancer Inst. 2005, 97, 103–115. [Google Scholar] [CrossRef]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Kong, A.N. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Jayasinghe, M.; Prathiraja, O.; Caldera, D.; Jena, R.; Coffie-Pierre, J.A.; Silva, M.S.; Siddiqui, O.S. Colon Cancer Screening Methods: 2023 Update. Cureus J. Med. Sci. 2023, 15, e37509. [Google Scholar] [CrossRef]
- Morii, Y.; Tsubaki, M.; Takeda, T.; Otubo, R.; Seki, S.; Yamatomo, Y.; Imano, M.; Satou, T.; Shimomura, K.; Nishida, S. Perifosine enhances the potential antitumor effect of 5-fluorourasil and oxaliplatin in colon cancer cells harboring the PIK3CA mutation. Eur. J. Pharmacol. 2021, 898, 173957. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Mitra, A.; Pathak, S.; Prasad, S.; Zhang, A.S.; Zhang, H.; Sun, X.F.; Banerjee, A. Recent Advancements, Limitations, and Future Perspectives of the use of Personalized Medicine in Treatment of Colon Cancer. Technol. Cancer Res. Treat. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Wang, D.Y.; Thrift, A.P.; Zarrin-Khameh, N.; Wichmann, A.; Armstrong, G.N.; Thompson, P.A.; Bondy, M.L.; Musher, B.L. Rising Incidence of Colorectal Cancer Among Young Hispanics in Texas. J. Clin. Gastroenterol. 2017, 51, 34–42. [Google Scholar] [CrossRef]
- James, L.E.A. Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Ng, S.C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of lipid oxidation products in quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Li, S.T.; Ding, M.; Feng, M.M.; Fan, X.X.; Li, Z.Y. Polyunsaturated Fatty Acids in Quinoa Induce Ferroptosis of Colon Cancer by Suppressing Stemness. J. Agr. Food Chem. 2024, 72, 16152–16162, Correction in J. Agr. Food Chem. 2025, 73, 3280–3281. [Google Scholar] [CrossRef]
- Nagano, O.; Okazaki, S.; Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 2013, 32, 5191–5198. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc- and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Shang, H.J.; Sun, J.W.; Zheng, Z.; Sun, S.J.; Yan, X.M. Study on the Effect of Quinoa Saponins on Human Colon Cancer HT-29 Cells. Food Sci. Nutr. 2025, 13, e4669. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, X.C.; Shi, J.Y.; Cui, K.L.; Yang, R.P.; Liu, F.M.; Shan, S.H.; Israr, G.; Li, Z.Y. Terpenoids of quinoa bran suppresses colorectal cancer by inducing cell apoptosis. Food Biosci. 2023, 53, 102615. [Google Scholar] [CrossRef]
- Feng, M.M.; Fan, X.X.; Shi, J.Y.; Shan, S.H.; Li, S.T.; He, S.L.; Ding, M.; Li, Z.Y. Terpenoids from quinoa reverse drug resistance of colon cancer by upregulating miR-495-3p. J. Sci. Food Agr. 2024, 104, 8916–8927. [Google Scholar] [CrossRef]
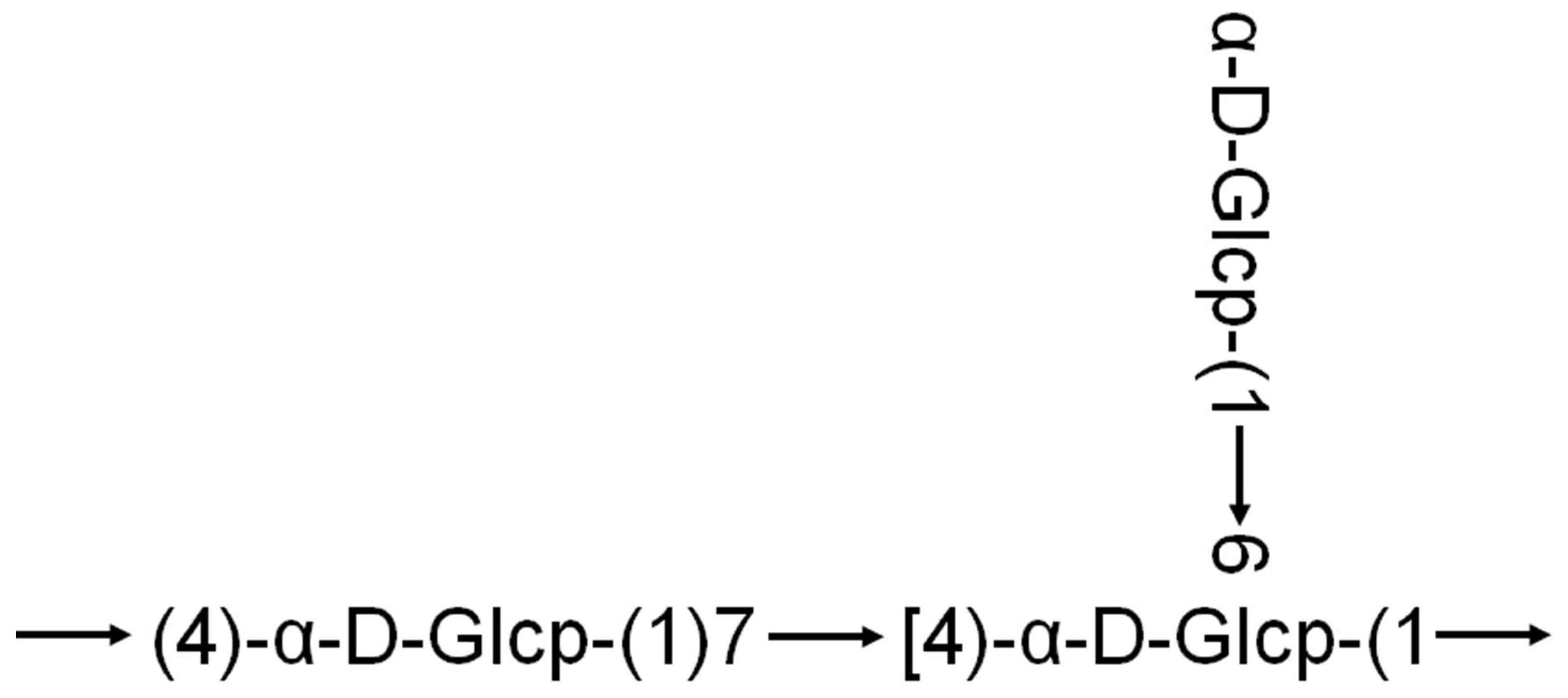
| Structural Feature | Functional Implication | Supporting Studies |
|---|---|---|
| Molecular weight (10–100 kDa) | Influences fermentation rate and SCFA production | [30,31,32] |
| Arabinose/Galactose branching | Enhances immunomodulatory activity | [27,28] |
| Triple-helix conformation | Improves antioxidant and prebiotic effects | [30,31] |
| Degree of esterification | Negatively correlated with anti-glycation and immunostimulation | [27] |
| α-/β-configured glycosidic linkages | Determines microbiota selectivity | [28] |
| Disease | Study Type | Quinoa Component | References |
|---|---|---|---|
| Celiac disease | Clinical | Whole quinoa (gluten-free diet) | [74] |
| Colitis/gut inflammation (DSS, LPS, systemic models) | Animal | Polysaccharides | [60,69,70] |
| Saponins | [61,63] | ||
| Bran soluble dietary fiber | [71] | ||
| Colorectal cancer | Animal | PUFAs | [86] |
| Terpenoids | [90,91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Zheng, J.; Wang, Z.; Lin, S.; Jia, H.; Pei, H.; Ju, R. Quinoa and Colonic Health: A Review of Bioactive Components and Mechanistic Insights. Curr. Issues Mol. Biol. 2025, 47, 815. https://doi.org/10.3390/cimb47100815
Pan Y, Zheng J, Wang Z, Lin S, Jia H, Pei H, Ju R. Quinoa and Colonic Health: A Review of Bioactive Components and Mechanistic Insights. Current Issues in Molecular Biology. 2025; 47(10):815. https://doi.org/10.3390/cimb47100815
Chicago/Turabian StylePan, Yan, Jimin Zheng, Zhixuan Wang, Shaohua Lin, Hongliang Jia, Hairun Pei, and Ronghui Ju. 2025. "Quinoa and Colonic Health: A Review of Bioactive Components and Mechanistic Insights" Current Issues in Molecular Biology 47, no. 10: 815. https://doi.org/10.3390/cimb47100815
APA StylePan, Y., Zheng, J., Wang, Z., Lin, S., Jia, H., Pei, H., & Ju, R. (2025). Quinoa and Colonic Health: A Review of Bioactive Components and Mechanistic Insights. Current Issues in Molecular Biology, 47(10), 815. https://doi.org/10.3390/cimb47100815






