Activation of Angiogenic TGF-β1 by Salbutamol Enhances Wound Contraction and Improves Healing in a Streptozotocin-Induced Diabetic Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ethical Approval and Animal Procurement
2.2.2. Grouping and Induction of Diabetes
2.3. Wound Creation and Hemostasis
2.4. Experimental Design and Treatment
2.5. Post-Treatment Monitoring
2.6. Measurement of the Wound Area and Percentage Contraction
2.7. Wound Standardization
2.8. Biochemical Analysis
2.9. Hexosamine
2.10. Cytokine Estimation
2.11. Molecular Docking Analysis
2.12. Statistical Analysis
3. Results
3.1. Wound Contraction
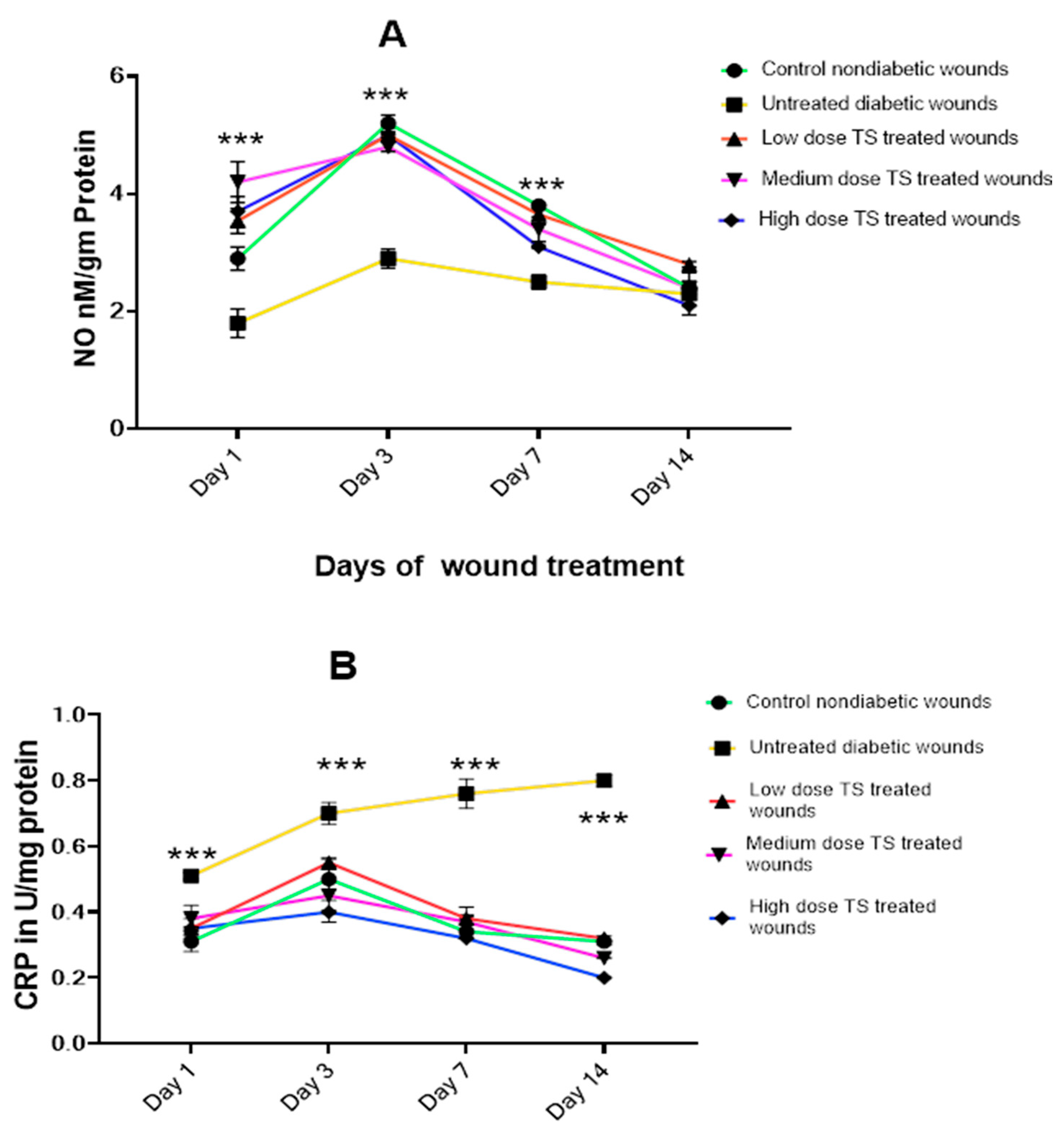
3.2. Oxidative Markers Involved in Wound Healing
3.2.1. Wound Exudate
3.2.2. Wound Tissue Oxidant Markers
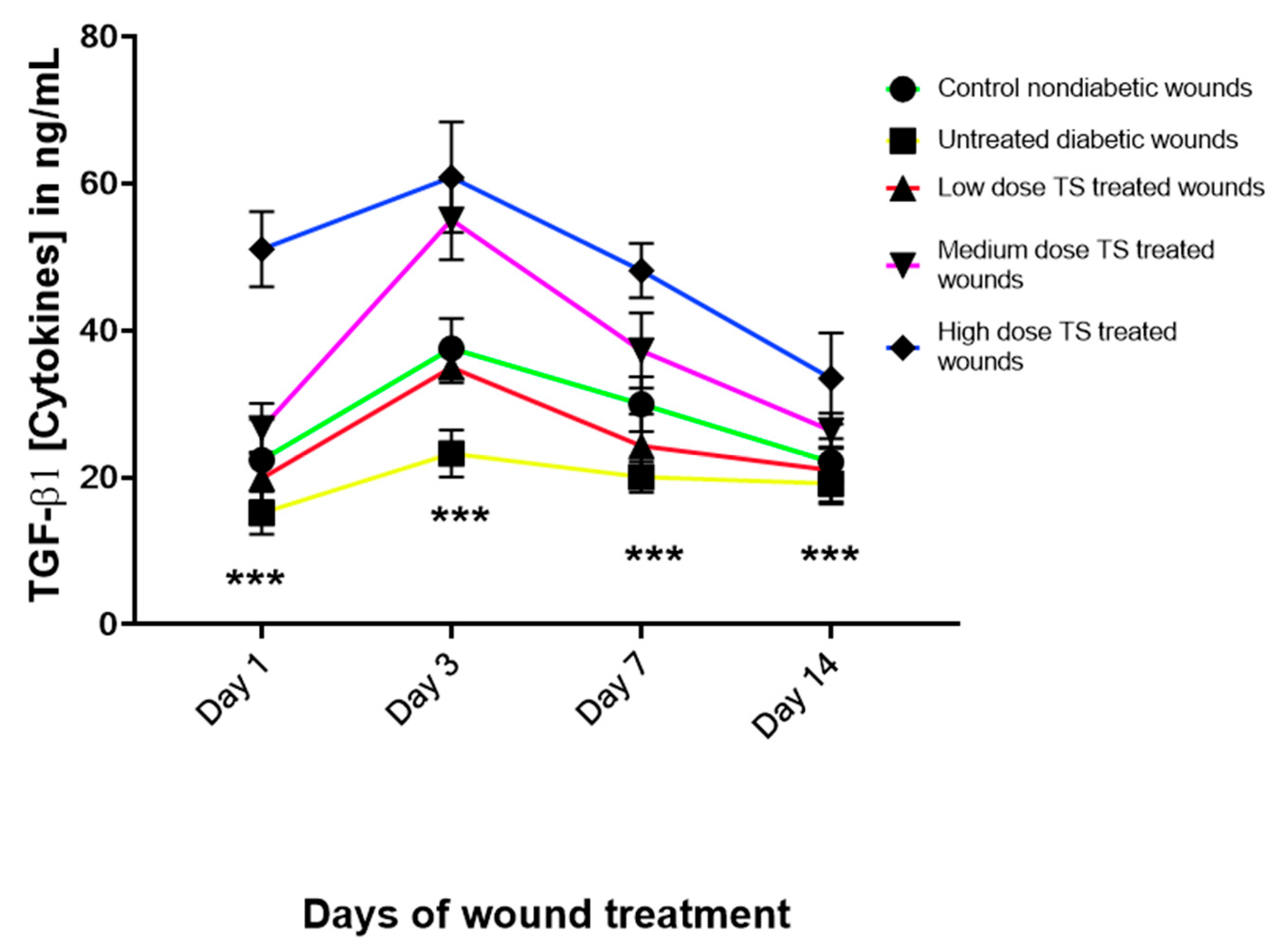
3.3. Levels of Angiogenic Marker Transforming Growth Factor Beta 1 (TGF-
3.4. Proinflammatory Cytokine Concentrations
3.5. Hexosamine Activity in Diabetic Excision Wounds
3.6. Molecular Docking of Salbutamol and TGF-β1
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Mi, B.; Xiong, Y.; Fu, Z.; Zhou, W.; Liu, W.; Liu, G.; Dai, G. Angiogenesis during diabetic wound repair: From mechanism to therapy opportunity. Burn. Trauma 2025, 13, tkae052. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Cardoso, S.H.; de Oliveira, C.R.; Guimaraes, A.S.; Nascimento, J.; de Oliveira Dos Santos Carmo, J.; de Souza Ferro, J.N.; de Carvalho Correia, A.C.; Barreto, E. Synthesis of newly functionalized 1,4-naphthoquinone derivatives and their effects on wound healing in alloxan-induced diabetic mice. Chem. Biol. Interact. 2018, 291, 55–64. [Google Scholar] [CrossRef]
- Lang, X.; Li, L.; Li, Y.; Feng, X. Effect of Diabetes on Wound Healing: A Bibliometrics and Visual Analysis. J. Multidiscip. Healthc. 2024, 17, 1275–1289. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Daskalopoulou, I.; Frountzi, S.; Papadimitriou, E. A Systematic Review on the Role of Adrenergic Receptors in Angiogenesis Regulation in Health and Disease. Int. J. Transl. Med. 2021, 1, 353–365. [Google Scholar] [CrossRef]
- Galasso, G.; De Rosa, R.; Ciccarelli, M.; Sorriento, D.; Del Giudice, C.; Strisciuglio, T.; De Biase, C.; Luciano, R.; Piccolo, R.; Pierri, A.; et al. beta2-Adrenergic receptor stimulation improves endothelial progenitor cell-mediated ischemic neoangiogenesis. Circ. Res. 2013, 112, 1026–1034. [Google Scholar] [CrossRef]
- Rajic, J.; Grdovic, N.; Markovic, A.; Skoro, N.; Dinic, S.; Uskokovic, A.; Arambasic Jovanovic, J.; Dordevic, M.; Saric, A.; Vidakovic, M.; et al. Plasma-Activated Water Improve Wound Healing in Diabetic Rats by Influencing the Inflammatory and Remodelling Phase. Int. J. Mol. Sci. 2025, 26, 1265. [Google Scholar] [CrossRef]
- Stohl, L.L.; Zang, J.B.; Ding, W.; Manni, M.; Zhou, X.K.; Granstein, R.D. Norepinephrine and adenosine-5’-triphosphate synergize in inducing IL-6 production by human dermal microvascular endothelial cells. Cytokine 2013, 64, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Tilan, J.; Kitlinska, J. Sympathetic Neurotransmitters and Tumor Angiogenesis-Link between Stress and Cancer Progression. J. Oncol. 2010, 2010, 539706. [Google Scholar] [CrossRef] [PubMed]
- Le Provost, G.S.; Pullar, C.E. β2-adrenoceptor activation modulates skin wound healing processes to reduce scarring. J. Invest. Dermatol. 2015, 135, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Jemec, G.B.; Ullman, S.; Goodfield, M.; Bygum, A.; Olesen, A.B.; Berth-Jones, J.; Nyberg, F.; Cramers, M.; Faergemann, J.; Andersen, P.; et al. A randomized controlled trial of R-salbutamol for topical treatment of discoid lupus erythematosus. Br. J. Dermatol. 2009, 161, 1365–1370. [Google Scholar] [CrossRef]
- Yiblet, T.G.; Tsegaw, A.; Ahmed, N.; Dagnew, S.B.; Tadesse, T.Y.; Kifle, Z.D. Evaluation of Wound Healing Activity of 80% Methanol Root Crude Extract and Solvent Fractions of Stephania abyssinica (Dill. & A. Rich.) Walp. (Menispermaceae) in Mice. J. Exp. Pharmacol. 2022, 14, 255–273. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress and Skin Microbiome are Critical for Initiation and Development of Chronic Wounds in Diabetic Mice. Sci. Rep. 2019, 9, 19318. [Google Scholar] [CrossRef]
- Blume, P.; Bowlby, M.; Schmidt, B.M.; Donegan, R. Safety and Efficacy of Becaplermin Gel in the Treatment of Diabetic Foot Ulcers. Chronic Wound Care M. 2014, 1, 11–14. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Gluud, C.; Nicola, S.; Simancas-Racines, D.; Reveiz, L.; Oliva, P.; Cedeño-Taborda, J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 2015, 2015, CD008548. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Akbarzadeh-Atashkhosrow, A.; Maghsudi, M.; Najafi, M. Effects of salbutamol on the inflammatory parameters and angiogenesis in the rat air pouch model of inflammation. Res. Pharm. Sci. 2017, 12, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Trimarco, B.; Iaccarino, G. Adrenergic mechanism in the control of endothelial function. Transl. Med. UniSa 2011, 1, 213–228. [Google Scholar] [PubMed]
- Lemmens, S.; Kusters, L.; Bronckaers, A.; Geurts, N.; Hendrix, S. The β2-Adrenoceptor Agonist Terbutaline Stimulates Angiogenesis via Akt and ERK Signaling. J. Cell. Physiol. 2017, 232, 298–308. [Google Scholar] [CrossRef]
- Iaccarino, G.; Ciccarelli, M.; Sorriento, D.; Galasso, G.; Campanile, A.; Santulli, G.; Cipolletta, E.; Cerullo, V.; Cimini, V.; Altobelli, G.G.; et al. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: A novel role for the endothelial adrenergic system. Circ. Res. 2005, 97, 1182–1189. [Google Scholar] [CrossRef]
- Pan, L.; Tang, J.; Liu, H.; Cheng, B. Sympathetic nerves: How do they affect angiogenesis, particularly during wound healing of soft tissues? Clin. Hemorheol. Microcirc. 2016, 62, 181–191. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Monte-Alto-Costa, A. Simultaneous blockade of alpha and beta adrenoceptors impairs cutaneous wound healing in rats. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 349–352. [Google Scholar] [CrossRef]
- Lefrandt, J.D.; Bosma, E.; Oomen, P.H.; Hoeven, J.H.; Roon, A.M.; Smit, A.J.; Hoogenberg, K. Sympathetic mediated vasomotion and skin capillary permeability in diabetic patients with peripheral neuropathy. Diabetologia 2003, 46, 40–47. [Google Scholar] [CrossRef]
- Sivaraj, D.; Noishiki, C.; Kosaric, N.; Kiwanuka, H.; Kussie, H.C.; Henn, D.; Fischer, K.S.; Trotsyuk, A.A.; Greco, A.H.; Kuehlmann, B.A.; et al. Nitric oxide-releasing gel accelerates healing in a diabetic murine splinted excisional wound model. Front. Med. 2023, 10, 1060758. [Google Scholar] [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef]
- Yu, C.O.; Leung, K.S.; Fung, K.P.; Lam, F.F.; Ng, E.S.; Lau, K.M.; Chow, S.K.; Cheung, W.H. The characterization of a full-thickness excision open foot wound model in n5-streptozotocin (STZ)-induced type 2 diabetic rats that mimics diabetic foot ulcer in terms of reduced blood circulation, higher C-reactive protein, elevated inflammation, and reduced cell proliferation. Exp. Anim. 2017, 66, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the Biological Effects of Lyophilized Hydrophilic Extract of Rhus coriaria on Myeloperoxidase (MPO) Activity, Wound Healing, and Microbial Infections of Skin Wound Tissues. Evid. Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef] [PubMed]
- Nall, A.V.; Brownlee, R.E.; Colvin, C.P.; Schultz, G.; Fein, D.; Cassisi, N.J.; Nguyen, T.; Kalra, A. Transforming growth factor beta 1 improves wound healing and random flap survival in normal and irradiated rats. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Tang, T.; Qi, W.Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Kwan, H.H.; Kowalski, J.; Prionas, S.D.; Allison, A.C. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 1992, 140, 539–544. [Google Scholar]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef]
- Anushree, U.; Punj, P.; Vasumathi; Bharati, S. Phosphorylated chitosan accelerates dermal wound healing in diabetic wistar rats. Glycoconj. J. 2023, 40, 19–31. [Google Scholar] [CrossRef]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complement. Altern. Med. 2012, 12, 226. [Google Scholar] [CrossRef]
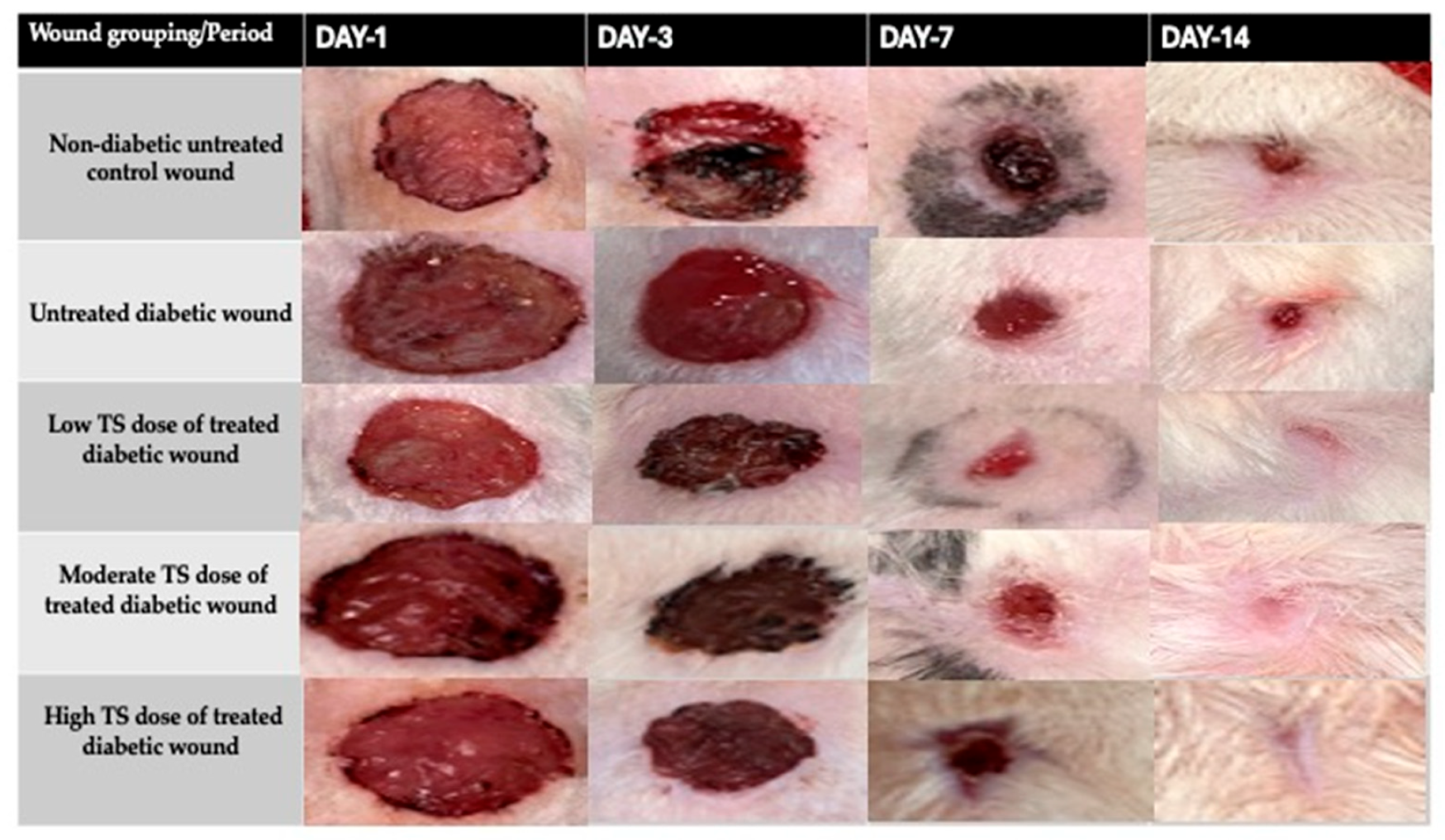




| Treatment Groups | Day 1 | Day 3 | Day 7 | Day 14 |
|---|---|---|---|---|
| Non-diabetic Control | A = 1.85 ± 0.58 cm2 WC = 0% | A = 1.53 ± 0.55 cm2 WC = 17% * | A = 1.081 ± 0.34 cm2 WC = 41% | A = 0.175 ± 0.10 cm2 WC = 91% |
| Untreated diabetic | A = 1.68 ± 0.36 cm2 WC = 0% | A = 1.54 ± 0.38 cm2 WC = 8.3% | A= 1.111 ± 0.19 cm2 WC = 34% | A = 0.291 ± 0.2 cm2 WC = 83% |
| Low-dose TS | A = 1.92 ± 0.33 cm2 WC = 0% | A = 1.41 ± 0.09 cm2 WC = 26.7% *** | A = 0.9 ± 0.11 cm2 WC = 53.18% ** | WC = 100% *** |
| Medium-dose TS | A = 1.95 ± 0.059 cm2 WC = 0% | A = 1.54 ± 0.2 cm2 WC = 20.9% * | A = 0.895 ± 0.11 cm2 WC = 54.1% ** | WC = 100% *** |
| High-dose TS | A = 2.6 ± 0.39 cm2 WC = 0% | A = 1.99 ± 0.01 cm2 SWC = 23.5% ** | A = 1.1 ± 0.07 cm2 WC = 57.7% *** | WC = 100% *** |
| Ligand | PubChem ID | Binding Energy | Ligand Efficiency | Intermolecular Energy | Docked Amino Acid Residue (Bond Length) |
|---|---|---|---|---|---|
| Salbutamol | 2083 | −5.96 | −0.35 | −6.95 | Chain A: ALA′75′(3.25 Å) Chain A: ALA′75′(3.10 Å) Chain B: GLY′46′(2.97 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeka, P.M.; Al Mouslem, A.K.; Almutawa, H.; Albandri, M.; Alhmoud, H.; Alhelal, M.; Alhassan, Z.; Alhamar, A. Activation of Angiogenic TGF-β1 by Salbutamol Enhances Wound Contraction and Improves Healing in a Streptozotocin-Induced Diabetic Rat Model. Curr. Issues Mol. Biol. 2025, 47, 820. https://doi.org/10.3390/cimb47100820
Emeka PM, Al Mouslem AK, Almutawa H, Albandri M, Alhmoud H, Alhelal M, Alhassan Z, Alhamar A. Activation of Angiogenic TGF-β1 by Salbutamol Enhances Wound Contraction and Improves Healing in a Streptozotocin-Induced Diabetic Rat Model. Current Issues in Molecular Biology. 2025; 47(10):820. https://doi.org/10.3390/cimb47100820
Chicago/Turabian StyleEmeka, Promise M., Abdulaziz K. Al Mouslem, Hussien Almutawa, Malek Albandri, Hussain Alhmoud, Mohammed Alhelal, Zakaria Alhassan, and Abdullah Alhamar. 2025. "Activation of Angiogenic TGF-β1 by Salbutamol Enhances Wound Contraction and Improves Healing in a Streptozotocin-Induced Diabetic Rat Model" Current Issues in Molecular Biology 47, no. 10: 820. https://doi.org/10.3390/cimb47100820
APA StyleEmeka, P. M., Al Mouslem, A. K., Almutawa, H., Albandri, M., Alhmoud, H., Alhelal, M., Alhassan, Z., & Alhamar, A. (2025). Activation of Angiogenic TGF-β1 by Salbutamol Enhances Wound Contraction and Improves Healing in a Streptozotocin-Induced Diabetic Rat Model. Current Issues in Molecular Biology, 47(10), 820. https://doi.org/10.3390/cimb47100820






