Challenges of Ozone Therapy in Periodontal Regeneration: A Narrative Review and Possible Therapeutic Improvements
Abstract
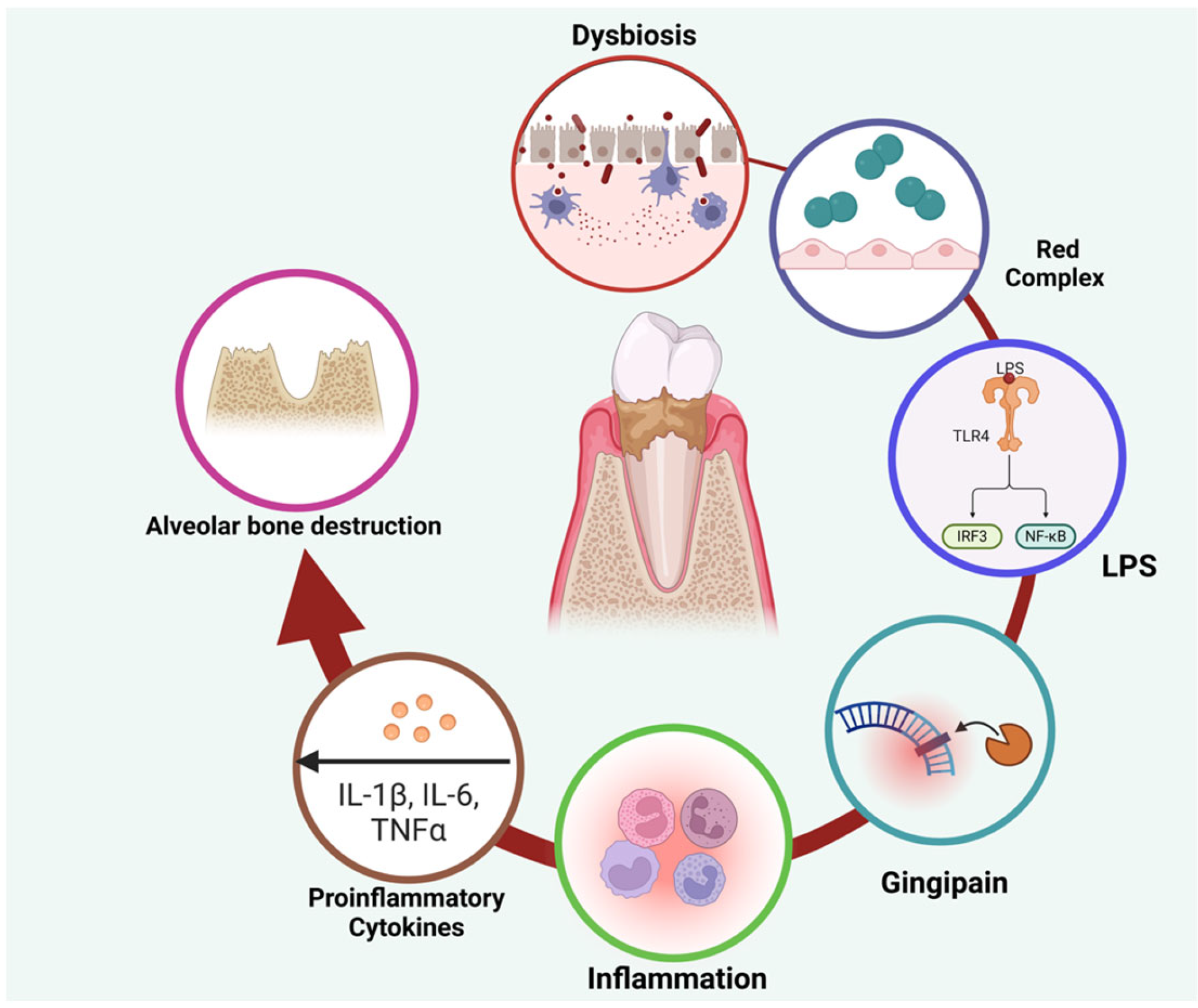
1. Background
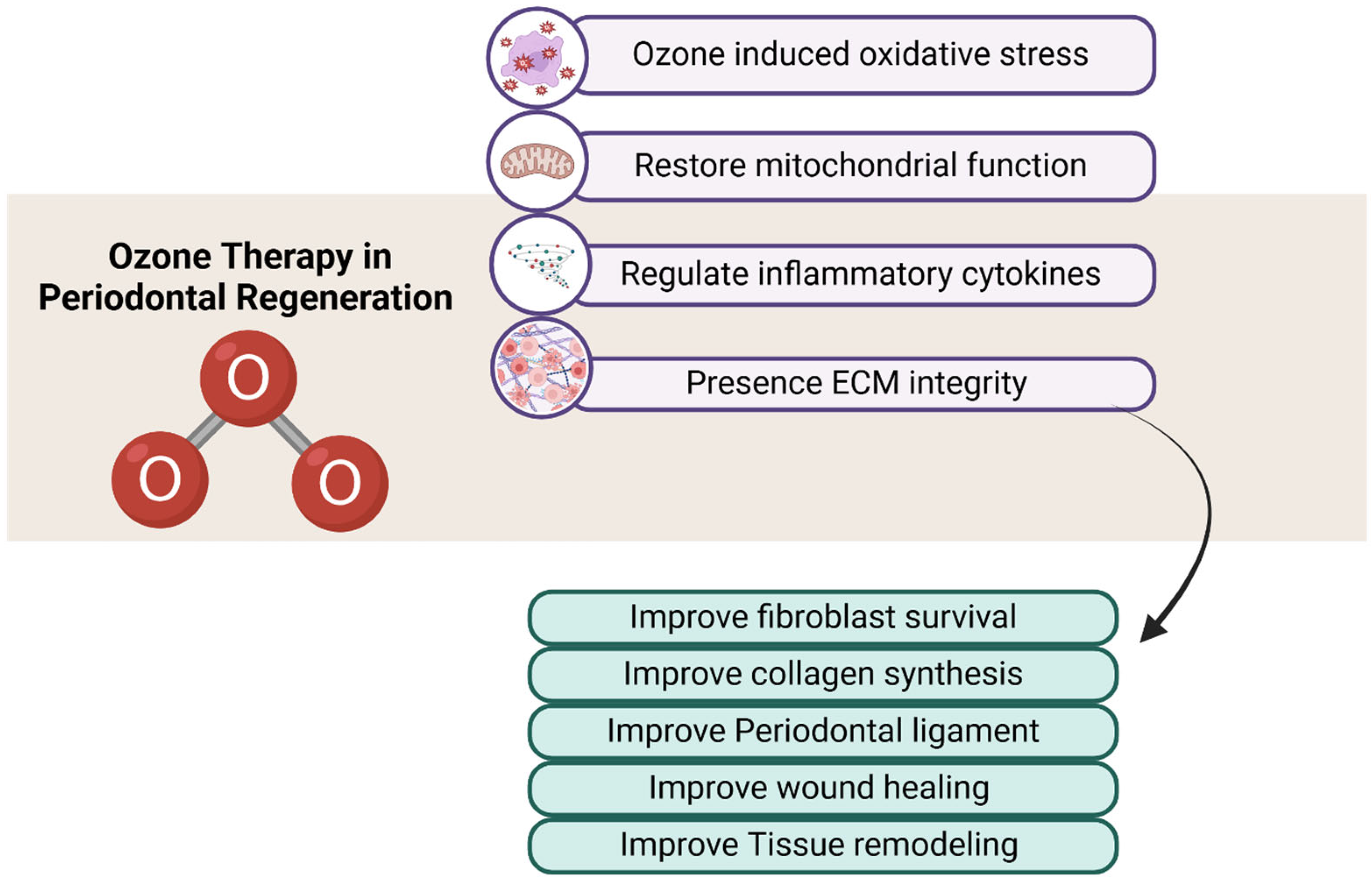
2. Ozone Therapy: A Paradigm Shift in Periodontal Treatment
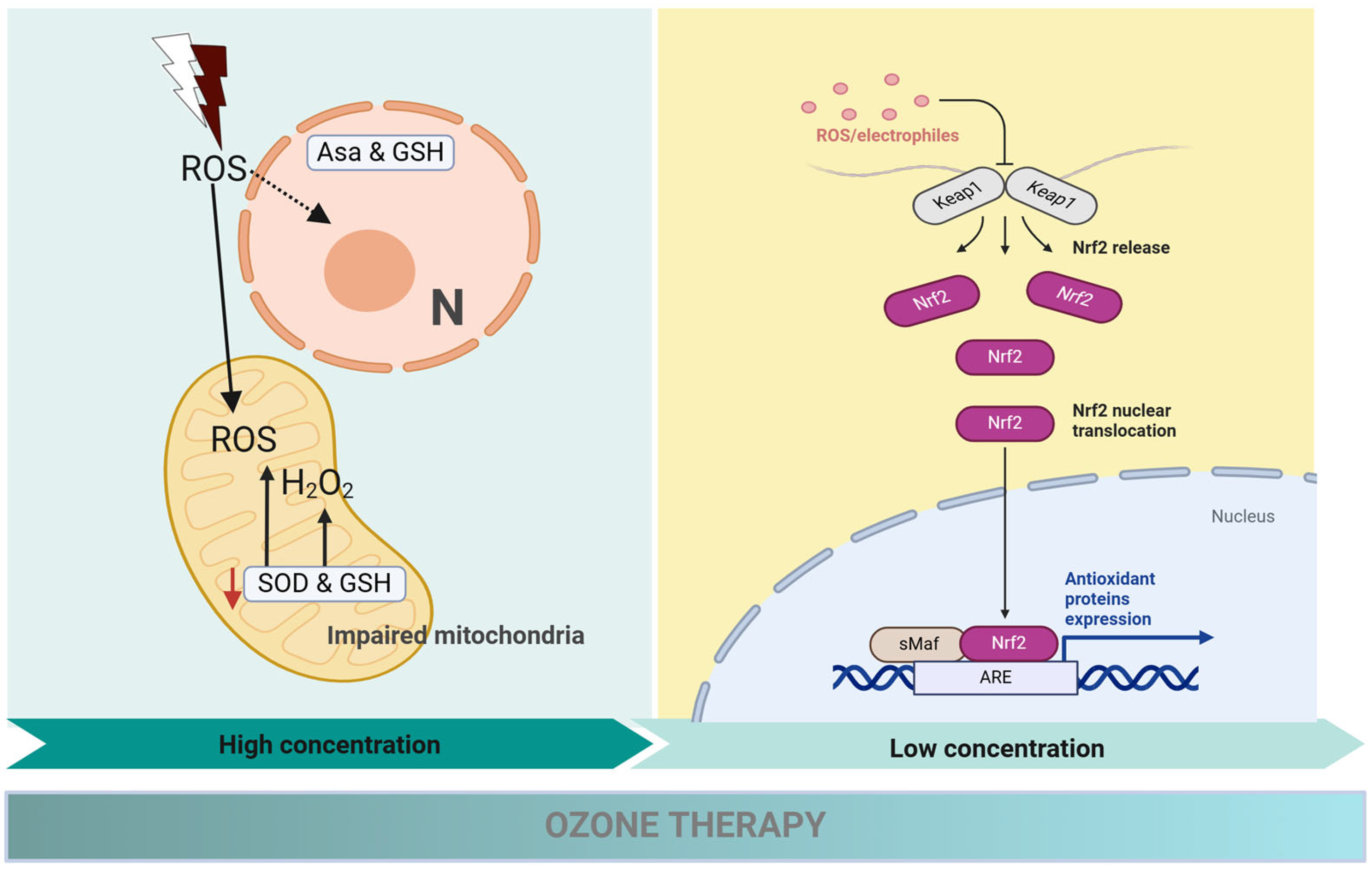
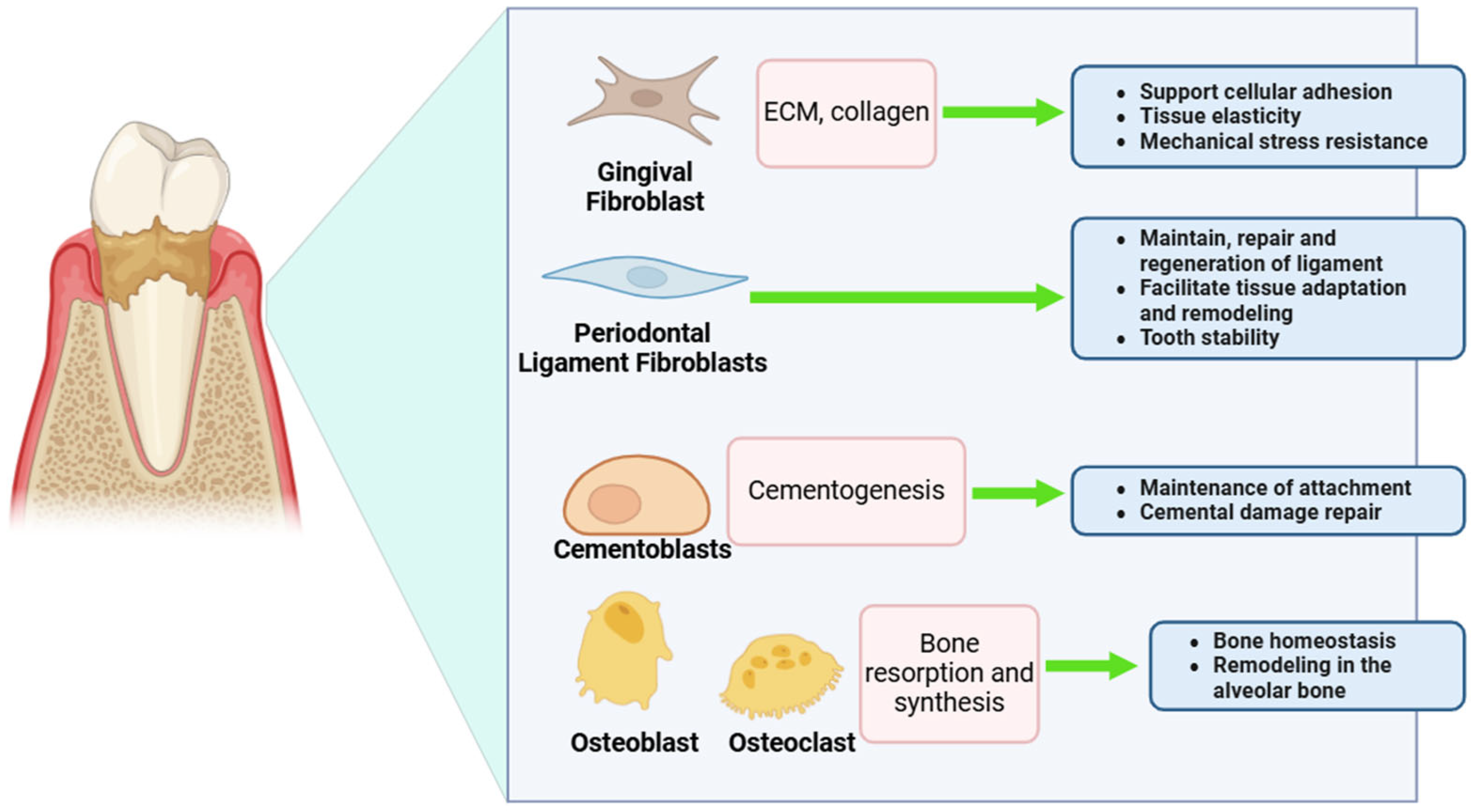
3. Challenges of Ozone Therapy in Periodontal Fibroblasts
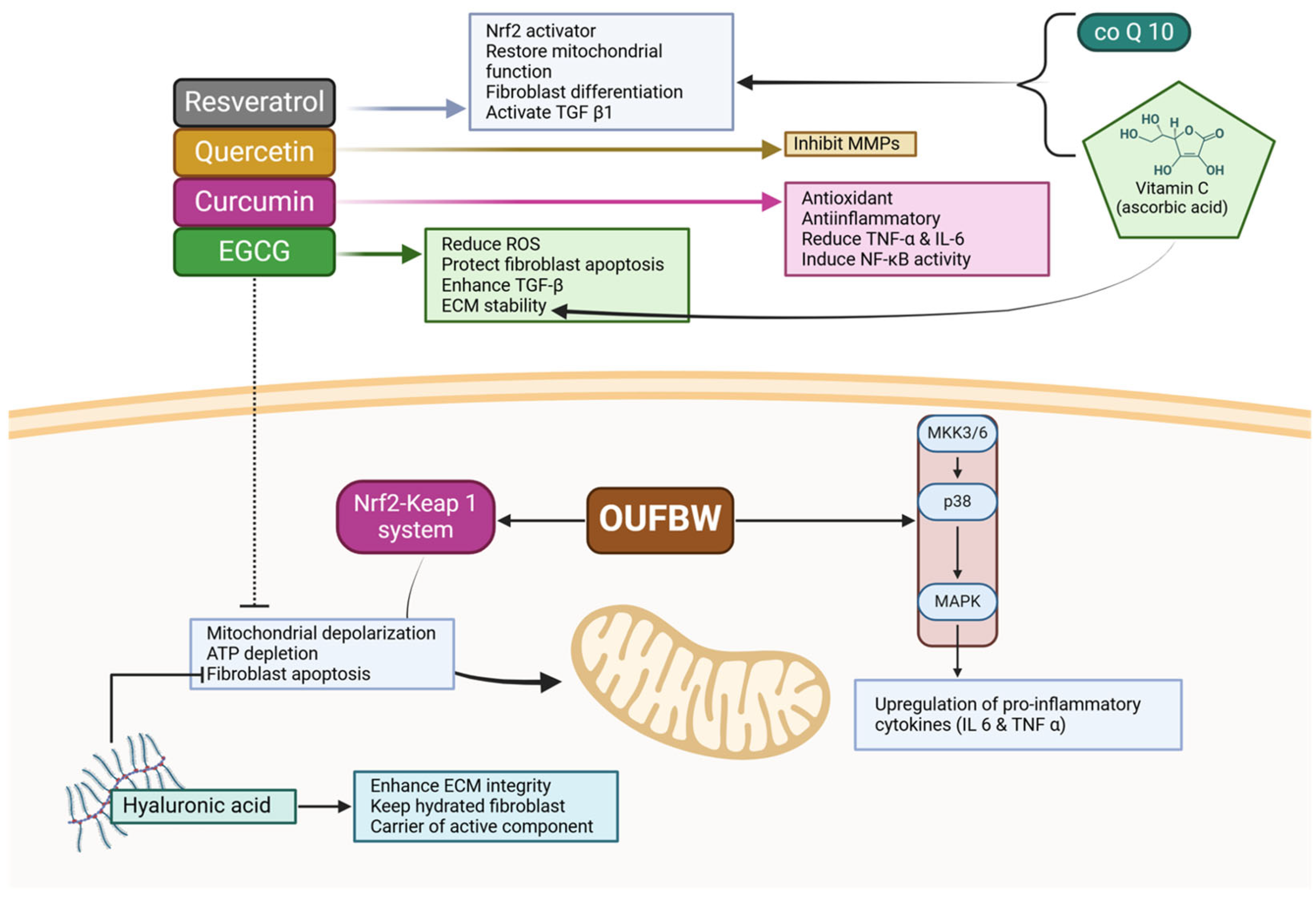
4. Counteracting Ozone’s Limitations with Bioactive Compounds
5. Toward a Bioactive-Ozone Therapeutic Model
6. A Novel Bioactive-Ozone Nanocarrier System for Enhanced Periodontal Regeneration
7. Conclusions
- Limitations and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | Antioxidant Response Element |
| ATP | Adenosine Triphosphate |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin-3-Gallate |
| EMD | Enamel Matrix Derivative |
| GSH | Glutathione |
| GTR | Guided Tissue Regeneration |
| HA | Hyaluronic Acid |
| IL | Interleukin |
| LAPT | Laser-Assisted Periodontal Therapy |
| MMPs | Matrix Metalloproteinases |
| NF-κB | Nuclear Factor Kappa B |
| PRF | Platelet-Rich Fibrin |
| ROS | Reactive Oxygen Species |
| SRP | Scaling and Root Planing |
| SOD | Superoxide Dismutase |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Raitapuro-Murray, T.; Molleson, T.I.; Hughes, F.J. The prevalence of periodontal disease in a Romano-British population c. 200–400 AD. Br. Dent. J. 2014, 217, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-Da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez-Avilés, F. European workshop in periodontal health and cardiovascular disease--scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12 (Suppl. B), B3–B12. [Google Scholar] [CrossRef]
- Casanova, L.; Hughes, F.J.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2018, 20, 6008. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Dalessandri, D.; Migliorati, M.; Indelicato, F. New Frontiers on Adjuvants Drug Strategies and Treatments in Periodontitis. Sci. Pharm. 2021, 89, 46. [Google Scholar] [CrossRef]
- Lamont, T.; Worthington, H.V.; Clarkson, J.E.; Beirne, P.V. Routine scale and polish for periodontal health in adults. Cochrane Database Syst. Rev. 2018, 12, CD004625. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Alassy, H.; Pizarek, J.A.; Kormas, I.; Pedercini, A.; Wolff, L.F. Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: A narrative review. Front. Oral Maxillofac. Med. 2021, 3, 16. [Google Scholar] [CrossRef]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef]
- Eickholz, P.; Koch, R.; Göde, M.; Nickles, K.; Kocher, T.; Lorenz, K.; Kim, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; et al. Clinical benefits of systemic amoxicillin/metronidazole may depend on periodontitis stage and grade: An exploratory sub-analysis of the ABPARO trial. J. Clin. Periodontol. 2023, 50, 1239–1252. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Rohani, M. Antibiotic resistance and the alternatives to conventional antibiotics: The role of probiotics and microbiota in combating antimicrobial resistance. Microbiol. Res. 2023, 267, 127275. [Google Scholar] [CrossRef]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal Regeneration With Enamel Matrix Derivative in Reconstructive Periodontal Therapy: A Systematic Review. J. Periodontol. 2011, 83, 707–720. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef]
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal Wound Healing and Regeneration: Insights for Engineering New Therapeutic Approaches. Front. Dent. Med. 2022, 3, 815810. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Sachelarie, L.; Cristea, R.; Burlui, E.; Hurjui, L.L. Laser Technology in Dentistry: From Clinical Applications to Future Innovations. Dent. J. 2024, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef] [PubMed]
- Emingil, G.; Gürkan, A.; Tervahartiala, T.; Hernandez, M.; Özgül, S.; Sorsa, T.; Alassiri, S. Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism. Dent. J. 2019, 7, 9. [Google Scholar] [CrossRef]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr. Med. Chem. 2021, 28, 3032–3058. [Google Scholar] [CrossRef]
- Bilichodmath, S.; Deepthi, R. Ozone therapy in periodontics: A meta-analysis. Contemp. Clin. Dent. 2020, 11, 108–115. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Dasnadi, S.P.; Islam, M.S.; Chaitanya, N.C.; Mohammed, R.; Farghal, N.S.; Gobara, B.; Rahman, M.M. The Impact of Ozone on Periodontal Cell Line Viability and Function. Curr. Issues Mol. Biol. 2025, 47, 72. [Google Scholar] [CrossRef]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Huth, K.C.; Quirling, M.; Lenzke, S.; Paschos, E.; Kamereck, K.; Brand, K.; Hickel, R.; Ilie, N. Effectiveness of ozone against periodontal pathogenic microorganisms. Eur. J. Oral Sci. 2011, 119, 204–210. [Google Scholar] [CrossRef]
- Serra, M.E.G.; Baeza-Noci, J.; Abdala, C.V.M.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. The role of ozone treatment as integrative medicine. An evidence and gap map. Front. Public Health 2023, 10, 1112296. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Martínez-Sánchez, G.; Llontop, P.; Aguiar-Bujanda, D.; Fernández-Pérez, L.; Santana-Rodríguez, N. Modulation of Oxidative Stress by Ozone Therapy in the Prevention and Treatment of Chemotherapy-Induced Toxicity: Review and Prospects. Antioxidants 2019, 8, 588. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Hernández-Orozco, E.; Rodríguez-Martínez, E.; Valdés-Fuentes, M.; Cornejo-Trejo, V.; Pérez-Pacheco, N.; Do-rado-Martínez, C.; Zequeida-Carmona, D.; Espinosa-Caleti, I. Ozone Pollution, Oxidative Stress, Regulatory T Cells and Antioxidants. Antioxidants 2022, 11, 1553. [Google Scholar] [CrossRef]
- Anshida, V.P.; Kumari, R.A.; Murthy, C.S.; Samuel, A. Extracellular matrix degradation by host matrix metalloproteinases in restorative dentistry and endodontics: An overview. J. Oral Maxillofac. Pathol. 2020, 24, 352–360. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Huang, J.; Yang, W.; Tao, R. Effects of ozone therapy as an adjuvant in the treatment of periodontitis: A systematic review and meta-analysis. BMC Oral Health 2025, 25, 335. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Signoriello, A.; Brancato, G.; Brancato, R.; Messina, E.; Faccioni, P.; Marcoccia, S.; Nardi, G.M.; Lombardo, G. Effectiveness of Ozone Therapy in Non-Surgical Periodontal Treatment: A Meta-Analysis of Topical Applications. J. Clin. Med. 2025, 14, 5124. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, Z.; Oskaybas, M.N.; Alkan, A.B.; Cakmak, O.; Alkan, B.A. The effects of ozone therapy on periodontal therapy: A randomized placebo-controlled clinical trial. Oral Dis. 2019, 25, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.; Dubbelman, T.M.; Raap, T.K.; Van Steveninck, J. Toxic effects of ozone on murine L929 fibroblasts. Enzyme inacti-vation and glutathione depletion. Biochem. J. 1987, 242, 707–712. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Chánová, E.; Syková, E.; Dejneka, A.; Kubinová, Š. Cell death induced by ozone and various non-thermal plasmas: Therapeutic perspectives and limitations. Sci. Rep. 2014, 4, 7129. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Mitochondrial Dysfunction, Its Oxidative Stress-Induced Pathologies and Redox Bioregulation through Low-Dose Medical Ozone: A Systematic Review. Molecules 2024, 29, 2738. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wen, W. Mitochondrial Dysfunction in Diabetic Periodontitis: Mechanisms and Therapeutic Potential. J. Inflamm. Res. 2025, 18, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Leewananthawet, A.; Arakawa, S.; Okano, T.; Kinoshita, R.D.; Ashida, H.; Izumi, Y.; Suzuki, T. Ozone ultrafine bubble water induces the cellular signaling involved in oxidative stress responses in human periodontal ligament fibroblasts. Sci. Technol. Adv. Mater. 2019, 20, 590–599. [Google Scholar] [CrossRef]
- Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. [Google Scholar] [CrossRef]
- Carmo, R.A.D.; Cörner, A.C.O.; Martins, E.F.; Ouverney, G.; Júnior, J.C.T.; Robbs, B.K.; Pascoal, V.D.B.; Esposito, E.; Capelo, L.P.; Camargo, G.A.d.C.G. Effect of ozone oil and non-surgical periodontal treatment in patients with type 2 diabetes. In-vivo and in-vitro studies with fibroblasts and Candida albicans. J. Appl. Oral Sci. 2025, 33, e20240080. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.; Costanzo, M.; Lacavalla, M.A.; Galiè, M.; Angelini, O.; Tabaracci, G.; Malatesta, M. Low Ozone Concentrations Differentially Affect the Structural and Functional Features of Non-Activated and Activated Fibroblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 10133. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.-J.; Wan, S.-C.; Li, X.; Chen, G. Ozone: Complicated effects in central nervous system diseases. Med. Gas. Res. 2024, 15, 44–57. [Google Scholar] [CrossRef]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Rahman, M.M.; Mohamed, R.; Priya, S.P.; Chaitanya, N.C.; Islam, M.S.; Gobara, B. Natural Bioactive Compounds in the Management of Periodontal Diseases: A Comprehensive Review. Molecules 2024, 29, 3044. [Google Scholar] [CrossRef]
- D’amico, E.; Cinquini, C.; Petrini, M.; Barone, A.; Iezzi, G.; D’ercole, S.; De Filippis, B.; Pierfelice, T.V. The Application of Resveratrol Derivatives in Oral Cells Reduces the Oxidative Stress Induced by Glucocorticoids. Metabolites 2024, 14, 350. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Chaitanya, N.C.S.K.; Mohammed, R.; Priya, S.P.; Padmanabhan, V.; Ahmed, A.; Dasnadi, S.P.; Islam, S.; Gismalla, B.G.; et al. New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential. Molecules 2025, 30, 807. [Google Scholar] [CrossRef]
- Khan, A.; Kothiwale, S.V. Evaluation on the efficacy of processed hydrated and dehydrated amnion chorion membrane on the proliferation of periodontal ligament fibroblasts. Cell Tissue Bank. 2023, 25, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Hirano, M. Coenzyme Q and mitochondrial disease. Dev. Disabil. Res. Rev. 2010, 16, 183–188. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, K.M.F.; Cosgarea, R.; Sculean, A.; Doerfer, C. Can vitamins improve periodontal wound healing/regeneration? Periodontology 2000 2024, 94, 539–602. [Google Scholar] [CrossRef] [PubMed]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- D’amico, E.; Aceto, G.M.; Petrini, M.; Cinquini, C.; D’ercole, S.; Iezzi, G.; Pierfelice, T.V. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? Int. J. Mol. Sci. 2025, 26, 592. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Yu, T.; Song, G.; Xu, T.; Xin, T.; Lin, Y.; Han, B. Nano-Based Drug Delivery Systems for Periodontal Tissue Re-generation. Pharmaceutics 2022, 14, 2250. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef]
- Gherardi, G.; Corbioli, G.; Ruzza, F.; Rizzuto, R. CoQ10 and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dys-functions. Nutrients 2022, 14, 4326. [Google Scholar] [CrossRef]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Zazuli, Z.; Hartati, R.; Rowa, C.R.; Asyarie, S. Satrialdi The Potential Application of Nanocarriers in Delivering Topical Antioxidants. Pharmaceuticals 2025, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. WIREs Nanomed. Nanobiotechnology 2022, 14, e1756. [Google Scholar] [CrossRef]
- Yang, R.; Yan, L.; Xu, T.; Zhang, K.; Lu, X.; Xie, C.; Fu, W. Injectable bioadhesive hydrogel as a local nanomedicine depot for targeted regulation of inflammation and ferroptosis in rheumatoid arthritis. Biomaterials 2024, 311, 122706. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Priya, S.P.; Mohammed, R.; Chaitanya, N.C.; Padmanabhan, V.; El Bahra, S.; Rahman, M.M.; Gismalla, B.G. Microbial Dynamics in Periodontal Regeneration: Understanding Microbiome Shifts and the Role of Antifouling and Bactericidal Materials: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 12196–12213. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Gupta, N.; Khan, S.; Ali, S.A.; Kulsum, O. Nanotechnology In Periodontal Therapy: A Groundbreaking Frontier In Modern Dentistry. Nanotechnol. Percept. 2024, 20, 598–606. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, N.T.; Babiker, R.; Padmanabhan, V.; Islam, M.S.; Priya, S.P.; Chaitanya, N.C.S.K.; Mohammed, R.; Dasnadi, S.P.; Ahmed, A.; Gismalla, B.G.; et al. Challenges of Ozone Therapy in Periodontal Regeneration: A Narrative Review and Possible Therapeutic Improvements. Curr. Issues Mol. Biol. 2025, 47, 811. https://doi.org/10.3390/cimb47100811
Hashim NT, Babiker R, Padmanabhan V, Islam MS, Priya SP, Chaitanya NCSK, Mohammed R, Dasnadi SP, Ahmed A, Gismalla BG, et al. Challenges of Ozone Therapy in Periodontal Regeneration: A Narrative Review and Possible Therapeutic Improvements. Current Issues in Molecular Biology. 2025; 47(10):811. https://doi.org/10.3390/cimb47100811
Chicago/Turabian StyleHashim, Nada Tawfig, Rasha Babiker, Vivek Padmanabhan, Md Sofiqul Islam, Sivan Padma Priya, Nallan C. S. K. Chaitanya, Riham Mohammed, Shahistha Parveen Dasnadi, Ayman Ahmed, Bakri Gobara Gismalla, and et al. 2025. "Challenges of Ozone Therapy in Periodontal Regeneration: A Narrative Review and Possible Therapeutic Improvements" Current Issues in Molecular Biology 47, no. 10: 811. https://doi.org/10.3390/cimb47100811
APA StyleHashim, N. T., Babiker, R., Padmanabhan, V., Islam, M. S., Priya, S. P., Chaitanya, N. C. S. K., Mohammed, R., Dasnadi, S. P., Ahmed, A., Gismalla, B. G., & Rahman, M. M. (2025). Challenges of Ozone Therapy in Periodontal Regeneration: A Narrative Review and Possible Therapeutic Improvements. Current Issues in Molecular Biology, 47(10), 811. https://doi.org/10.3390/cimb47100811









