Exploring the Anticancer Potential of Coriolus versicolor in Breast Cancer: A Review
Abstract
1. Introduction
1.1. Breast Cancer: Epidemiology, Clinical Significance, Limitations of Current Therapies
1.2. Growing Interest in Supportive Therapies and Natural Products in Oncology
1.3. Coriolus versicolor: Biological and Ethnomedicinal Characteristics
1.4. Chemical Composition of the Coriolus versicolor Mushroom
1.4.1. Polysaccharides
1.4.2. Low-Molecular-Weight Secondary Metabolites Isolated from C. versicolor and Their Biological Activity
2. Materials and Methods
2.1. Objective and Rationale of the Article
2.2. Inclusion and Exclusion Criteria
2.3. PubMed Search Strategy
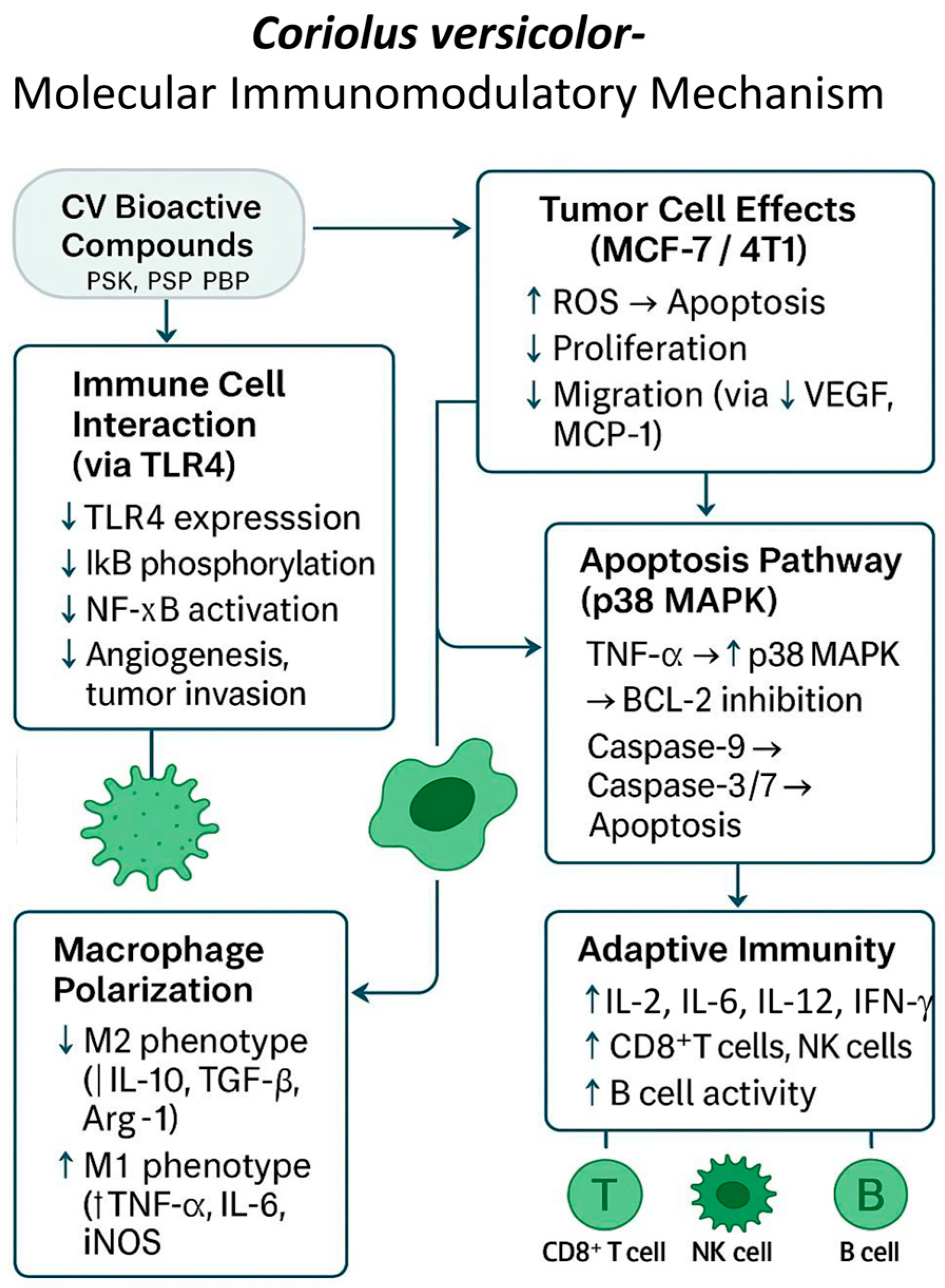
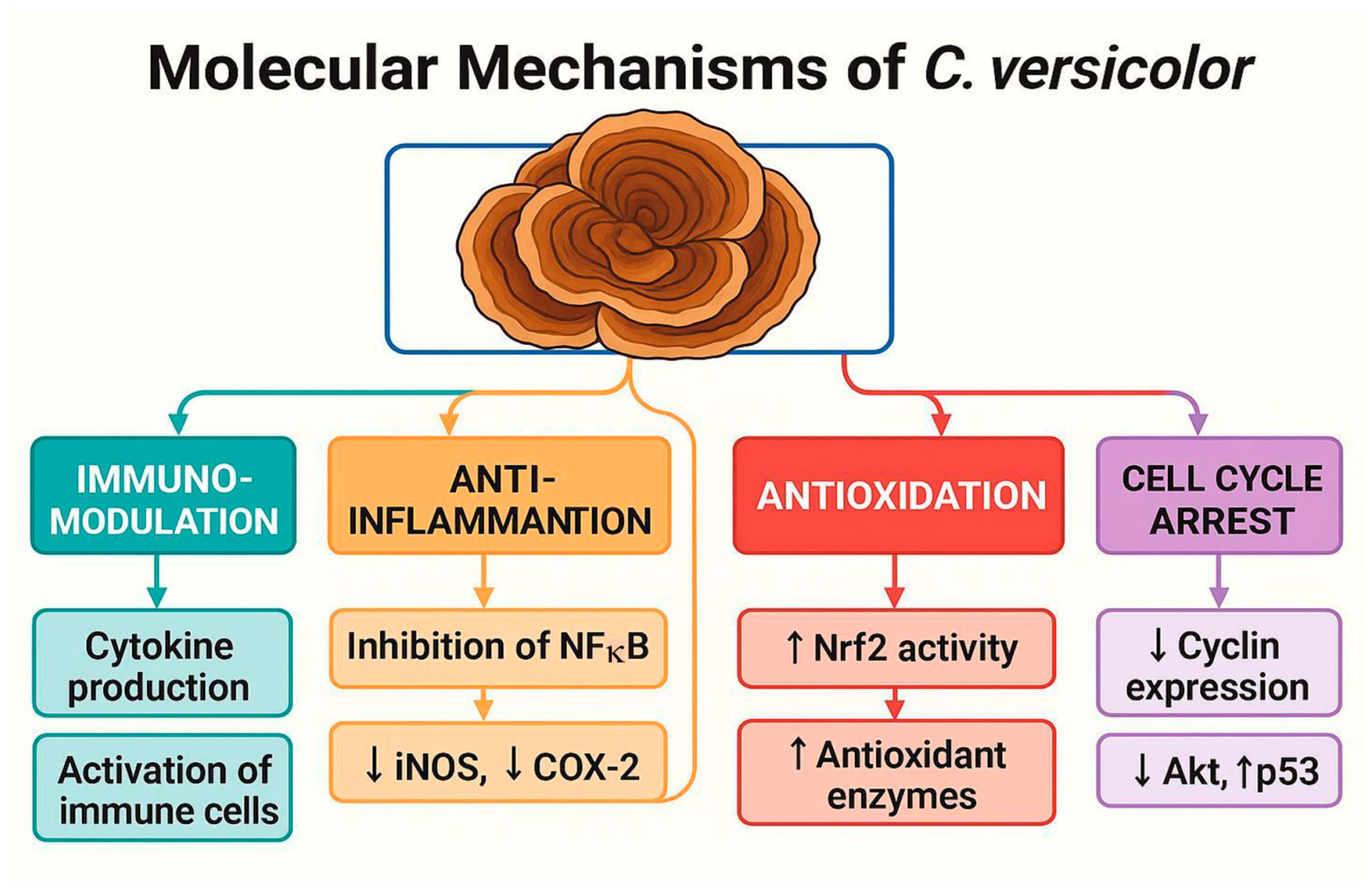
3. Mechanistic Insights into the Antitumor Activity of Coriolus versicolor
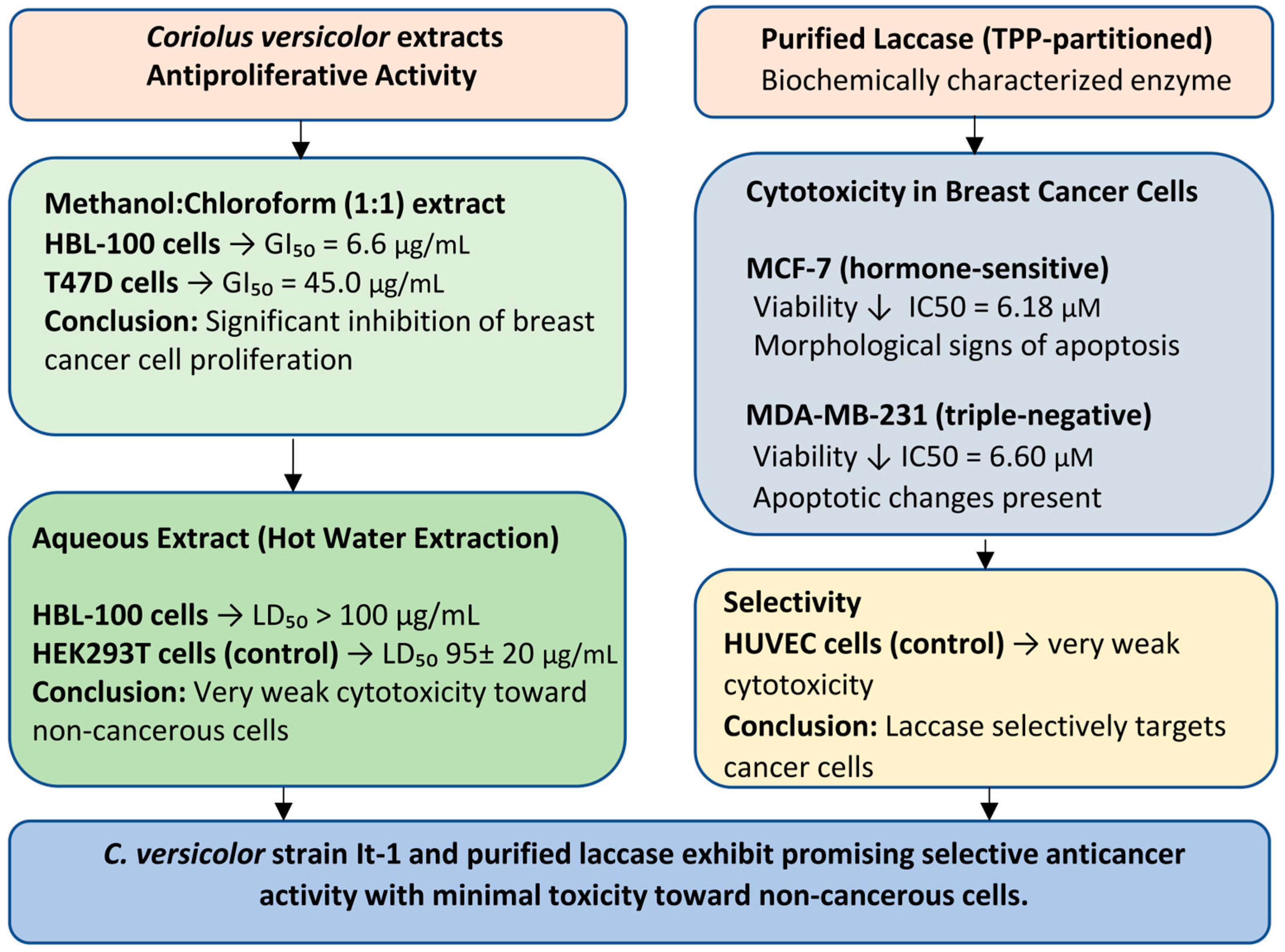
3.1. Cytotoxic and Anti-Proliferative Activity
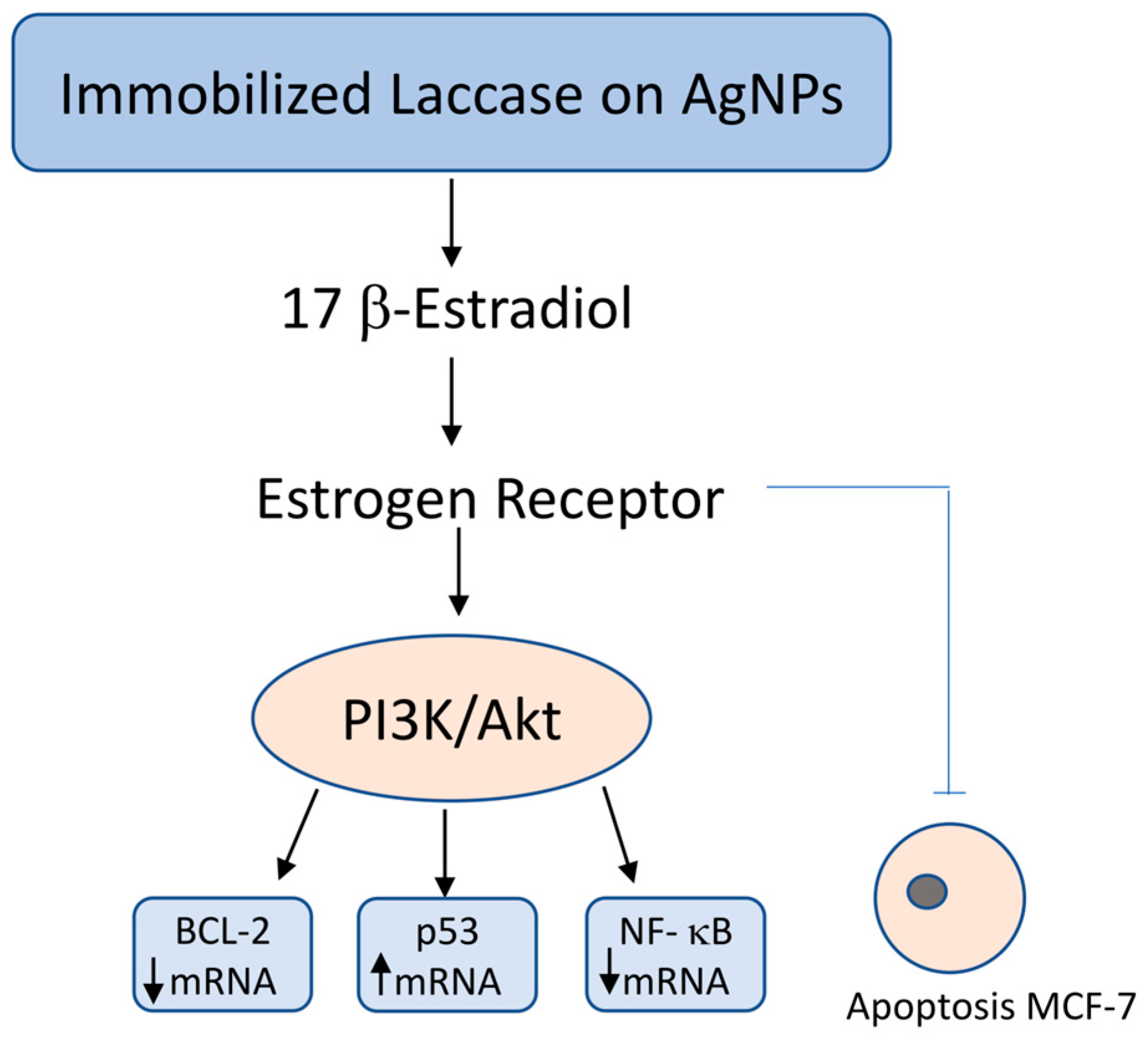
3.2. Apoptosis Induction and Estrogen Signaling Disruption
3.3. Necroptosis Activation
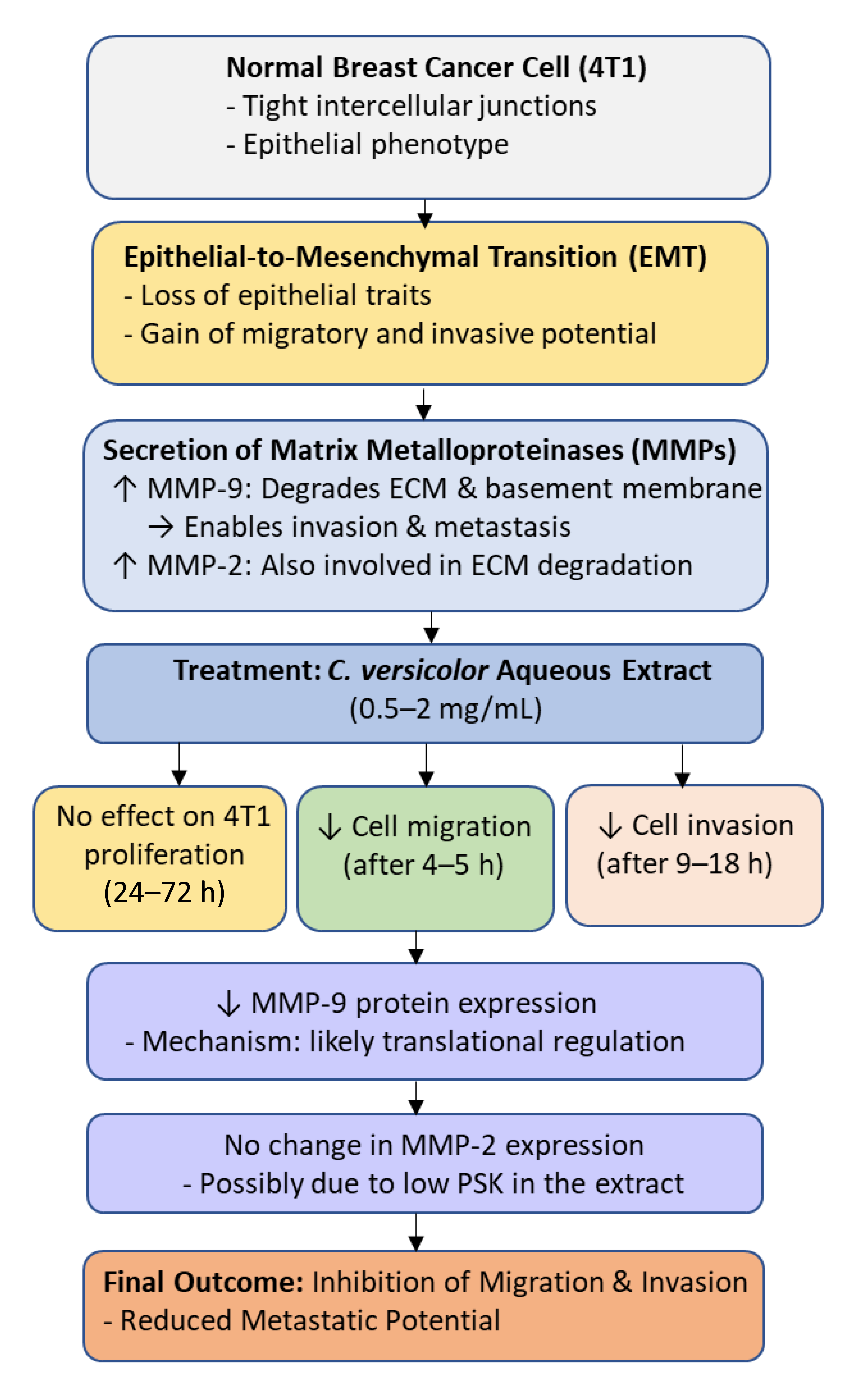
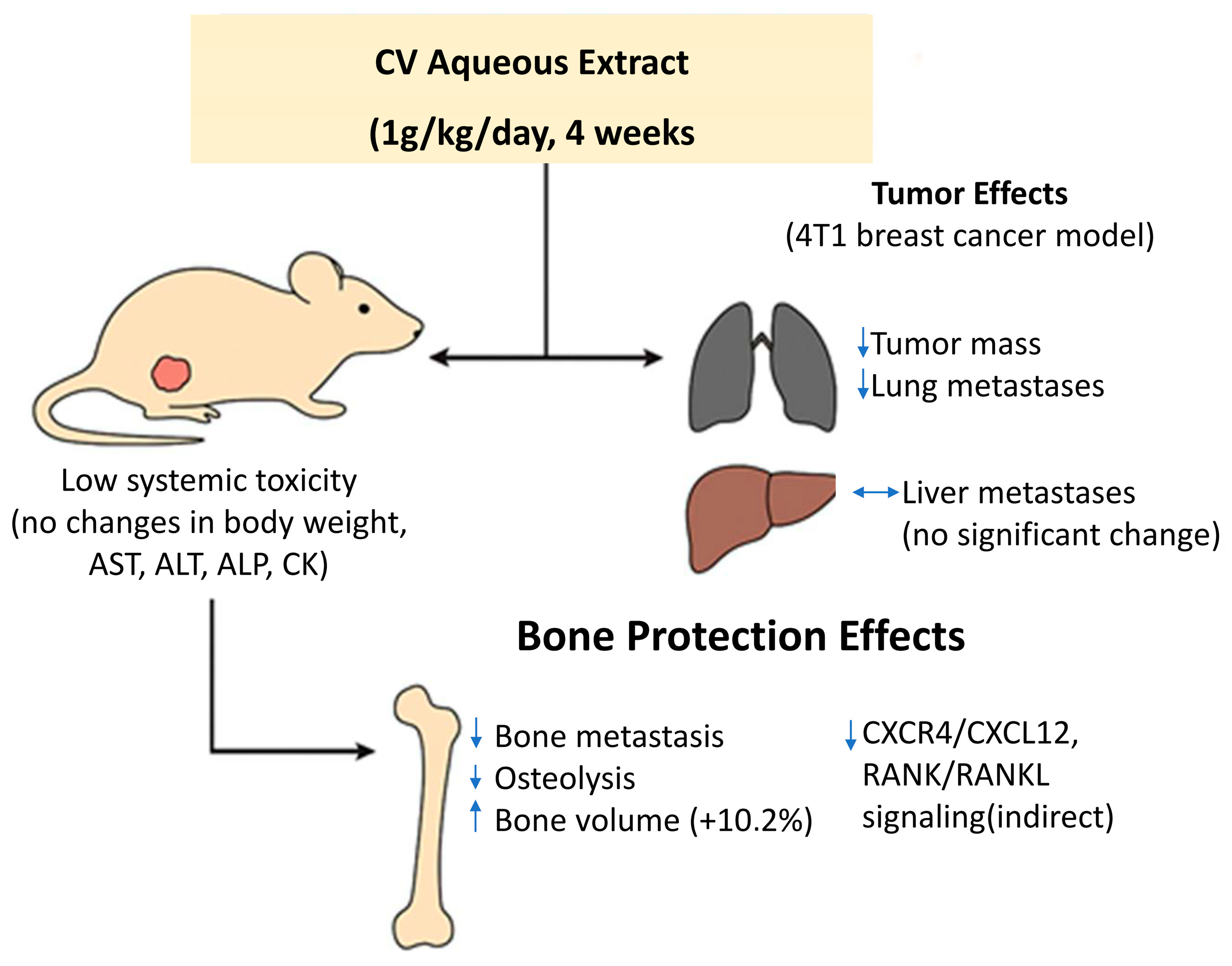
3.4. Anti-Metastatic Effects
3.5. Immunomodulation
| No. | Reference | CV Formula | Research Model | Research Methods | Research Objective | Results/Effect Mechanism | Conclusions |
|---|---|---|---|---|---|---|---|
| 1 | Yuzugullu Karakus Y, et al. 2025 [49] | TPP separated laccase | MCF-7, MDA-MB-231 | WST-1 ELISA analysis of Annexin V | assessment of the cytotoxic potential of laccase | laccase at high concentrations inhibited the viability of MCF-7 and MDA-MB-231 cells and caused apoptotic morphological changes in cells | laccase treatment was significantly more effective in MCF-7 cells than in MDA-MB-231 cells |
| 2 | Chauhan PS, et al. 2019 [54] | immobilized laccase from C. versicolor on Pluronic-stabilized silver nanoparticles | MCF-7 | semi qRT-PCR experiments | to assess the anticancer efficacy of immobilized laccase in breast cancer MCF-7 cells | inhibition of MCF-7 cells proliferation through β-estradiol degradation and cell apoptosis | described new strategy increasing the activity of immobilized laccase provides new opportunities for its use in the development of hormone-dependent breast cancer therapy |
| 3 | Pawlikowska M, et al. 2020 [59] | CV capsules/PBPs | MCF-7 | MTT HT-microscopy, DCF-DA, qRT-PCR, IF-based assay | to evaluate whether PBP extract triggers the necroptotic pathway in MCF-7 breast cancer cells | PBPs from CV induced RIPK1/RIPK3/MLKL-dependent necroptosis in MCF-7 cell line; | stimulating necroptosis has potential as an anticancer therapy |
| 4 | Kowalczewska M, et al. 2016 [78] | CV capsules, PBPs extract | MCF-7 | MTT qRT-PCR; | to investigate the immunomodulatory and anticancer properties of the PBPs isolated from commercially available capsules of C. versicolor | the PBPs induced a significant decrease in breast cancer MCF-7 cells growth, in TNF-α-dependent manner; the level of IL-1β and IL-6 was not affected | further studies are needed to clarify the mechanism of PBPs in therapy and prevention of human cancers |
| 5 | Jędrzejewski T, Sobocińska J, et al. 2020 [66] | CV extract | MCF-7 | MTT, LDH, ROS levels—DCFH-DA, wound-healing assay (Scratch Assay), cytokine and matrix metalloproteinase assays-ELISA, WB | to study the effect of CV extract on MCF-7 breast cancer cells in a pro-inflammatory microenvironment mimicked by lipopolysaccharide (LPS) | inhibition of the production of pro-inflammatory and pro-angiogenic factors by LPS-stimulated cells; reduction in the expression of TLR4 and p-IκB; anti-migratory and cytotoxic effect, an increase in ROS production | CV extract is cytotoxic to MCF-7 cancer cells and inhibits the expression of pro-tumor factors associated with inflammation |
| 6 | Shnyreva AV, et al. 2018 [33] | CV hot water extract; methanol: chloroform (1:1) extract | HBL-100, T-47D | MTT | to evaluate antiproliferative properties of CV extracts | the methanol–chloroform extract of C. versicolor (strain It-1) inhibited the growth of HBL-100 and T-47D cells | C. versicolor shows potential as a natural source for research and production of new chemical compounds with antiproliferative activity. |
| 7 | Yang CL, et al. 2023 [27] | CV extract 95% ethanol and small molecules from CV | MDA-MB-231 | tumor cell invasion assay | to examine the anti-invasive effect of (SMCV)/bioactive compound | direct reduction in the invasive capacity of malignant MDA-MB-231 cells | SMCV bioactive compound has therapeutic potential, additional studies needed |
| 8 | Jędrzejewski T, et al. 2025 [20] | CV extract; the MycoMedica Company (Police nad Metují, Czech Republic). | 4T1 | MTT, ELISA (total protein, cytokine concentrations) | to assess whether triple-negative breast cancer (TNBC) cells incubated in the presence of CV extract can release factors that lead to a change in the macrophage subtype from pro-tumor to anti-tumor | TNBC cells stimulated with CV extract create a microenvironment that promotes reduction in macrophage viability and migration, intracellular ROS production and production of proangiogenic cytokines, and changes macrophage polarization toward an antitumor subtype | CV extract has the potential to be used in the treatment of TNBC-associated macrophages |
| 9 | Jędrzejewski T, et al. 2020 [69] | CV capsules, PBPs extract | 4T1 | MTT ELISA | study of communication between triple-negative mouse breast cancer 4T1 cells and macrophages in the presence of PBPs | PBPs inhibit communication between 4T1 cells and macrophages by regulating the production of angiogenic and inflammatory factors and promote the M1 macrophage phenotype in the neoplastic microenvironment | PBPs show chemopreventive potential and are promising agents for preventing the progression of TNBC |
| 10 | Luo KW, et al. 2014 [62] | CV aqueous extract (0.125–2 mg/mL); 1 g/kg, orally administered daily for 4 weeks | 4T1, Female BALB/c mice (6–8 weeks of age) bearing orthotopic 4T1 tumors | MTT, scratch wound healing assay, transwell migration assay, gelatin zymography, WB, assessing the hematobiochemical markers in blood, H&E staining, micro-computed tomography (m-CT) analysis, ELISA | to examine the antitumor and antimetastatic properties of CV aqueous extract in 4T1 mouse breast cancer cells and in a 4T1 tumor-bearing mouse model | antitumor, antimetastatic and immunomodulatory effects in a mouse model of metastatic breast cancer; inhibition of the proliferation of 4T1 cells, cell migration and invasion; suppression of the activity of MMP-9 enzyme and protein level In vivo studies: effective reduction in tumor weight and lung metastasis; immunomodulatory effects | studies provided evidence supporting the use of CV aqueous extract as a health supplement in breast cancer patients |
| 11 | Torkelson CJ, et al. 2012 [21] | CV freeze-dried mycelial powder (Fungi Perfecti, Inc., Olympia, WA, USA) encapsulated by Beehive Botanicals (Hayward, WI, USA). | Phase I clinical trial; 9 women diagnosed with stage I, II, or III breast cancer | a phase I, two-center, dose escalation study; monitoring for: adverse events (AEs) using clinical and laboratory methods, dose-limiting toxicity (DLT) criteria defined as any Grade 2 or greater treatment-related toxicity as scored using the NCI’s Common Terminology Criteria for Adverse Events V 3.0 (CTCAE). | to assess the safety and tolerability of CV in women with breast cancer after radiotherapy; a collection of preliminary data comparing baseline values before and after radiotherapy, complete blood count with differential, immunological measurements during and after treatment, NK (natural killer) cell activity, regulatory T cell test complete, T/B/NK cell subset assay, phagocytic index, and cytokine level | CV was well tolerated. Immunological results indicated increased lymphocyte counts, increased NK cell functional activity, dose-dependent increases in CD8+ T cells and CD19+ B cells | CV therapy administered orally may improve the immune status of immunocompromised breast cancer patients undergoing standard oncological therapy |
4. Therapeutic Potential and Limitations of Coriolus versicolor
5. Limitations
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4T1 | Murine triple-negative breast cancer (TNBC) cells (Mimics human breast cancer stage IV) |
| AEs | Adverse events |
| CTCAE | Common Terminology Criteria for Adverse Events V 3.0 |
| CV | Coriolus versicolor |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate staining |
| DLT | Dose-limiting toxicity |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HBL-100 | Human breast tumor cell line |
| HT | Holotomographic microscopy |
| LDH | Lactate dehydrogenase, cell in vitro viability assay |
| LPS | Lipopolysaccharide |
| MCF-7 | Human breast adenocarcinoma cell line with positive estrogen and progesterone receptors |
| MDA-MB-231 | Adenocarcinoma, highly aggressive, invasive and poorly differentiated triple-negative breast cancer (TNBC) cell line |
| MLKL | Mixed lineage kinase domain-like protein |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) cell in vitro viability assay |
| NCI | National Cancer Institute |
| Nec-1 | Specific inhibitor necrostatin-1 |
| NK | Natural killer |
| PBPs | Protein-bound polysaccharides |
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| RIPK1 | Receptor-interacting protein1 kinase |
| RIPK3 | Receptor-interacting protein 3 kinase |
| ROS | Reactive Oxygen Species |
| T47D | Ductal carcinoma, a cell line derived from the pleural effusion of infiltrating ductal breast cancer |
| TLR 4 | Toll-like receptor |
| TPP | Three-phase partitioning |
| WB | Western blot analysis |
| WST-1 | Water-soluble tetrazolium salt, in vitro viability and proliferation assay |
References
- Jiang, J.; Thyagarajan-Sahu, A.; Loganathan, J.; Eliaz, I.; Terry, C.; Sandusky, G.E.; Sliva, D. BreastDefendTM Prevents Breast-to-Lung Cancer Metastases in an Orthotopic Animal Model of Triple-Negative Human Breast Cancer. Oncol. Rep. 2012, 28, 1139–1145. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals 2022, 15, 176. [Google Scholar] [CrossRef]
- Porter, P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N. Engl. J. Med. 2008, 358, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef]
- Fakhri, N.; Chad, M.A.; Lahkim, M.; Houari, A.; Dehbi, H.; Belmouden, A.; El Kadmiri, N. Risk Factors for Breast Cancer in Women: An Update Review. Med. Oncol. 2022, 39, 197. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Judasz, E.; Lisiak, N.; Kopczyński, P.; Taube, M.; Rubiś, B. The Role of Telomerase in Breast Cancer’s Response to Therapy. Int. J. Mol. Sci. 2022, 23, 12844. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.-W.; Ding, Y.; Chen, Y.-F.; Cai, Y.-W.; Wang, L.-P.; Huang, L.; Liu, C.-C.; Shao, Z.-M.; Yu, K.-D. Breast Cancer: Pathogenesis and Treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic Therapy for Early-Stage Breast Cancer: Learning from the Past to Build the Future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer☆. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Tommasi, C.; Balsano, R.; Corianò, M.; Pellegrino, B.; Saba, G.; Bardanzellu, F.; Denaro, N.; Ramundo, M.; Toma, I.; Fusaro, A.; et al. Long-Term Effects of Breast Cancer Therapy and Care: Calm after the Storm? J. Clin. Med. 2022, 11, 7239. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural Products for Cancer Chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Figueiredo, L.; Régis, W.C.B. Medicinal Mushrooms in Adjuvant Cancer Therapies: An Approach to Anticancer Effects and Presumed Mechanisms of Action. Nutrire 2017, 42, 28. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes Versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular Targets of Dietary Agents for Prevention and Therapy of Cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Mushrooms—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/cam/patient/mushrooms-pdq (accessed on 3 July 2025).
- Dowaraka-Persad, B.; Neergheen, V.S. Mushroom-Derived Compounds as Metabolic Modulators in Cancer. Molecules 2023, 28, 1441. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Spisz, P.; Walczak-Skierska, J.; Pomastowski, P.; Wrotek, S. In Vitro Treatment of Triple-Negative Breast Cancer Cells with an Extract from the Coriolus versicolor Mushroom Changes Macrophage Properties Related to Tumourigenesis. Immunol. Res. 2024, 73, 14. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes Versicolor in Women with Breast Cancer. Int. Sch. Res. Not. 2012, 2012, 251632. [Google Scholar] [CrossRef]
- Cui, J.; Chisti, Y. Polysaccharopeptides of Coriolus versicolor: Physiological Activity, Uses, and Production. Biotechnol. Adv. 2003, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.K.W.; Ho, S.S.S.; Chow, A.H.L. Coriolus Versicolor: A Medicinal Mushroom with Promising Immunotherapeutic Values. J. Clin. Pharmacol. 2002, 42, 976–984. [Google Scholar] [CrossRef]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Bauer, R.; Binder, M.; Giachini, A.J.; Hosaka, K.; Justo, A.; Larsson, E.; Larsson, K.H.; Lawrey, J.D.; Miettinen, O.; et al. 14 Agaricomycetes. In Systematics and Evolution: Part A; McLaughlin, D.J., Spatafora, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 373–429. ISBN 978-3-642-55318-9. [Google Scholar]
- Jiang, J.; Sliva, D. Novel Medicinal Mushroom Blend Suppresses Growth and Invasiveness of Human Breast Cancer Cells. Int. J. Oncol. 2010, 37, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.-H.; Chik, S.C.-C.; Lau, A.S.-Y.; Chan, G.C.-F. Coriolus Versicolor and Its Bioactive Molecule Are Potential Immunomodulators against Cancer Cell Metastasis via Inactivation of MAPK Pathway. J. Ethnopharmacol. 2023, 301, 115790. [Google Scholar] [CrossRef] [PubMed]
- Sułkowska-Ziaja, K.; Muszyńska, B.; Sałaciak, K.; Gawalska, A. Trametes versicolor (L.) Lloyd as a source of biologically active compounds with a wide spectrum of action and application. Postępy Fitoterapii 2016, 4, S274–S281. [Google Scholar]
- Ming, O. Chinese-English Manual of Common-Used in Traditional Chinese Medicine; Joint Publishing Co.: Hong Kong, China; Guangdong Science & Technology Publishing House: Hong Kong, China, 1989; ISBN 978-962-04-0763-5. [Google Scholar]
- Oba, K.; Teramukai, S.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K for Patients with Curative Resections of Gastric Cancer. Cancer Immunol. Immunother. 2007, 56, 905–911. [Google Scholar] [CrossRef]
- Ohwada, S.; Ikeya, T.; Yokomori, T.; Kusaba, T.; Roppongi, T.; Takahashi, T.; Nakamura, S.; Kakinuma, S.; Iwazaki, S.; Ishikawa, H.; et al. Adjuvant Immunochemotherapy with Oral Tegafur/Uracil plus PSK in Patients with Stage II or III Colorectal Cancer: A Randomised Controlled Study. Br. J. Cancer 2004, 90, 1003–1010. [Google Scholar] [CrossRef]
- Sakamoto, J.; Morita, S.; Oba, K.; Matsui, T.; Kobayashi, M.; Nakazato, H.; Ohashi, Y. Meta-Analysis Group of the Japanese Society for Cancer of the Colon Rectum Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K for Patients with Curatively Resected Colorectal Cancer: A Meta-Analysis of Centrally Randomized Controlled Clinical Trials. Cancer Immunol. Immunother. 2006, 55, 404–411. [Google Scholar] [CrossRef]
- Shnyreva, A.V.; Shnyreva, A.A.; Espinoza, C.; Padrón, J.M.; Trigos, Á. Antiproliferative Activity and Cytotoxicity of Some Medicinal Wood-Destroying Mushrooms from Russia. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal Mushrooms as a Source of Antitumor and Immunomodulating Polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Borchers, A.T.; Krishnamurthy, A.; Keen, C.L.; Meyers, F.J.; Gershwin, M.E. The Immunobiology of Mushrooms. Exp. Biol. Med. 2008, 233, 259–276. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Jiang, Y.; Liu, Y.; Luo, H.; Hao, C.; Zeng, P.; Zhang, L. Preclinical and Clinical Studies of Coriolus versicolor Polysaccharopeptide as an Immunotherapeutic in China. Discov. Med. 2017, 23, 207–219. [Google Scholar]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom Extracts and Compounds with Suppressive Action on Breast Cancer: Evidence from Studies Using Cultured Cancer Cells, Tumor-Bearing Animals, and Clinical Trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 Agonist PSK Activates Human NK Cells and Enhances the Antitumor Effect of HER2-Targeted Monoclonal Antibody Therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Musa Hago, A.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, Characterization, and Antitumor Activity of a Novel Glucan from the Fruiting Bodies of Coriolus versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Han, W.W.; Pan, Y.J. Studies on chemical structure of polysaccharide from fruit body of Coriolus versicolor. YaoXue XueBao 2001, 36, 664–667. [Google Scholar]
- Rau, U.; Kuenz, A.; Wray, V.; Nimtz, M.; Wrenger, J.; Cicek, H. Production and Structural Analysis of the Polysaccharide Secreted by Trametes (Coriolus) versicolor ATCC 200801. Appl. Microbiol. Biotechnol. 2009, 81, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-Glucans from Grifola Frondosa and Ganoderma Lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; LiQun, S.; Zhong, M.; Huang, M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018, 17, 200–209. [Google Scholar] [CrossRef]
- Zaidman, B.-Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal Mushroom Modulators of Molecular Targets as Cancer Therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Wang, S.-R.; Zhang, L.; Chen, H.-P.; Li, Z.-H.; Dong, Z.-J.; Wei, K.; Liu, J.-K. Four New Spiroaxane Sesquiterpenes and One New Rosenonolactone Derivative from Cultures of Basidiomycete Trametes versicolor. Fitoterapia 2015, 105, 127–131. [Google Scholar] [CrossRef]
- Eliza, W.L.Y.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent. Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.; Kataria, H.; Dhawan, I. Literature search for research planning and identification of research problem. Indian J. Anaesth. 2016, 60, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Yuzugullu Karakus, Y.; Isik, S.; Kale Bakir, E.; Turkmenoglu, A.; Deveci Ozkan, A. Characterization of the Three-Phase Partitioned Laccase from Trametes versicolor Strain with Antiproliferative Activity against Breast Cancer Cells. Int. J. Biol. Macromol. 2025, 286, 138504. [Google Scholar] [CrossRef] [PubMed]
- Guest, T.C.; Rashid, S. Anticancer Laccases: A Review. J. Clin. Exp. Oncol. 2018, 5, 2. [Google Scholar] [CrossRef]
- Han, R.; Gu, S.; Zhang, Y.; Luo, A.; Jing, X.; Zhao, L.; Zhao, X.; Zhang, L. Estrogen Promotes Progression of Hormone-Dependent Breast Cancer through CCL2-CCR2 Axis by Upregulation of Twist via PI3K/AKT/NF-κB Signaling. Sci. Rep. 2018, 8, 9575. [Google Scholar] [CrossRef]
- Lewis-Wambi, J.S.; Jordan, V.C. Estrogen Regulation of Apoptosis: How Can One Hormone Stimulate and Inhibit? Breast Cancer Res. 2009, 11, 206. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Kim, C.-F.; Leung, K.-N.; Fung, K.-P.; Tse, T.-F.; Chan, H.; Lau, C.B.-S. Differential Anti-Tumor Activity of Coriolus versicolor (Yunzhi) Extract through P53- and/or Bcl-2-Dependent Apoptotic Pathway in Human Breast Cancer Cells. Cancer Biol. Ther. 2005, 4, 638–644. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kumarasamy, M.; Sosnik, A.; Danino, D. Enhanced Thermostability and Anticancer Activity in Breast Cancer Cells of Laccase Immobilized on Pluronic-Stabilized Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39436–39448. [Google Scholar] [CrossRef]
- Fu, B.; Lou, Y.; Wu, P.; Lu, X.; Xu, C. Emerging Role of Necroptosis, Pyroptosis, and Ferroptosis in Breast Cancer: New Dawn for Overcoming Therapy Resistance. Neoplasia 2024, 55, 101017. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.; Vieira-Cordeiro, L.; Enns, S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediat. Inflamm. 2015, 2015, 128076. [Google Scholar] [CrossRef]
- Oberst, A. Death in the Fast Lane: What’s next for Necroptosis? FEBS J. 2016, 283, 2616–2625. [Google Scholar] [CrossRef]
- Hänggi, K.; Ruffell, B. Cell Death, Therapeutics, and the Immune Response in Cancer. Trends Cancer 2023, 9, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, M.; Jędrzejewski, T.; Brożyna, A.A.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus versicolor Induce RIPK1/RIPK3/MLKL-Mediated Necroptosis in ER-Positive Breast Cancer and Amelanotic Melanoma Cells. Cell. Physiol. Biochem. 2020, 54, 591–604. [Google Scholar] [CrossRef]
- Shao, H.; Varamini, P. Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells 2022, 11, 388. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Pandya, S.; Ghosh, K.; Nadkarni, A. Matrix Metalloproteinase and Its Drug Targets Therapy in Solid and Hematological Malignancies: An Overview. Mutat. Res. Rev. Mutat. Res. 2013, 753, 7–23. [Google Scholar] [CrossRef]
- Luo, K.-W.; Yue, G.G.-L.; Ko, C.-H.; Lee, J.K.-M.; Gao, S.; Li, L.-F.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. In Vivo and in Vitro Anti-Tumor and Anti-Metastasis Effects of Coriolus versicolor Aqueous Extract on Mouse Mammary 4T1 Carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Morisaki, T.; Matsunaga, H.; Sato, N.; Uchiyama, A.; Hashizume, K.; Nagumo, F.; Tadano, J.; Katano, M. Protein-Bound Polysaccharide PSK Inhibits Tumor Invasiveness by down-Regulation of TGF-Beta1 and MMPs. Clin. Exp. Metastasis 2000, 18, 343–352. [Google Scholar] [CrossRef]
- Azim, H.A.; Kamal, N.S.; Azim, H.A. Bone Metastasis in Breast Cancer: The Story of RANK-Ligand. J. Egypt. Natl. Cancer Inst. 2012, 24, 107–114. [Google Scholar] [CrossRef]
- Jekabsons, M.B.; Merrell, M.; Skubiz, A.G.; Thornton, N.; Milasta, S.; Green, D.; Chen, T.; Wang, Y.-H.; Avula, B.; Khan, I.A.; et al. Breast Cancer Cells That Preferentially Metastasize to Lung or Bone Are More Glycolytic, Synthesize Serine at Greater Rates, and Consume Less ATP and NADPH than Parent MDA-MB-231 Cells. Cancer Metab. 2023, 11, 4. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Heppner, G.H.; Miller, F.R.; Shekhar, P.M. Nontransgenic Models of Breast Cancer. Breast Cancer Res. 2000, 2, 331–334. [Google Scholar] [CrossRef]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 Model for the Study of Late Stage Breast Cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus versicolor Fungus Disrupt the Crosstalk Between Breast Cancer Cells and Macrophages through Inhibition of Angiogenic Cytokines Production and Shifting Tumour-Associated Macrophages from the M2 to M1 Subtype. Cell. Physiol. Biochem. 2020, 54, 615–628. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Piotrowski, J.; Kozak, W. Protein-Bound Polysaccharides from Coriolus Versicolor Attenuate LPS-Induced Synthesis of pro-Inflammatory Cytokines and Stimulate PBMCs Proliferation. Immunol. Lett. 2016, 178, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The Two Faces of IL-6 in the Tumor Microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N.; Milovanović, J. Interleukin-8 in Breast Cancer Progression. J. Interferon Cytokine Res. 2013, 33, 563–570. [Google Scholar] [CrossRef]
- Awasthi, S. Toll-like Receptor-4 Modulation for Cancer Immunotherapy. Front. Immunol. 2014, 5, 328. [Google Scholar] [CrossRef]
- Ikebe, M.; Kitaura, Y.; Nakamura, M.; Tanaka, H.; Yamasaki, A.; Nagai, S.; Wada, J.; Yanai, K.; Koga, K.; Sato, N.; et al. Lipopolysaccharide (LPS) Increases the Invasive Ability of Pancreatic Cancer Cells through the TLR4/MyD88 Signaling Pathway. J. Surg. Oncol. 2009, 100, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-J.; Zhou, Y.-H.; Yuan, Y.; Li, D.; Wu, F.-H.; Wang, Q.; Zhu, J.-H.; Yan, B.; Wei, J.-J.; Zhang, G.-M.; et al. Triggering of Toll-like Receptor 4 on Metastatic Breast Cancer Cells Promotes Avβ3-Mediated Adhesion and Invasive Migration. Breast Cancer Res. Treat. 2012, 133, 853–863. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A Study on Immunomodulatory Mechanism of Polysaccharopeptide Mediated by TLR4 Signaling Pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhang, H.; Ren, P.; Cheng, X.; Hong, F.; Liu, J.; Zhang, R.; Zhao, J.; Gou, D. Immunostimulatory Effects Mechanism of Polysaccharide Extracted from Acanthopanax Senticosus on RAW 264.7 Cells through Activating the TLR/MAPK/NF-κB Signaling Pathway. Sci. Rep. 2025, 15, 13440. [Google Scholar] [CrossRef]
- Kowalczewska, M.; Piotrowski, J.; Jędrzejewski, T.; Kozak, W. Polysaccharide Peptides from Coriolus versicolor Exert Differential Immunomodulatory Effects on Blood Lymphocytes and Breast Cancer Cell Line MCF-7 in Vitro. Immunol. Lett. 2016, 174, 37–44. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Ho, C.Y.; Lau, C.B.S.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.L.; Chow, M.S.S. Differential Effect of Coriolus versicolor (Yunzhi) Extract on Cytokine Production by Murine Lymphocytes in Vitro. Int. Immunopharmacol. 2004, 4, 1549–1557. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ma, D.; Tan, Y.-X.; Wang, H.-Y.; Cai, Z. Role Necroptosis Cancer: A Double-Edged Sword? Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 259–266. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Swain, R.; Belonce, S.; Jacobs, R.J. Therapeutic Effects of Medicinal Mushrooms on Gastric, Breast, and Colorectal Cancer: A Scoping Review. Cureus 2023, 15, e37574. [Google Scholar] [CrossRef] [PubMed]

| Compound Class | Specific Compounds/Examples | Biological Activities | References |
|---|---|---|---|
| Polysaccharides | Polysaccharopeptide (PSP), Polysaccharide-K (PSK) | Immunomodulatory, anti-tumor, TLR2 agonist, NK cell activation | [16,24,38,39,40] |
| β-Glucans | β-(1→3)-D-glucan, β-(1→6)-D-glucan | Immune stimulation, apoptosis induction | [3,39,42,43] |
| Proteins and Glycoproteins | Lectins, Fungal immunomodulatory proteins (FIPs) | Anticancer, immunostimulatory | [37,43,44] |
| Phenolic Compounds | Gallic acid, Catechin | Antioxidant, radical scavenging | [3,19,45] |
| Triterpenoids | Ergosterol, Lanostane-type triterpenes | Anticancer, anti-inflammatory | [3,19,43] |
| Sesquiterpenes | Spiroaxane derivatives, Rosenonolactone derivative | Potential antitumor properties | [46] |
| Sterols | Ergosterol | Cytotoxic, antioxidant | [3,19] |
| Enzymes | Laccase, Peroxidases | Biodegradation, antioxidant | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaja-Sołtys, M.; Jaszek, M. Exploring the Anticancer Potential of Coriolus versicolor in Breast Cancer: A Review. Curr. Issues Mol. Biol. 2025, 47, 808. https://doi.org/10.3390/cimb47100808
Ziaja-Sołtys M, Jaszek M. Exploring the Anticancer Potential of Coriolus versicolor in Breast Cancer: A Review. Current Issues in Molecular Biology. 2025; 47(10):808. https://doi.org/10.3390/cimb47100808
Chicago/Turabian StyleZiaja-Sołtys, Marta, and Magdalena Jaszek. 2025. "Exploring the Anticancer Potential of Coriolus versicolor in Breast Cancer: A Review" Current Issues in Molecular Biology 47, no. 10: 808. https://doi.org/10.3390/cimb47100808
APA StyleZiaja-Sołtys, M., & Jaszek, M. (2025). Exploring the Anticancer Potential of Coriolus versicolor in Breast Cancer: A Review. Current Issues in Molecular Biology, 47(10), 808. https://doi.org/10.3390/cimb47100808






