HCX3 Mitigates LPS-Induced Inflammatory Responses in Macrophages by Suppressing the Activation of the NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
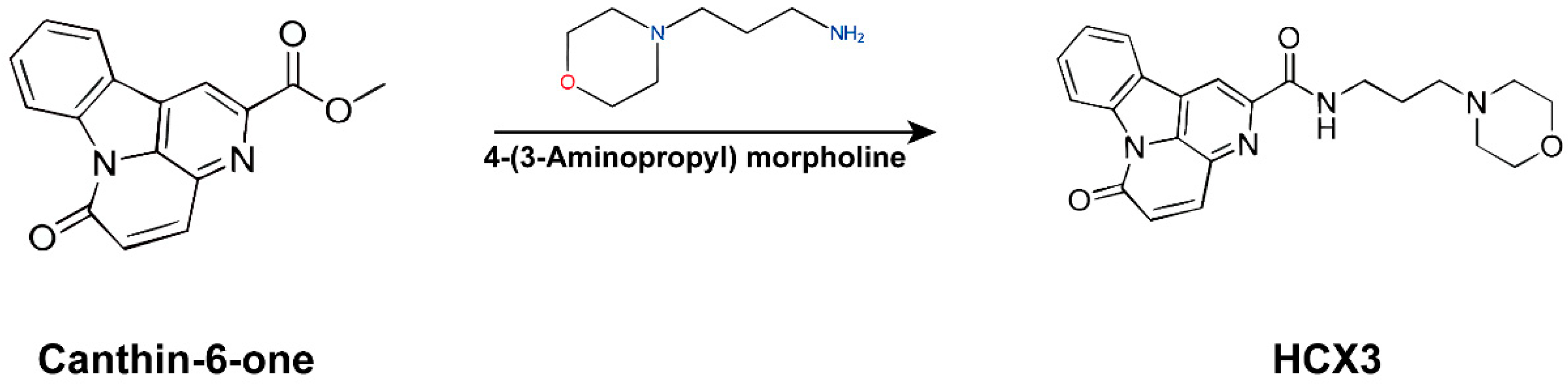
2.1. Materials and Reagents
2.2. Cell Culture
2.3. Network Pharmacology
2.4. CCK8 Assay
2.5. Western Blot
2.6. ELISA
2.7. Quantitative Polymerase Chain Reaction (qPCR)
| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
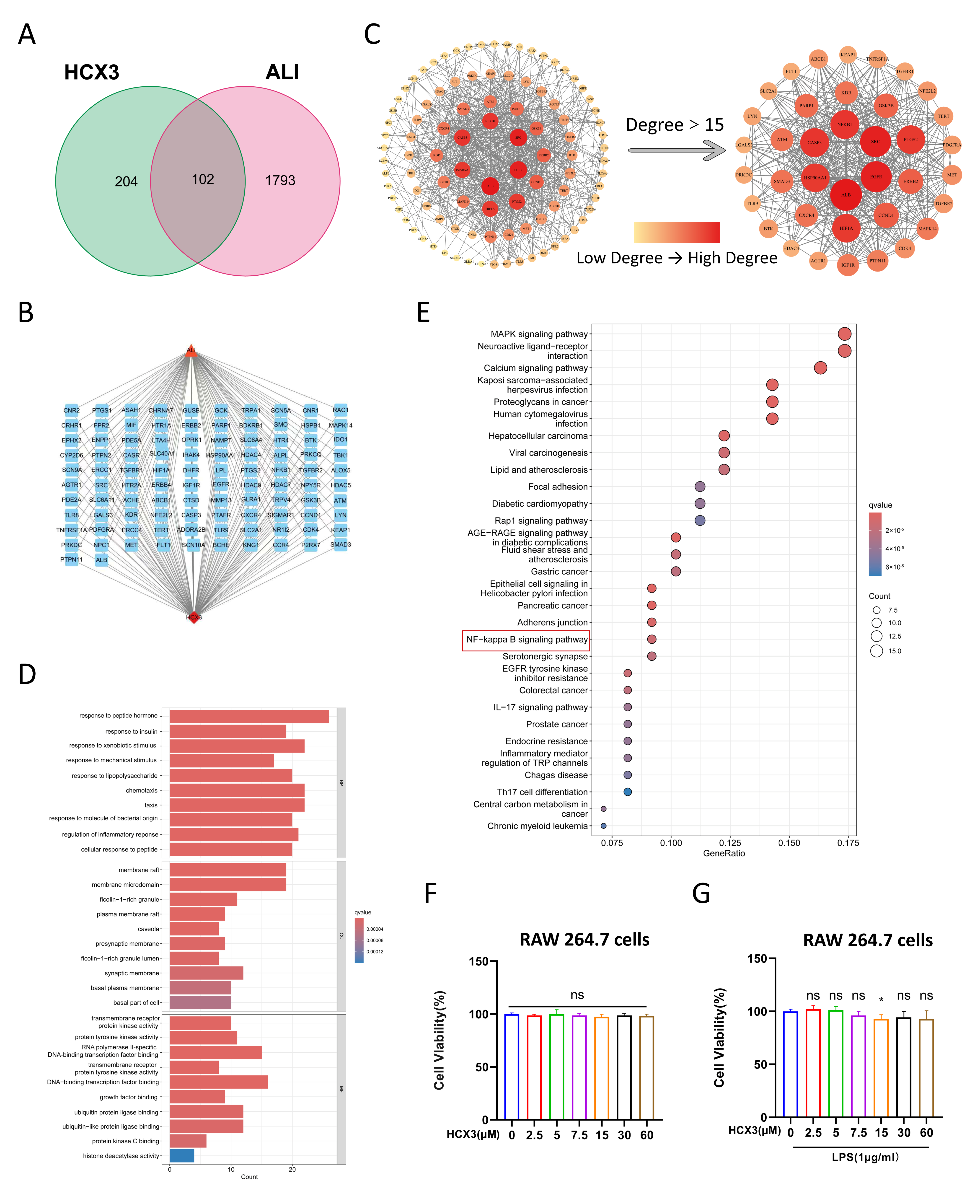
3.1. Network Pharmacological Analysis Reveals a Potential Role of HCX3 in Modulating Inflammatory Responses
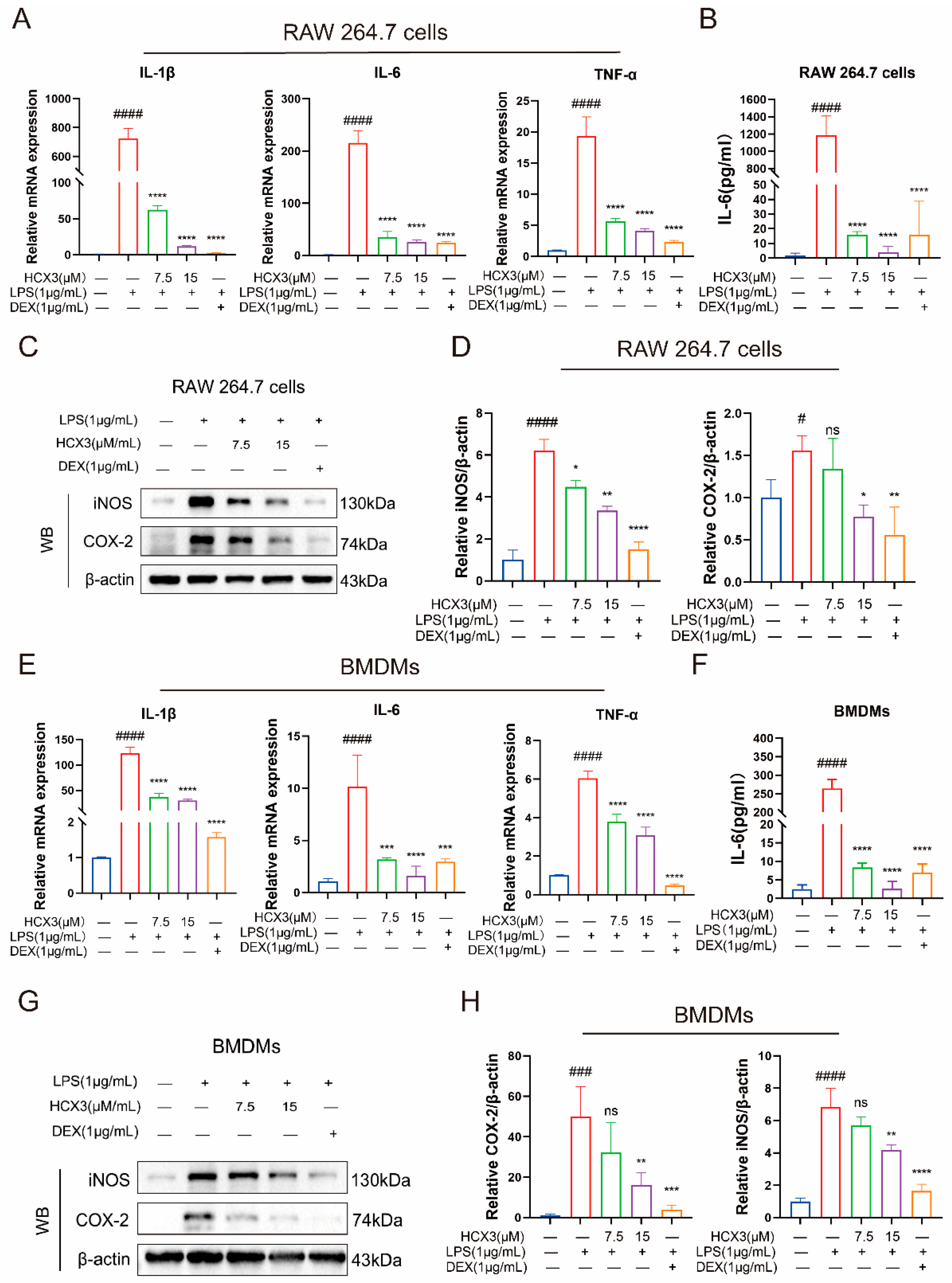
3.2. The Effect of HCX3 on Cell Viability of Macrophages
3.3. HCX3 Inhibits the Production of Pro-Inflammatory Mediators by Macrophages
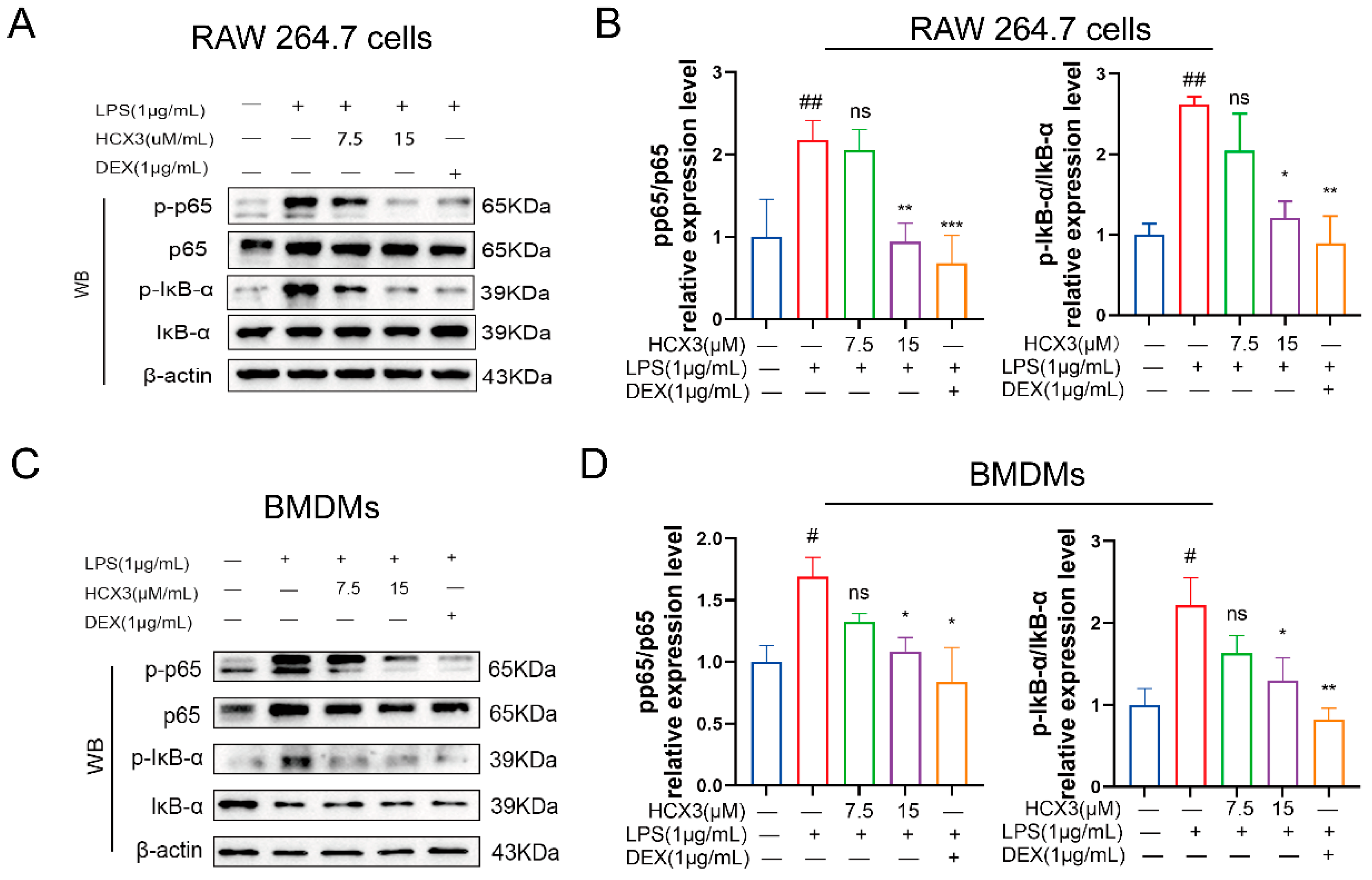
3.4. HCX3 Inhibits the NF-κB Signaling Pathway
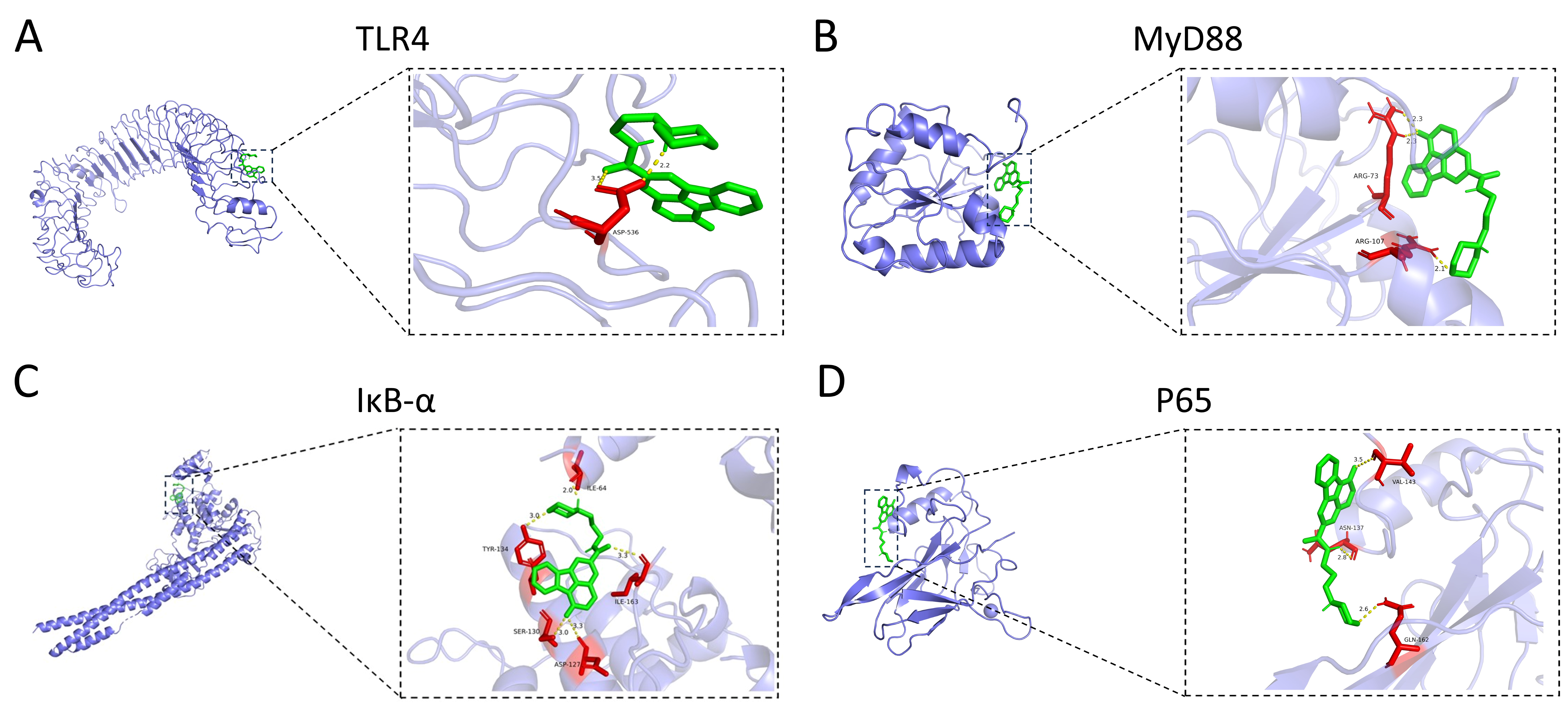
3.5. HCX3 Is Molecularly Docked to Key Proteins of the NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, J.; Xu, G.; Lin, Y.; Zhou, B.; Luo, Z.; Yu, S.; Lu, J. Inhibition of TRPV4 attenuates ferroptosis against LPS-induced ALI via Ca2+ pathway. Turk. J. Biol. 2022, 46, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu. Rev. Pathol. 2011, 6, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.; Martin, L.; Rossaint, R. The acute respiratory distress syndrome: Pathophysiology, current clinical practice, and emerging therapies. Expert Rev. Respir. Med. 2018, 12, 1021–1029. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Dong, J.; Li, X.; Ye, T.; Zeng, F.; Jiang, M.; Shi, J.; Wang, X.; Zhang, L. Isatin improves oligoasthenospermia caused by busulfan by regulating GSH/GPX4 axis to inhibit ferroptosis. Front. Pharmacol. 2024, 15, 1489956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Meng, L.; He, Q.; Ren, C.; Li, C. Valsartan attenuates LPS-induced ALI by modulating NF-κB and MAPK pathways. Front. Pharmacol. 2024, 15, 1321095. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.-T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front. Pharmacol. 2021, 12, 790072. [Google Scholar] [CrossRef]
- Lee, J.-W.; Park, J.-W.; Shin, N.-R.; Park, S.-Y.; Kwon, O.-K.; Park, H.A.; Lim, Y.; Ryu, H.W.; Yuk, H.J.; Kim, J.H.; et al. Picrasma quassiodes (D. Don) Benn. attenuates lipopolysaccharide (LPS)-induced acute lung injury. Int. J. Mol. Med. 2016, 38, 834–844. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef]
- Volk, A.; Li, J.; Xin, J.; You, D.; Zhang, J.; Liu, X.; Xiao, Y.; Breslin, P.; Li, Z.; Wei, W.; et al. Co-inhibition of NF-κB and JNK is synergistic in TNF-expressing human AML. J. Exp. Med. 2014, 211, 1093–1108. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Hu, K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- Gan, A.; Chen, H.; Lin, F.; Wang, R.; Wu, B.; Yan, T.; Jia, Y. Sanzi Yangqin Decoction improved acute lung injury by regulating the TLR2-mediated NF-κB/NLRP3 signaling pathway and inhibiting the activation of NLRP3 inflammasome. Phytomedicine Int. J. Phytother. Phytopharm. 2025, 139, 156438. [Google Scholar] [CrossRef]
- Gouda, M.M.; Shaikh, S.B.; Bhandary, Y.P. Inflammatory and Fibrinolytic System in Acute Respiratory Distress Syndrome. Lung 2018, 196, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Zhang, Y.; Zhang, J.; Wang, H.; Luo, Y.; Chan, H.F.; Tao, Y.; Chen, Z.; Li, M. Nanomedicine to advance the treatment of bacteria-induced acute lung injury. J. Mater. Chem. B 2021, 9, 9100–9115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, L.; Xia, Q.; Tian, L.; Qi, J.; Cao, M. Radix Astragali and Radix Angelicae Sinensis in the Treatment of Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-analysis. Front. Pharmacol. 2020, 11, 415. [Google Scholar] [CrossRef]
- Hecker, M.; Walmrath, H.-D.; Seeger, W.; Mayer, K. Clinical Aspects of Acute Lung Insufficiency (ALI/TRALI). Transfus. Med. Hemotherapy 2008, 35, 80–88. [Google Scholar] [CrossRef]
- Lu, T.; Denehy, L.; Cao, Y.; Cong, Q.; Wu, E.; Granger, C.L.; Ni, J.; Edbrooke, L. A 12-Week Multi-Modal Exercise Program: Feasibility of Combined Exercise and Simplified 8-Style Tai Chi Following Lung Cancer Surgery. Integr. Cancer Ther. 2020, 19, 1534735420952887. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, J.; Su, Q.; Liang, J.; Zhao, L.; Zhang, Y.; Sun, W. Antihypertensive effects of extract from Picrasma quassiodes (D. Don) Benn. in spontaneously hypertensive rats. J. Ethnopharmacol. 2013, 145, 187–192. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, C.; He, J.; Chen, L.; Zhang, Y.; Sun, W. The possible mechanisms of Picrasma quassiodes (D. Don) Benn. in the treatment of colitis induced by 2,4,6-trinitrobenzene sulfonic acid in mice. J. Ethnopharmacol. 2013, 145, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shu, X.; Jing, F.; Wang, X.; Lin, C.; Luo, A. Preparative separation of alkaloids from Picrasma quassioides (D. Don) Benn. by conventional and pH-zone-refining countercurrent chromatography. Mol. Basel Switz. 2014, 19, 8752–8761. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, N.; Jian, Y.; Liu, Y.; Zhao, L.; He, L.; Liu, Q.; Li, M. The pro-inflammatory effect of Staphylokinase contributes to community-associated Staphylococcus aureus pneumonia. Commun. Biol. 2022, 5, 618. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, A.; Wang, Y.; Sun, W.; Zhou, X.; Xu, Q.; Mao, L.; Zhang, J. Canthin-6-Ones: Potential Drugs for Chronic Inflammatory Diseases by Targeting Multiple Inflammatory Mediators. Mol. Basel Switz. 2023, 28, 3381. [Google Scholar] [CrossRef]
- Ding, J.; Sun, T.; Wu, H.; Zheng, H.; Wang, S.; Wang, D.; Shan, W.; Ling, Y.; Zhang, Y. Novel Canthin-6-one Derivatives: Design, Synthesis, and Their Antiproliferative Activities via Inducing Apoptosis, Deoxyribonucleic Acid Damage, and Ferroptosis. ACS Omega 2023, 8, 31215–31224. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Han, Y.; Kim, M.-H.; Boo, M.; Cho, K.-J.; Kim, H.-L.; Lee, I.-S.; Jung, J.H.; Kim, W.; Um, J.-Y.; et al. The multi-herbal decoction SH003 alleviates LPS-induced acute lung injury by targeting inflammasome and extracellular traps in neutrophils. Phytomed. Int. J. Phytother. Phytopharm. 2024, 133, 155926. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Attia, S.M.; Shabanah, O.A.; Ahmad, S.F. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol. Invest. 2016, 45, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yin, Z.; Feng, T.; Zhang, M.; Zhou, Z.; Zhou, Y. An integrated network pharmacology and RNA-Seq approach for exploring the preventive effect of Lonicerae japonicae flos on LPS-induced acute lung injury. J. Ethnopharmacol. 2021, 264, 113364. [Google Scholar] [CrossRef]
- Mao, L.; Dhar, A.; Meng, G.; Fuss, I.; Montgomery-Recht, K.; Yang, Z.; Xu, Q.; Kitani, A.; Strober, W. Blau syndrome NOD2 mutations result in loss of NOD2 cross-regulatory function. Front. Immunol. 2022, 13, 988862. [Google Scholar] [CrossRef]
- Tingle, B.I.; Tang, K.G.; Castanon, M.; Gutierrez, J.J.; Khurelbaatar, M.; Dandarchuluun, C.; Moroz, Y.S.; Irwin, J.J. ZINC-22—A Free Multi-Billion-Scale Database of Tangible Compounds for Ligand Discovery. J. Chem. Inf. Model. 2023, 63, 1166–1176. [Google Scholar] [CrossRef]
- Kong, X.; Liu, C.; Zhang, Z.; Cheng, M.; Mei, Z.; Li, X.; Liu, P.; Diao, L.; Ma, Y.; Jiang, P.; et al. BATMAN-TCM 2.0: An enhanced integrative database for known and predicted interactions between traditional Chinese medicine ingredients and target proteins. Nucleic Acids Res. 2024, 52, D1110–D1120. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database J. Biol. Databases Curation 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.L.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zhao, D.; Yu, X.; Shen, X.; Zhou, Y.; Wang, S.; Qiu, Y.; Chen, Y.; Zhu, F. TTD: Therapeutic Target Database describing target druggability information. Nucleic Acids Res. 2024, 52, D1465–D1477. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, J.M.; Whirl-Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1417. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. Clifton N.J. 2017, 1607, 627–641. [Google Scholar] [CrossRef]
- Wang, Y.; Bryant, S.H.; Cheng, T.; Wang, J.; Gindulyte, A.; Shoemaker, B.A.; Thiessen, P.A.; He, S.; Zhang, J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017, 45, D955–D963. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, J.; Sun, H.; Xu, L. Plumbagin ameliorates LPS-induced acute lung injury by regulating PI3K/AKT/mTOR and Keap1-Nrf2/HO-1 signalling pathways. J. Cell. Mol. Med. 2024, 28, e18386. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Park, J.-S.; Heo, J.D.; Kim, G.S. Scutellarein Inhibits LPS-Induced Inflammation through NF-κB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules 2022, 27, 3782. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Deng, Y.; Dang, J.; Liu, Y.; Zhao, J.; Liang, R.; Zeng, D.; Wu, W.; Xiong, Y.; et al. miRNA-148a-containing GMSC-derived EVs modulate Treg/Th17 balance via IKKB/NF-κB pathway and treat a rheumatoid arthritis model. JCI Insight 2024, 9, e177841. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Kong, X.; Guo, J. Exploring immune-related pathogenesis in lung injury: Providing new insights into ALI/ARDS. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 175, 116773. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, G.; Liu, Y.; Liu, L.; Zhang, T.; Liu, P.; Tu, Q.; Zhang, X.; Luo, S.; Yao, L.; et al. The transcription factor KLF14 regulates macrophage glycolysis and immune function by inhibiting HK2 in sepsis. Cell. Mol. Immunol. 2022, 19, 504–515. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Z.-A.; Yan, S.; Lei, W.; Wu, H.; Lu, X.-A.; Chen, Y.; Li, J.; Wang, Y.; Yu, M.; et al. CXADR-like membrane protein protects against heart injury by preventing excessive pyroptosis after myocardial infarction. J. Cell. Mol. Med. 2020, 24, 13775–13788. [Google Scholar] [CrossRef] [PubMed]
- He-Yang, J.; Zhang, W.; Liu, J.; Xue, P.; Zhou, X. Human breast milk oligosaccharides attenuate necrotizing enterocolitis in rats by suppressing mast cell accumulation, DPPI activity and TLR4 expression in ileum tissue, and regulating mitochondrial damage of Caco-2 cells. Int. Immunopharmacol. 2020, 88, 106881. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, A.; Jabłonowska, E.; Studzińska, M.; Kamerys, J.; Paradowska, E. The TLR9 2848C/T Polymorphism Is Associated with the CMV DNAemia among HIV/CMV Co-Infected Patients. Cells 2021, 10, 2360. [Google Scholar] [CrossRef]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef]
- Shin, N.-R.; Shin, I.-S.; Song, H.-H.; Hong, J.-M.; Kwon, O.-K.; Jeon, C.-M.; Kim, J.-H.; Lee, S.-W.; Lee, J.-K.; Jin, H.; et al. Callicarpa japonica Thunb. reduces inflammatory responses: A mouse model of lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2015, 26, 174–180. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Yang, J.-J.; Yang, M.-L.; Li, Y.-C.; Kuan, Y.-H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Chang, M.; Zhao, Q.; Zhou, Z.; Huang, X.; Guo, F.; Huan, J. Genistein-3’-sodium sulphonate protects against lipopolysaccharide-induced lung vascular endothelial cell apoptosis and acute lung injury via BCL-2 signalling. J. Cell. Mol. Med. 2020, 24, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Wigenstam, E.; Elfsmark, L.; Koch, B.; Bucht, A.; Jonasson, S. Acute respiratory changes and pulmonary inflammation involving a pathway of TGF-β1 induction in a rat model of chlorine-induced lung injury. Toxicol. Appl. Pharmacol. 2016, 309, 44–54. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Shi, J.; Wu, L.; Li, L.; Ling, Y.; Mao, L.; Zhang, J. HCX3 Mitigates LPS-Induced Inflammatory Responses in Macrophages by Suppressing the Activation of the NF-κB Signaling Pathway. Curr. Issues Mol. Biol. 2025, 47, 809. https://doi.org/10.3390/cimb47100809
Wu Q, Shi J, Wu L, Li L, Ling Y, Mao L, Zhang J. HCX3 Mitigates LPS-Induced Inflammatory Responses in Macrophages by Suppressing the Activation of the NF-κB Signaling Pathway. Current Issues in Molecular Biology. 2025; 47(10):809. https://doi.org/10.3390/cimb47100809
Chicago/Turabian StyleWu, Qianyi, Jiyuan Shi, Luojin Wu, Lingxi Li, Yong Ling, Liming Mao, and Jie Zhang. 2025. "HCX3 Mitigates LPS-Induced Inflammatory Responses in Macrophages by Suppressing the Activation of the NF-κB Signaling Pathway" Current Issues in Molecular Biology 47, no. 10: 809. https://doi.org/10.3390/cimb47100809
APA StyleWu, Q., Shi, J., Wu, L., Li, L., Ling, Y., Mao, L., & Zhang, J. (2025). HCX3 Mitigates LPS-Induced Inflammatory Responses in Macrophages by Suppressing the Activation of the NF-κB Signaling Pathway. Current Issues in Molecular Biology, 47(10), 809. https://doi.org/10.3390/cimb47100809






