Integrating Morphological, Pathogenic, and Molecular Approaches to Characterize Fusarium Root Rot Pathogens of Common Bean in Egypt
Abstract
1. Introduction
2. Materials and Methods
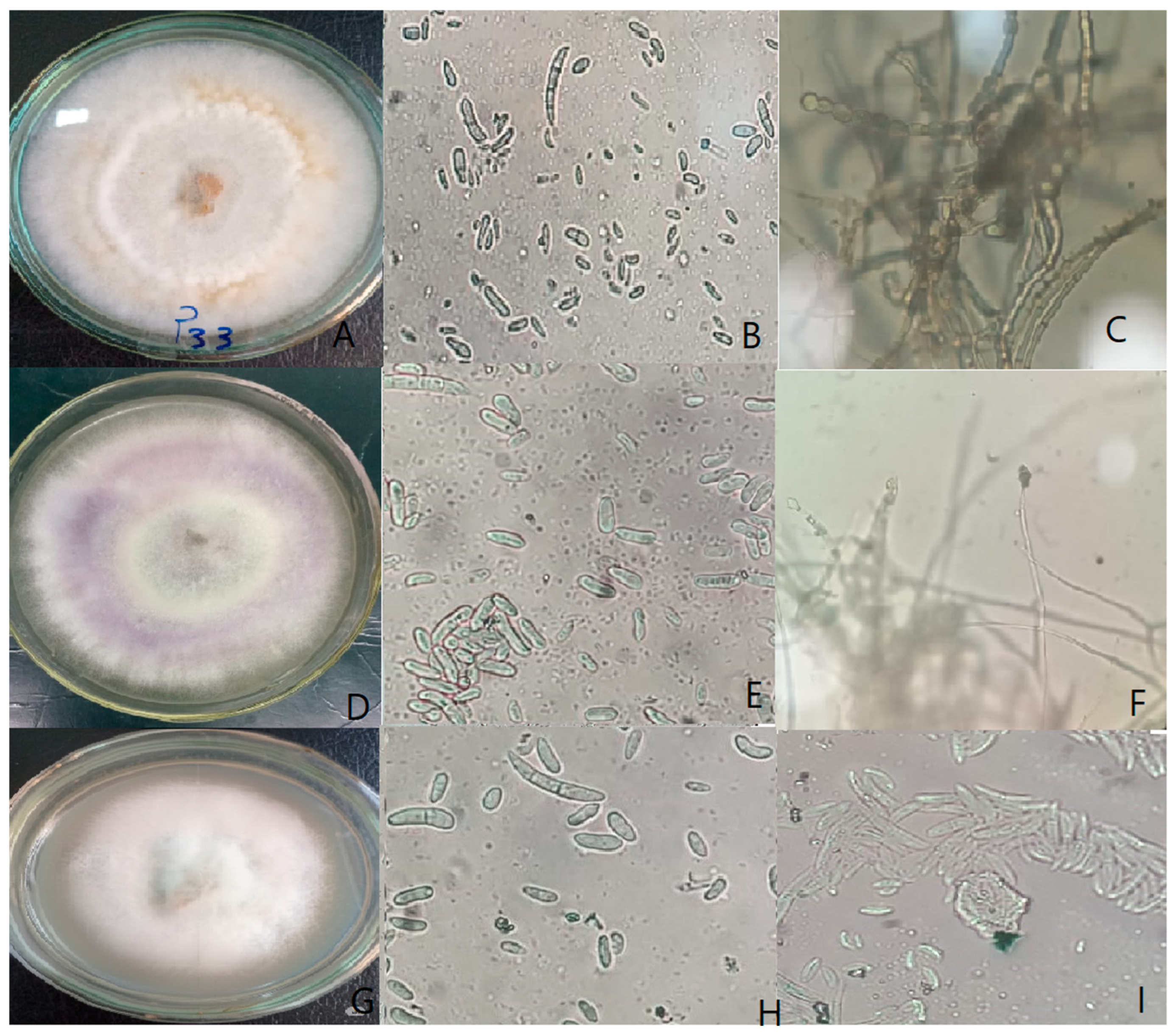
2.1. Isolation and Morphological Characterization of Fusarium spp.
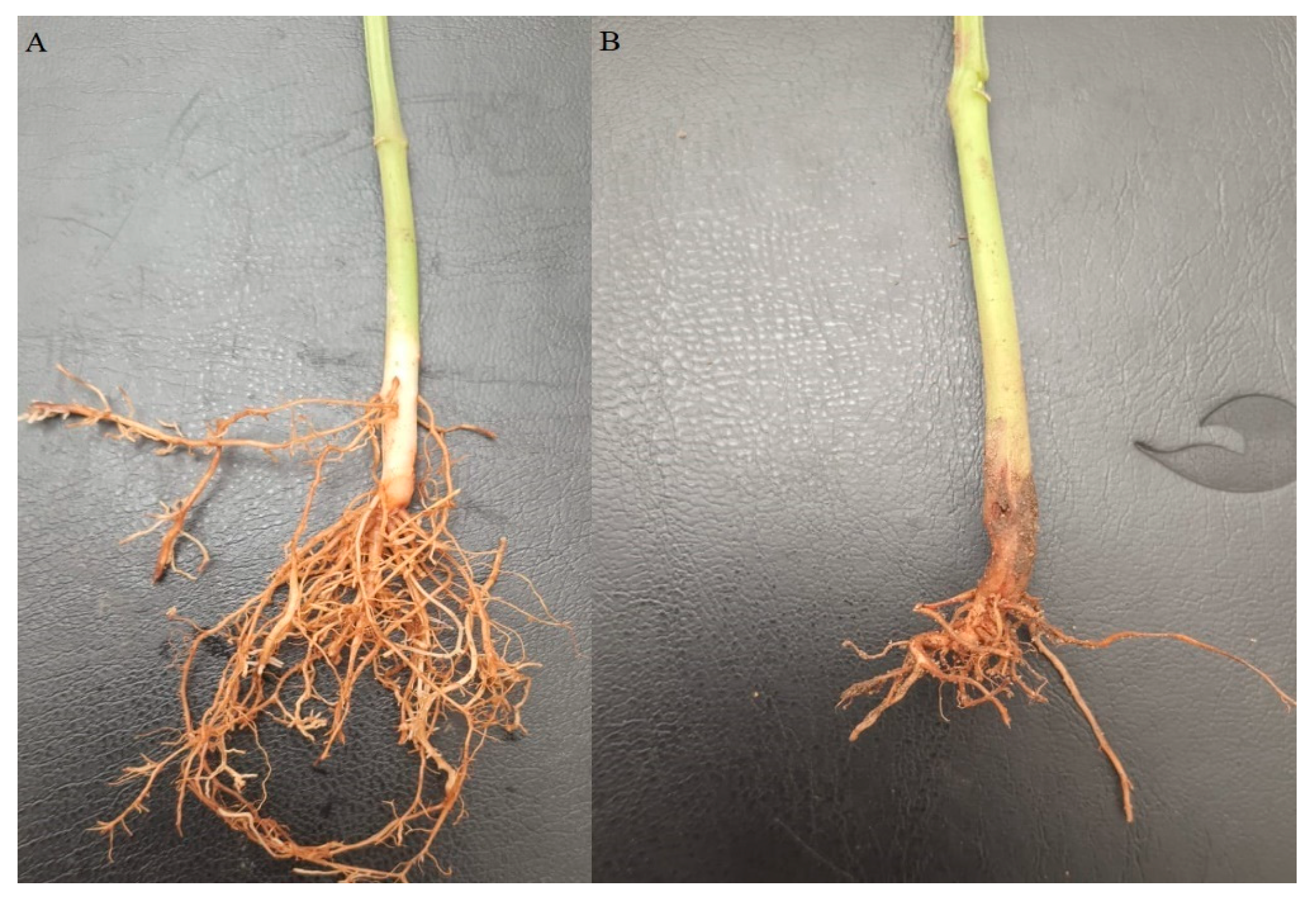
2.2. Pathogenicity Test
2.3. Disease Assessment
2.4. DNA Extraction
2.5. Molecular and Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Frequency and Morphological Characterization of Fusarium Isolates
3.2. Pre-Emergence (%) Damping-Off of Fusarium Species with Different Cultivars of Common Beans
3.3. Post-Emergence (%) Damping-Off of Fusarium Species with Different Cultivars of Common Beans
3.4. Disease Incidence Percentage on Fusarium Isolates with Different Cultivars of Common Beans
3.5. Disease Severity Percentage on Fusarium Isolates with Different Cultivars of Common Beans
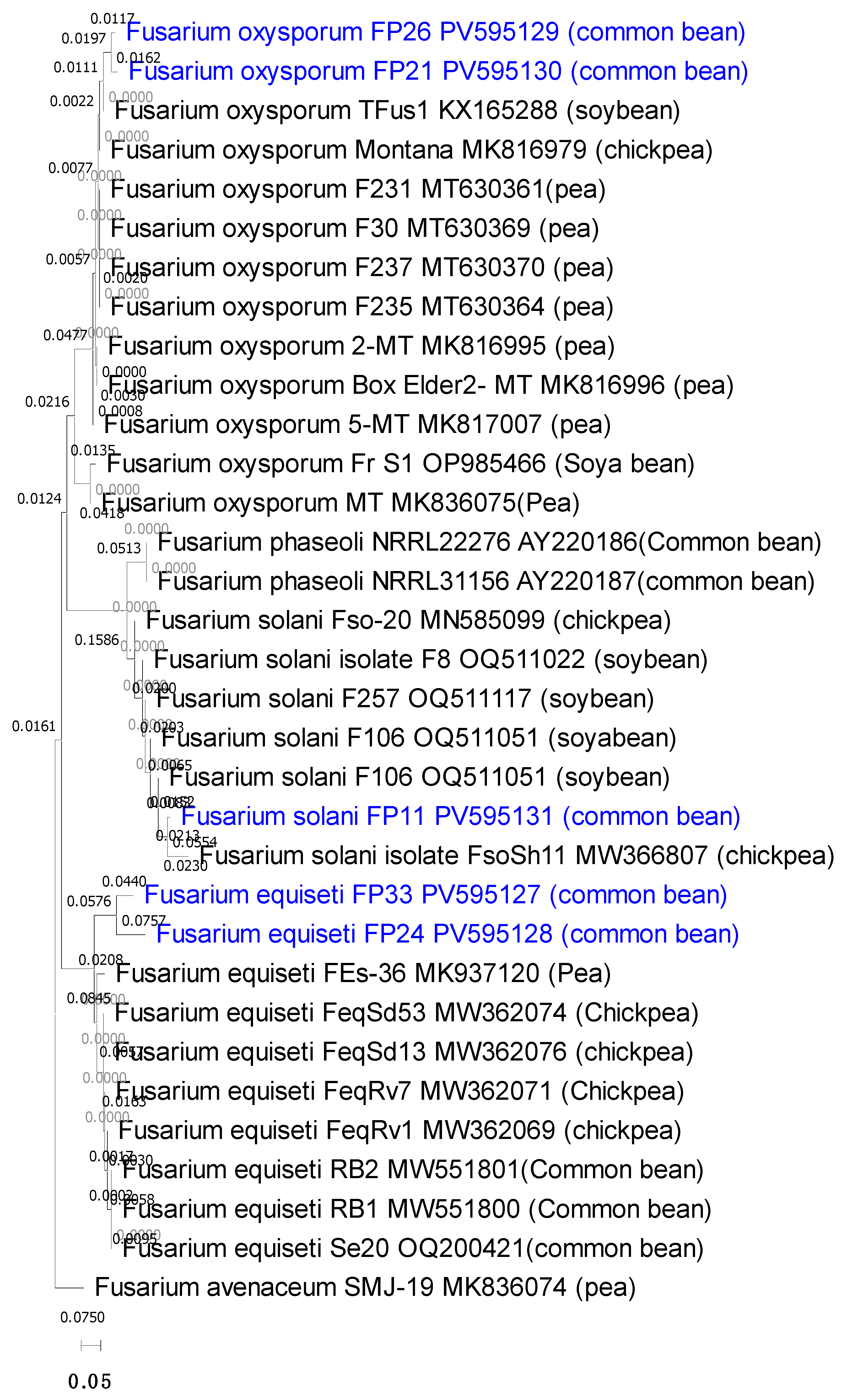
3.6. Molecular Identification and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Meziadi, C.; Richard, M.M.S.; Derquennes, A.; Thareau, V.; Blanch Romero, A. Development of molecular markers linked to disease resistance genes in common bean based on whole genome sequence. Plant Sci. 2016, 242, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Machiani, M.A.; Rezaei-Chiyaneh, E.; Javanmard, A.; Maggi, F.; Morshedloo, M.R. Evaluation of common bean (Phaseolus vulgaris L.) seed yield and quali-quantitative production of the essential oils from fennel (Foeniculum vulgare Mill.) and dragonhead (Dracocephalum moldavica L.) in intercropping system under humic acid application. J. Clean. Prod. 2019, 235, 112–122. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT Statistics Database. Available online: http://www.fao.org/faostat (accessed on 21 April 2025).
- Mostafa, F.W. Integrated Control for White Rot Caused by Sclerotinia sclerotiorum (Lib.) de Bary and Rust Caused by Uromyces appendiculatus (Pers.) Unger of Bean (Phaseolus vulgaris L.) Plant. Ph.D. Thesis, Faculty of Agriculture, Fayoum University, Fayoum, Egypt, 2014; 184p. [Google Scholar]
- Singh, S.P.; Schwartz, H.F. Breeding common bean for resistance to diseases: A review. Crop Sci. 2010, 50, 2199–2223. [Google Scholar] [CrossRef]
- Vural, C.; Soylu, S.O. Prevalence and incidence of fungal disease agents affecting bean (Phaseolus vulgaris L.) plants. Res. Crops 2012, 13, 634–640. [Google Scholar]
- Marcenaro, D.; Valkonen, J.P. Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. INTA Rojo) in Nicaragua. PLoS ONE 2016, 11, e0168662. [Google Scholar] [CrossRef]
- Girma, F.; Fininsa, C.; Terefe, H.; Amsalu, B. Evaluation of common bean (Phaseolus vulgaris) genotypes for resistance to common bacterial blight and angular leaf spot diseases, and agronomic performances. Heliyon 2022, 8, e10425. [Google Scholar] [CrossRef]
- Rahmanzadeh, A.; Khahani, B.S.; Taghavi, M.; Khojasteh, M.; Osdaghi, E. Genome-wide meta-QTL analyses provide novel insight into disease resistance repertoires in common bean. BMC Genom. 2022, 23, 680. [Google Scholar] [CrossRef]
- Félix-Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Orozco-Mosqueda, M.C.; Santos-Villalobos, S.D. Draft genome sequence of Bacillus sp. strain FSQ1, a biological control agent against white mold in common bean (Phaseolus vulgaris L.). Curr. Res. Microb. Sci. 2022, 3, 100138. [Google Scholar] [CrossRef]
- Adomako, J.S.; Yeboah, J.F.; Asamoah, P.; Amankwaa-Yeboah, E.A.; Adjei, E.A.; Obeng, B.; Sakyiamah, M.; Lamptey, L.; Asibuo, B.J.Y. Survey of plant parasitic nematodes and disease severity of common bean lines evaluated for reaction to root knot nematodes infestation. Afr. Crop Sci. J. 2022, 30, 147–154. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, A.S.; Selem, E.; Mohsen, A.A.A.; Arnaout, S.M.A.I.; El-Sappah, A.H.; El-Tarabily, K.A.; Desoky, E.M. Spirulina platensis extract improves the production and defenses of the common bean grown in a heavy metals-contaminated saline soil. J. Environ. Sci. 2023, 129, 240–257. [Google Scholar] [CrossRef]
- Papathanasiou, F.; Ninou, E.; Mylonas, I.; Baxevanos, D.; Papadopoulou, F.; Avdikos, I.; Sistanis, I.; Koskosidis, A.; Vlachostergios, D.N.; Stefanou, S. The evaluation of common bean (Phaseolus vulgaris L.) genotypes under water stress based on physiological and agronomic parameters. Plants 2022, 11, 2432. [Google Scholar] [CrossRef]
- Zhang, Q.; Geng, J.; Du, Y.; Zhao, Q.; Zhang, W.; Fang, Q.; Yin, Z.; Li, J.; Yuan, X.; Fan, Y. Heat shock transcription factor (Hsf) gene family in common bean (Phaseolus vulgaris): Genome-wide identification, phylogeny, evolutionary expansion and expression analyses at the sprout stage under abiotic stress. BMC Plant Biol. 2022, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Musoke, S.; Mukankusi, C. Prevalence and incidence of four common bean root rots in Uganda. J. Exp. Agric. Int. 2018, 54, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Harter, L. A Fusarium disease of beans. Phytopathology 1929, 19, 84. [Google Scholar]
- Sampaio, A.M.; de Araújo, S.S.; Rubiales, D.; Vaz Patto, M.C. Fusarium wilt management in legume crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Ampaire, E. Farmers’ Indigenous Technical Knowledge of Bean Diseases Management and Communication Systems in Southwestern Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 2003; 127p. [Google Scholar]
- Opio, F.; Ugen, M.; Namayanja, A.; Mugagga, I.; Mawejje, D. Improving food security in southwestern Uganda by transferring and promoting resistant varieties and integrated management packages for BRR. In Biotechnology, Breeding, and Seed Systems for African Crops Conference; National Institute for Agriculture Research (IIAM): Maputo, Mozambique, 2007. [Google Scholar]
- Usmael, A.; Dejene, M.; Ayena, G. Assessment of root rot pathogens of common bean (Phaseolus vulgaris L.) and reaction of genotypes to the pathogens in West Hararghe Zone, Ethiopia. Open J. Plant Sci. 2023, 8, 037–055. [Google Scholar] [CrossRef]
- Deng, D.; Wu, W.; Duan, C.; Sun, S.; Zhu, Z. A novel pathogen Fusarium cuneirostrum causing common bean (Phaseolus vulgaris) root rot in China. J. Integr. Agric. 2024, 23, 166–176. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Orrell, T.; Kodati, S. Effect of virulence of root rot pathogens and cultivar resistance on disease occurrence in dry beans. Plant Health Prog. 2018, 19, 237–241. [Google Scholar] [CrossRef]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host–pathogen interaction in Fusarium oxysporum infections: Where do we stand? Mol. Plant Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef]
- Wang, C.H.; Sun, C.; Liu, S.Y. Preliminary study on pathogen of root rot of kidney bean and its control in Xinjiang. Arid Zone Res. 2010, 27, 380–384. (In Chinese) [Google Scholar]
- Liu, T.; Shen, Y.Q.; Liu, Z.; Han, D.; Cui, J.; Zuo, Y.H. Control effect of three kinds of seed-coating formulations on the root rot of kidney beans. Plant Prot. 2017, 43, 216–219. [Google Scholar]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-27646-4. [Google Scholar]
- Sennoi, R.; Jogloy, S.; Saksirirat, W.; Patanothai, A. Pathogenicity test of Sclerotium rolfsii, a causal agent of Jerusalem artichoke (Helianthus tuberosus L.) stem rot. Asian J. Plant Sci. 2010, 9, 281–284. [Google Scholar] [CrossRef]
- Ali, M.A.; Abd El Gwad, T.I.; Isamail, M.E.; Galal, A.A. Eco-friendly control traits of common bean root rot caused by Macrophomina phaseolina (Tossi) Goid and Fusarium equiseti using fungicide alternatives. New Val. J. Agric. Sci. 2022, 2, 530–543. [Google Scholar] [CrossRef]
- Shaban, W.I.; El-Bramawy, M.A. Impact of dual inoculation with Rhizobium and Trichoderma on damping-off, root rot diseases and plant growth parameters of some legumes field crop under greenhouse conditions. Int. Res. J. Agric. Sci. Soil Sci. 2011, 1, 98–108. [Google Scholar]
- Kator, L.; Hosea, Z.Y.; Oche, O.D. Sclerotium rolfsii: Causative organism of southern blight, stem rot, white mold and sclerotia rot disease. Ann. Biol. Res. 2015, 6, 78–89. [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Geiser, D.M.; Jiménez-Gasco, M.M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; O’Donnell, K. FUSARIUM-ID v.1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jenkins, S.; Labanska, M.; Amsterdam, S.; Clarkson, J.; Covington, J. Preliminary studies on detection of Fusarium basal rot infection in onions and shallots using electronic nose. Sensors 2021, 22, 5453. [Google Scholar] [CrossRef]
- Duvnjak, T.; Sudaric, A.; Matosa Kocar, M.; Cosic, J.; Vrandecic, K. First report of soybean fusarium wilt caused by Fusarium oxysporum in Croatia. Plant Dis. 2017, 101, 249. [Google Scholar] [CrossRef]
- Moparthi, S.; Agindotan, B.O.; Burrows, M.E. Identification and characterization of Fusarium spp. associated with root rot of dry pea in Montana. Plant Health Prog. 2019, 20, 215–219. [Google Scholar] [CrossRef]
- Olszak-Przybyś, H.; Korbecka-Glinka, G.; Patkowska, E. Identification and pathogenicity of Fusarium isolated from soybean in Poland. Pathogens 2023, 12, 1162. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Homma, Y.; Lattanzi, A.R. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex F. virguliforme in North America and F. tucumaniae in South America. Mycologia 2003, 95, 660–684. [Google Scholar] [CrossRef]
- Weerasooriya, S.; Bovill, M.S.; Benson, A.; Musick, A.M.; Ricotti, M. Devouring the Milky Way satellites: Modeling dwarf galaxies with Galacticus. Astrophys. J. 2023, 948, 87. [Google Scholar] [CrossRef]
- Moparthi, S.; Peluola, C.; Agindotan, B.; McPhee, K.; Burrows, M. First report of gray mold of chickpea caused by Botrytis euroamericana in the USA. Crop Prot. 2020, 137, 105297. [Google Scholar] [CrossRef]
- Mojerlou, S.; Sepehri, G.; Shahbaz, S. Identification of bean root rot causal and associated fungal agents in Khomein County, Markazi Province, Iran. Crop Prot. 2021, 10, 633–646. [Google Scholar]
- Darvishnia, M.; Hasanvand, E.; Pakbaz, S. Morphological and molecular identification of Fusarium associated with beans in Selseleh County. J. Genet. Res. 2023, 9, 222–231. [Google Scholar]
- Oudman, L. Identification, Characterization, and Management of Fusarium Root Rot Pathogens of Dry Beans in Michigan. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2018. [Google Scholar]
- Henriquez, M.A.; Conner, R.L.; Hou, A.; Balasubramanian, P.; McLaren, D.L.; Chang, K.F.; McRae, K.B. Reaction of dry bean cultivars grown in western Canada to root rot inoculation. Can. J. Plant Sci. 2014, 94, 1219–1230. [Google Scholar] [CrossRef]
- Zitnick-Anderson, K.; Oladzadabbasabadi, A.; Jain, S.; Modderman, C.; Osorno, J.M.; McClean, P.E.; Pasche, J.S. Sources of resistance to Fusarium solani and associated genomic regions in common bean diversity panels. Front. Genet. 2020, 11, 475. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Hashem, A.H.; Al-Askar, A.A.; AbdElgawad, H.; Attia, M.S. Efficient role of endophytic Aspergillus terreus in biocontrol of Rhizoctonia solani causing damping-off disease of Phaseolus vulgaris and Vicia faba. Microorganisms 2023, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Naseri, B.; Mousavi, S.S. Root rot pathogens in field soil, roots and seeds in relation to common bean (Phaseolus vulgaris), disease and seed production. Int. J. Pest Manag. 2015, 61, 60–67. [Google Scholar] [CrossRef]
- Diaz, L.M.; Arredondo, V.; Ariza-Suarez, D.; Aparicio, J.; Buendia, H.F.; Cajiao, C.; Raatz, B. Genetic analyses and genomic predictions of root rot resistance in common bean across trials and populations. Front. Plant Sci. 2021, 12, 629221. [Google Scholar] [CrossRef] [PubMed]
- Shahiba, A.M.; Pranay, G.; Sonaniya, P.; Kushwaha, J.S.; Aware, S.A. Modern approaches in plant breeding enhancing crop genetics. In Modern Approaches in Plant Breeding Enhancing Crop Genetics; Elite Publishing House: Delhi, India, 2023; pp. 15–33. [Google Scholar]
- Maina, P.K.; Wachira, P.M.; Okoth, S.A.; Kimenju, J.W. Cultural, morphological and pathogenic variability among Fusarium oxysporum f. sp. phaseoli causing wilt in French bean (Phaseolus vulgaris L.). J. Adv. Microbiol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Singh, H.B.; Singh, B.N.; Singh, S.P.; Nautiyal, C.S. Solid-state cultivation of Trichoderma harzianum NBRI-1055 for modulating natural antioxidants in soybean seed matrix. Bioresour. Technol. 2010, 101, 6444–6453. [Google Scholar] [CrossRef]
- Moparthi, S.; Burrows, M.; Mgbechi-Ezeri, J.; Agindotan, B. Fusarium spp. associated with root rot of pulse crops and their cross-pathogenicity to cereal crops in Montana. Plant Dis. 2021, 105, 548–557. [Google Scholar] [CrossRef]
- Olarte, R.A.; Hall, R.; Tabima, J.F.; Malvick, D.; Bushley, K. Genetic diversity and aggressiveness of Fusarium virguliforme isolates across the midwestern United States. Phytopathology 2022, 112, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Safarloo, Z.; Hemmati, R. Identification and study of pathogenicity of Fusarium species involved in bean root rot in Zanjan province. Appl. Res. Phytomed. 2014, 3, 77–92. [Google Scholar]
- Ahari Mostafavi, H.; Mirmajlessi, S.M.; Safaie, N.; Minassyan, V.; Fathollahi, H.; Dorri, H.R.; Mansouripour, S.M. The use of a gamma-irradiated mutant of Fusarium solani f. sp. phaseoli with reduced pathogenicity for the biological control of Fusarium root rot of bean (Phaseolus vulgaris) in field conditions. J. Agric. Sci. Technol. 2012, 14, 1415–1423. [Google Scholar]
- Naseri, B.; Marefat, A. Large-scale assessment of agricultural practices affecting Fusarium root rot and common bean yield. Eur. J. Plant Pathol. 2011, 131, 179–195. [Google Scholar] [CrossRef]
- El Hazzat, N.; Adnani, M.; Msairi, S.; El Alaoui, M.A.; Mouden, N.; Chliyeh, M.; Douira, A. Fusarium equiseti as one of the main Fusarium species causing wilt and root rot of chickpeas in Morocco. Acta Mycol. 2023, 57, 576. [Google Scholar] [CrossRef]
- Zhou, T.; DallaSanta, K.; Nazarenko, L.; Schmidt, G.A.; Jin, Z. The impact of increasing stratospheric radiative damping on the quasi-biennial oscillation period. Atmos. Chem. Phys. 2021, 21, 7395–7407. [Google Scholar] [CrossRef]

| Isolate Code | Governorate | Fusarium spp. | Pathogenic Reaction |
|---|---|---|---|
| FP1 | Qalyubia | F. proliferatum | Pathogenic |
| FP2 | Qalyubia | F. proliferatum | Pathogenic |
| FP3 | Qalyubia | F. equiseti | Non-pathogenic |
| FP4 | Qalyubia | F. verticillioides | Non-pathogenic |
| FP5 | Qalyubia | F. semitectum | Non-pathogenic |
| FP6 | Qalyubia | F. solani | Non-pathogenic |
| FP7 | Qalyubia | F. proliferatum | Non-pathogenic |
| FP8 | Qalyubia | F. proliferatum | Pathogenic |
| FP9 | Qalyubia | F. solani | Non-pathogenic |
| FP10 | Qalyubia | F. proliferatum | Pathogenic |
| FP11 | Qalyubia | F. solani | Pathogenic |
| FP12 | Qalyubia | F. equiseti | Non-pathogenic |
| FP13 | Qalyubia | F. verticillioides | Non-pathogenic |
| FP14 | Qalyubia | F. subglutinans | Pathogenic |
| FP15 | Qalyubia | F. semitectum | Pathogenic |
| FP16 | Qalyubia | F. solani | Non-pathogenic |
| FP17 | Qalyubia | F. verticillioides | Pathogenic |
| FP18 | Kafr-elsheikh | F. verticillioides | Pathogenic |
| FP19 | Kafr-elsheikh | F. subglutinans | Non-pathogenic |
| FP20 | Kafr-elsheikh | F. semitectum | Non-pathogenic |
| FP21 | Beni-Suef | F. oxysporum | Pathogenic |
| FP22 | Beni-Suef | F. subglutinans | Non-pathogenic |
| FP23 | Beni-Suef | F. solani | Non-pathogenic |
| FP24 | Beni-Suef | F. equiseti | Pathogenic |
| FP25 | Beni-Suef | F. tricinctum | Non-pathogenic |
| FP26 | Kafr-elsheikh | F. oxysporum | Pathogenic |
| FP27 | Qalyubia | F. anthophilum | Non-pathogenic |
| FP28 | Qalyubia | F. semitectum | Non-pathogenic |
| FP29 | Qalyubia | F. equiseti | Non-pathogenic |
| FP30 | Baheira | F. oxysporum | Non-pathogenic |
| FP31 | Kafr-elsheikh | F. solani | Non-pathogenic |
| FP32 | Kafr-elsheikh | F. verticillioides | Non-pathogenic |
| FP33 | Kafr-elsheikh | F. equiseti | Pathogenic |
| FP34 | Beheira | F. solani | Non-pathogenic |
| FP35 | Baheira | F. equiseti | Non-pathogenic |
| Common Bean Cultivars | ||||||
|---|---|---|---|---|---|---|
| Isolate code | Alpha | Samantha | Nebraska | Giza6 | Giza 12 | Cambo |
| FP18 | 11.11 ± 0.64 d | 11.11 ± 1.28 d | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP33 | 44.44 ± 2.57 a | 44.44 ± 5.13 a | 22.22 ± 2.57 c | 44.44 ± 2.57 a | 22.22 ± 3.85 c | 44.44 ± 5.9 a |
| FP24 | 33.33 ± 1.92 b | 33.33 ± 3.85 b | 11.11 ± 1.28 d | 44.44 ± 2.57 a | 22.22 ± 3.85 c | 44.44 ± 5.9 a |
| FP26 | 33.33 ± 1.92 b | 33.33 ± 3.85 b | 11.11 ± 1.28 d | 44.44 ± 2.57 a | 22.22 ± 3.85 c | 44.44 ± 5.9 a |
| FP1 | 11.11 ± 0.64 d | 22.22 ± 2.57 c | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP21 | 33.33 ± 1.92 b | 33.33 ± 3.85 b | 11.11 ± 1.28 d | 44.44 ± 2.57 a | 22.22 ± 3.85 c | 44.44 ± 5.9 a |
| FP2 | 11.11 ± 0.64 d | 11.11 ± 1.28 d | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP8 | 11.11 ± 0.64 d | 11.11 ± 1.28 d | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP10 | 11.11 ± 0.64 d | 11.11 ± 1.28 d | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP11 | 22.22 ± 1.28 c | 22.22 ± 2.57 c | 11.11 ± 1.28 d | 33.33 ± 1.92 b | 11.11 ± 1.92 d | 33.33 ± 4.43 b |
| FP17 | 22.22 ± 1.28 c | 22.22 ± 2.57 c | 11.11 ± 1.28 d | 33.33 ± 1.92 b | 11.11 ± 1.92 d | 33.33 ± 4.43 b |
| FP15 | 11.11 ± 0.64 d | 22.22 ± 2.57 c | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| FP14 | 11.11 ± 0.64 d | 11.11 ± 1.28 d | 11.11 ± 1.28 d | 22.22 ± 1.28 c | 11.11 ± 1.92 d | 22.22 ± 2.95 c |
| Control | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e |
| Common Bean Cultivars | ||||||
|---|---|---|---|---|---|---|
| Isolate Code | Alpha | Samantha | Nebraska | Giza6 | Giza 12 | Cambo |
| FP18 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP33 | 25 ± 1.44 a | 25 ± 2.89 a | 16.67 ± 1.92 b | 25 ± 1.44 a | 16.67 ± 2.89 b | 25 ± 3.32 a |
| FP24 | 20 ± 1.15 b | 20 ± 2.31 b | 14.29 ± 1.65 c | 25 ± 1.44 a | 16.67 ± 2.89 b | 25 ± 3.32 a |
| FP26 | 20 ± 1.15 b | 20 ± 2.31 b | 14.29 ± 1.65 c | 25 ± 1.44 a | 16.67 ± 2.89 b | 25 ± 3.32 a |
| FP1 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP21 | 20 ± 1.15 b | 20 ± 2.31 b | 14.29 ± 1.65 c | 25 ± 1.44 a | 16.67 ± 2.89 b | 25 ± 3.32 a |
| FP2 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP8 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP10 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP11 | 16.67 ± 0.96 b | 16.67 ± 1.92 b | 14.29 ± 1.65 c | 20 ± 1.15 b | 14.29 ± 2.48 c | 20 ± 2.66 b |
| FP17 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP15 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| FP14 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| Control | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| Common Bean Cultivars | ||||||
|---|---|---|---|---|---|---|
| Isolate Code | Alpha | Samantha | Nebraska | Giza6 | Giza 12 | Cambo |
| FP18 | 25 ± 0.72 c | 25 ± 0.43 c | 25 ± 1.37 c | 28.57 ± 2.47 b | 25 ± 2.89 c | 28.57 ± 1.65 b |
| FP33 | 100 ± 0 a | 100 ± 0 a | 33.33 ± 1.83 b | 100 ± 0 a | 33.33 ± 3.85 b | 100 ± 0 a |
| FP24 | 100 ± 0 a | 100 ± 0 a | 28.57 ± 1.57 b | 100 ± 0 a | 33.33 ± 3.85 b | 100 ± 0 a |
| FP26 | 100 ± 0 a | 100 ± 0 a | 28.57 ± 1.57 b | 100 ± 0 a | 33.33 ± 3.85 b | 100 ± 0 a |
| FP1 | 25 ± 0.72 c | 28.57 ± 0.49 b | 25 ± 1.37 c | 28.57 ± 2.47 b | 25 ± 2.89 c | 28.57 ± 1.65 b |
| FP21 | 100 ± 0 a | 100 ± 0 a | 28.57 ± 1.57 b | 100 ± 0 a | 33.33 ± 3.85 b | 100 ± 0 a |
| FP2 | 12.5 ± 0.36 c | 12.5 ± 0.22 c | 12.5 ± 0.69 c | 14.29 ± 1.24 c | 12.5 ± 1.44 c | 14.29 ± 0.83 c |
| FP8 | 12.5 ± 0.36 c | 12.5 ± 0.22 c | 0 ± 0 d | 14.29 ± 1.24 c | 0 ± 0 d | 14.29 ± 0.83 c |
| FP10 | 12.5 ± 0.36 c | 12.5 ± 0.22 c | 12.5 ± 0.69 c | 14.29 ± 1.24 c | 12.5 ± 1.44 c | 14.29 ± 0.83 c |
| FP11 | 100 ± 0 a | 100 ± 0 a | 25 ± 1.37 c | 100 ± 0 a | 25 ± 2.89 c | 100 ± 0 a |
| FP17 | 28.57 ± 0.82 b | 28.57 ± 0.49 b | 25 ± 1.37 c | 33.33 ± 2.89 b | 25 ± 2.89 c | 33.33 ± 1.92 b |
| FP15 | 25 ± 0.72 c | 28.57 ± 0.49 b | 25 ± 1.37 c | 28.57 ± 2.47 b | 25 ± 2.89 c | 28.57 ± 1.65 b |
| FP14 | 12.5 ± 0.36 c | 12.5 ± 0.22 c | 0 ± 0 d | 14.29 ± 1.24 c | 12.5 ± 1.44 c | 14.29 ± 0.83 c |
| Control | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| Common Bean Cultivars | ||||||
|---|---|---|---|---|---|---|
| Isolate Code | Alpha | Samantha | Nebraska | Giza6 | Giza 12 | Cambo |
| FP18 | 25 ± 2.89 c | 25 ± 1.15 c | 20 ± 1.27 d | 25.71 ± 3.27 c | 22.5 ± 1.69 d | 25.71 ± 1.48 c |
| FP33 | 65 ± 7.51 a | 70 ± 3.23 a | 30 ± 1.91 c | 90 ± 11.43 a | 33.33 ± 2.5 c | 80 ± 4.62 a |
| FP24 | 64 ± 7.39 a | 68 ± 3.14 a | 28.57 ± 1.81 c | 85 ± 10.8 a | 33.33 ± 2.5 c | 75 ± 4.33 a |
| FP26 | 60 ± 6.93 b | 64 ± 2.96 a | 25.71 ± 1.63 c | 80 ± 10.16 a | 25.71 ± 1.93 c | 70 ± 4.04 a |
| FP1 | 25 ± 2.89 c | 28.57 ± 1.32 c | 22.5 ± 1.43 d | 28.57 ± 3.63 c | 25 ± 1.88 c | 25.71 ± 1.48 c |
| FP21 | 56 ± 6.47 b | 60 ± 2.77 b | 22.86 ± 1.45 d | 75 ± 9.53 a | 25.71 ± 1.93 c | 65 ± 3.75 a |
| FP2 | 10 ± 1.15 d | 12.5 ± 0.58 d | 7.5 ± 0.48 e | 14.29 ± 1.82 d | 10 ± 0.75 d | 11.43 ± 0.66 d |
| FP8 | 7.5 ± 0.87 e | 10 ± 0.46 d | 0 ± 0 e | 11.43 ± 1.45 d | 0 ± 0 e | 8.57 ± 0.49 c |
| FP10 | 12.5 ± 1.44 d | 12.5 ± 0.58 d | 10 ± 0.64 d | 14.29 ± 1.82 d | 10 ± 0.75 d | 14.29 ± 0.83 d |
| FP11 | 51.43 ± 5.94 b | 60 ± 2.77 b | 22.5 ± 1.43 d | 70 ± 8.89 a | 25 ± 1.88 c | 63.33 ± 3.66 a |
| FP17 | 28.57 ± 3.3 c | 28.57 ± 1.32 c | 22.5 ± 1.43 d | 30 ± 3.81 c | 25 ± 1.88 c | 30 ± 1.73 c |
| FP15 | 25 ± 2.89 c | 28.57 ± 1.32 c | 22.5 ± 1.43 d | 28.57 ± 3.63 c | 25 ± 1.88 c | 28.57 ± 1.65 c |
| FP14 | 10 ± 1.15 d | 10 ± 0.46 d | 0 ± 0 e | 11.43 ± 1.45 d | 7.5 ± 0.56 e | 11.43 ± 0.66 d |
| Control | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e | 0 ± 0 e |
| Isolate Code | Fusarium Species | Governorate | Closest GenBank Match | Identity (%) | Query Coverage (%) | E-Value | GenBank Accession Number |
|---|---|---|---|---|---|---|---|
| FP33 | Fusarium equiseti | Kafr-elsheikh | MK937120 | 96.45 | 89% | 0.0 | PV595127 |
| FP24 | Fusarium equiseti | Beni-Suef | MW362076 | 97.13 | 94% | 0.0 | PV595128 |
| FP26 | Fusarium oxysporum | Kafr-elsheikh | KX165288 | 98.79 | 93% | 0.0 | PV595129 |
| FP21 | Fusarium oxysporum | Beni-Suef | MT 630364 | 99.69 | 93% | 0.0 | PV595130 |
| FP11 | Fusarium solani | Qalyubia | OQ511051 | 99.42 | 91% | 0.0 | PV595131 |
| Species from Isolate | Origin Plant | Isolation Source | References | Country | GenBank Accession Number |
|---|---|---|---|---|---|
| Fusarium oxysporum | pea | Root | [39] | United Kingdom | MT 630361 |
| Fusarium oxysporum | pea | Root | [39] | United Kingdom | MT 630369 |
| Fusarium oxysporum | pea | Root | [39] | United Kingdom | MT 630370 |
| Fusarium oxysporum | pea | Root | [39] | United Kingdom | MT 630364 |
| Fusarium oxysporum | soybean | Stem | [40] | Croatia | KX165288 |
| Fusarium oxysporum | chickpea | Root | [41] | Gallatin, Montana | MK816979 |
| Fusarium oxysporum | pea | Root | [41] | Daniels, Montana USA | MK816995 |
| Fusarium oxysporum | pea | Root | [41] | Sheridan, USA | MK816996 |
| Fusarium oxysporum | pea | Root | [41] | Daniels, Montana USA | MK817007 |
| Fusarium oxysporum | soybean | Seed | [42] | Poland | OP985466 |
| Fusarium oxysporum | pea | Root | [41] | Daniels, Montana USA | MK836075 |
| Fusarium phaseoli | Common bean | Root | [43] | USA | AY220186 |
| Fusarium phaseoli | Common bean | Root | [43] | USA | AY220187 |
| Fusarium solani | chickpea | Root | [43] | Gallatin, Montana USA | MN585099 |
| Fusarium solani | soybean | Root | [44] | Pennsylvania USA | OQ511117 |
| Fusarium solani | soybean | Root | [44] | Pennsylvania USA | OQ511022 |
| Fusarium solani | soybean | Root | [44] | Pennsylvania, USA | OQ511051 |
| Fusarium solani | chickpea | Root | [45] | Montana, USA | MW366807 |
| Fusarium equiseti | pea | seed | [41] | USA | MK937120 |
| Fusarium equiseti | chickpea | Root | [45] | USA | MW362071 |
| Fusarium equiseti | chickpea | Root | [45] | USA | MW362074 |
| Fusarium equiseti | chickpea | Root | [45] | USA | MW362076 |
| Fusarium equiseti | chickpea | Root | [45] | USA | MW362069 |
| Fusarium equiseti | Common bean | Root | [46] | Khomein, Iran | MW551801 |
| Fusarium equiseti | Common bean | Root | [46] | Khomein, Iran | MW551800 |
| Fusarium equiseti | Common bean | Root | [47] | Selseleh | OQ200421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, T.A.; Yasser, M.M.; Taha, N.A.; Alkhalifah, D.H.M.; Marzouk, M.A.; Hozzien, W.N.; Abdelghany, W.R. Integrating Morphological, Pathogenic, and Molecular Approaches to Characterize Fusarium Root Rot Pathogens of Common Bean in Egypt. Curr. Issues Mol. Biol. 2025, 47, 803. https://doi.org/10.3390/cimb47100803
Kamel TA, Yasser MM, Taha NA, Alkhalifah DHM, Marzouk MA, Hozzien WN, Abdelghany WR. Integrating Morphological, Pathogenic, and Molecular Approaches to Characterize Fusarium Root Rot Pathogens of Common Bean in Egypt. Current Issues in Molecular Biology. 2025; 47(10):803. https://doi.org/10.3390/cimb47100803
Chicago/Turabian StyleKamel, Taghrid A., Manal M. Yasser, Naglaa A. Taha, Dalal Hussien M. Alkhalifah, Marym A. Marzouk, Wael N. Hozzien, and Walaa R. Abdelghany. 2025. "Integrating Morphological, Pathogenic, and Molecular Approaches to Characterize Fusarium Root Rot Pathogens of Common Bean in Egypt" Current Issues in Molecular Biology 47, no. 10: 803. https://doi.org/10.3390/cimb47100803
APA StyleKamel, T. A., Yasser, M. M., Taha, N. A., Alkhalifah, D. H. M., Marzouk, M. A., Hozzien, W. N., & Abdelghany, W. R. (2025). Integrating Morphological, Pathogenic, and Molecular Approaches to Characterize Fusarium Root Rot Pathogens of Common Bean in Egypt. Current Issues in Molecular Biology, 47(10), 803. https://doi.org/10.3390/cimb47100803








