Phage Display Reveals VLRB-Mediated Recognition of Minimal Tumor Glycan Antigen Sialyl-Tn
Abstract
1. Introduction
2. Materials and Methods
2.1. Phage Display Library Screening for STn-Binding Ccombodies
2.1.1. Phage-Displayed Ccombody Library Construction
2.1.2. Antigen Coating and Blocking
2.1.3. Bead-Based Biopanning
2.1.4. Colony Isolation and Propagation
2.1.5. Phage ELISA Screening
2.1.6. Cloning and Expression of STn-Specific Ccombodies
2.1.7. Purification of Recombinant STn-Binding Ccombodies
2.2. Characterization of STn-Binding Ccombodies
2.2.1. Assessment of Ccombody Specificity via ELISA
2.2.2. EC50 Determination of Ccombody-sTn Binding
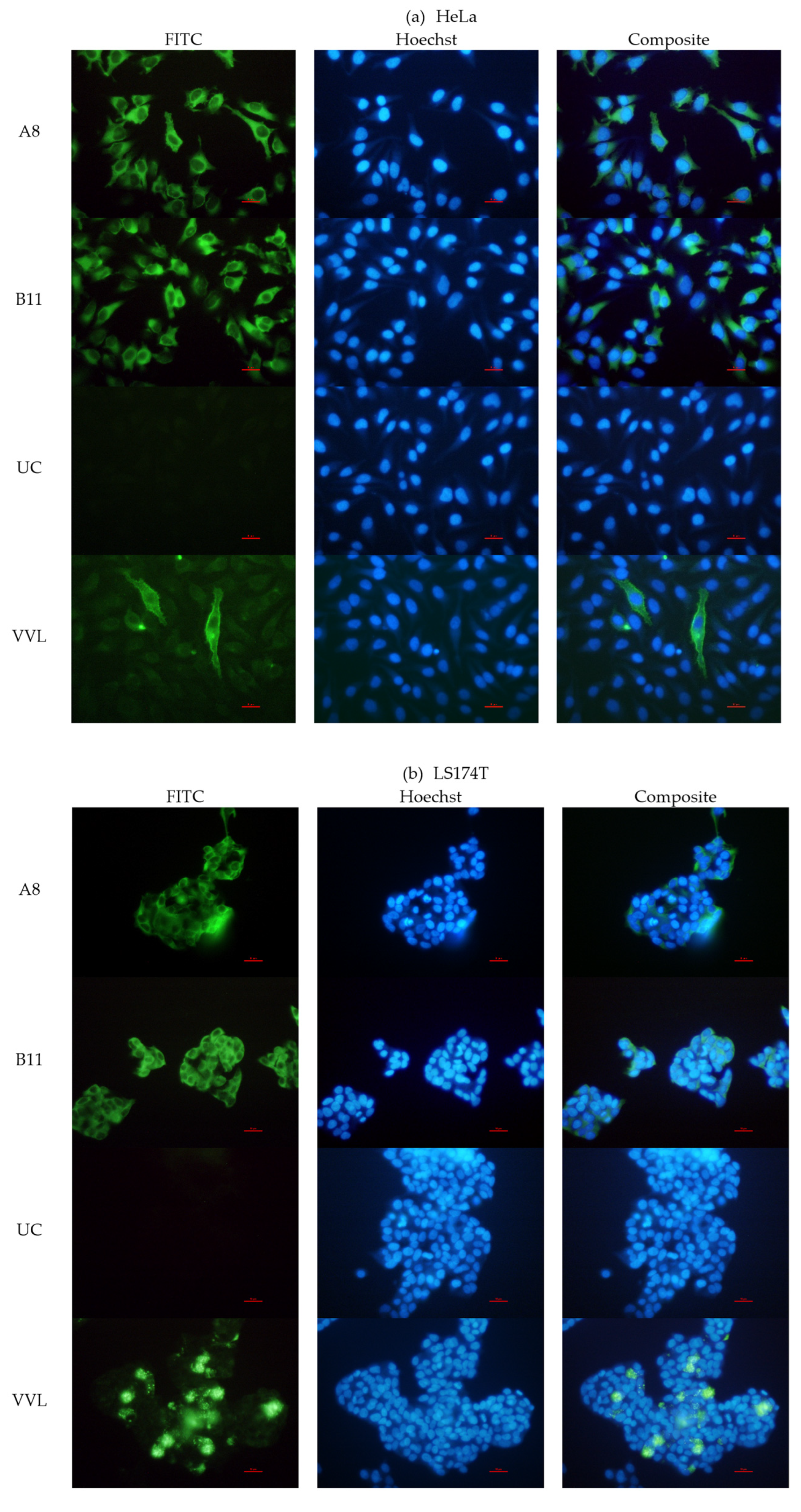
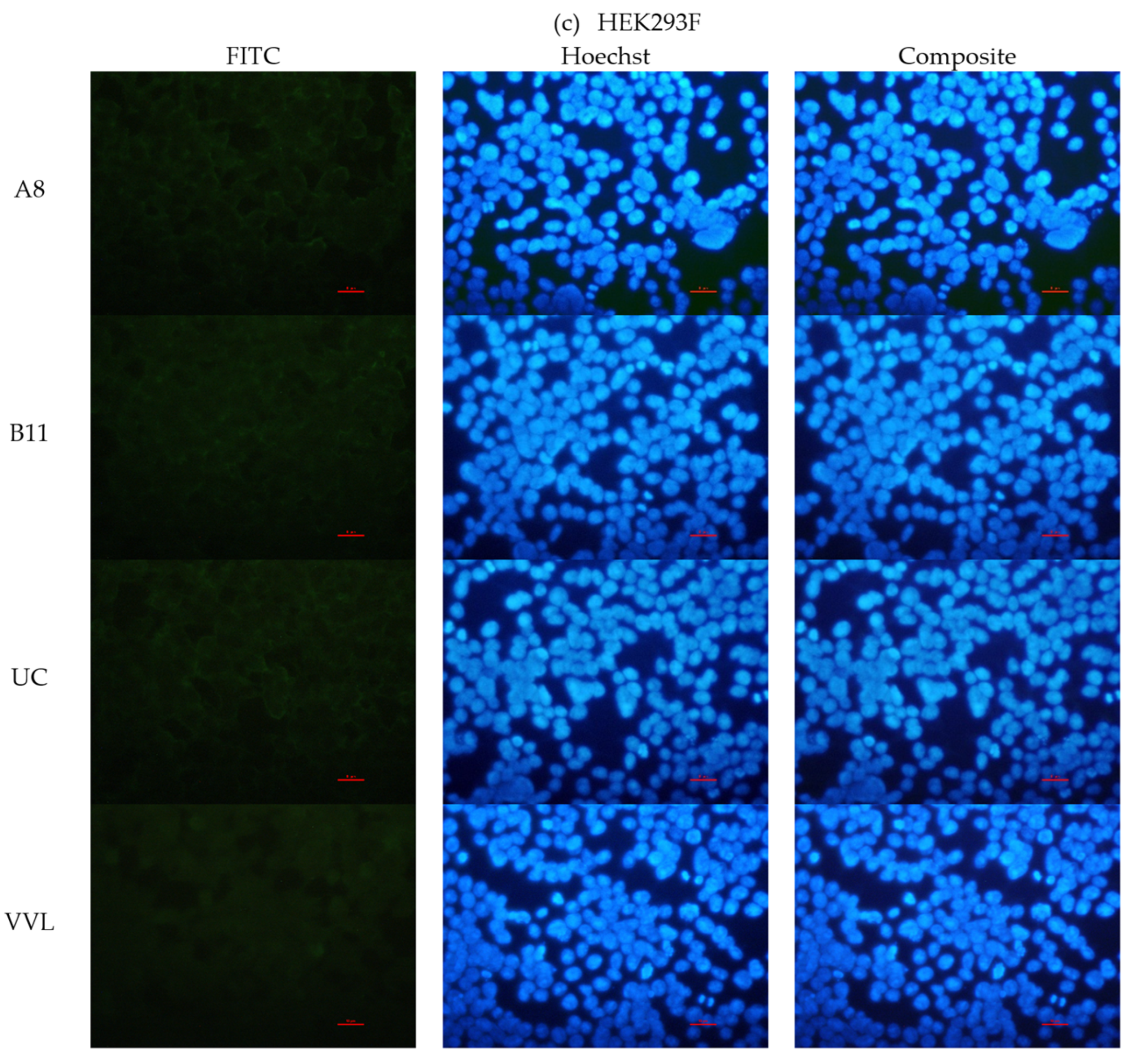
2.2.3. Immunofluorescence
2.2.4. Lectin Competition Assay
3. Results
3.1. Phage Display Library Screening
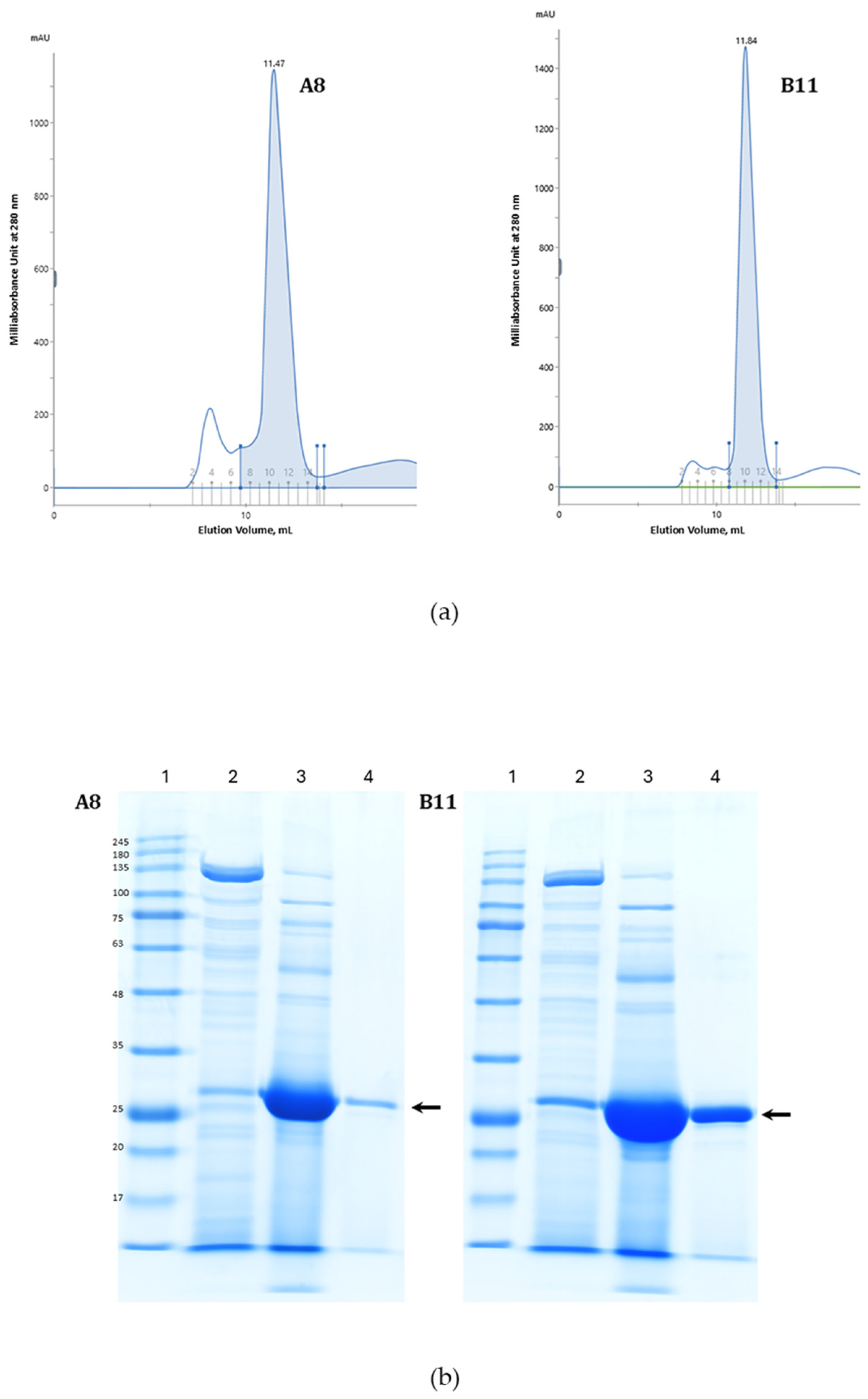
3.2. Ccombody Purification
3.3. Ccombody Characterization
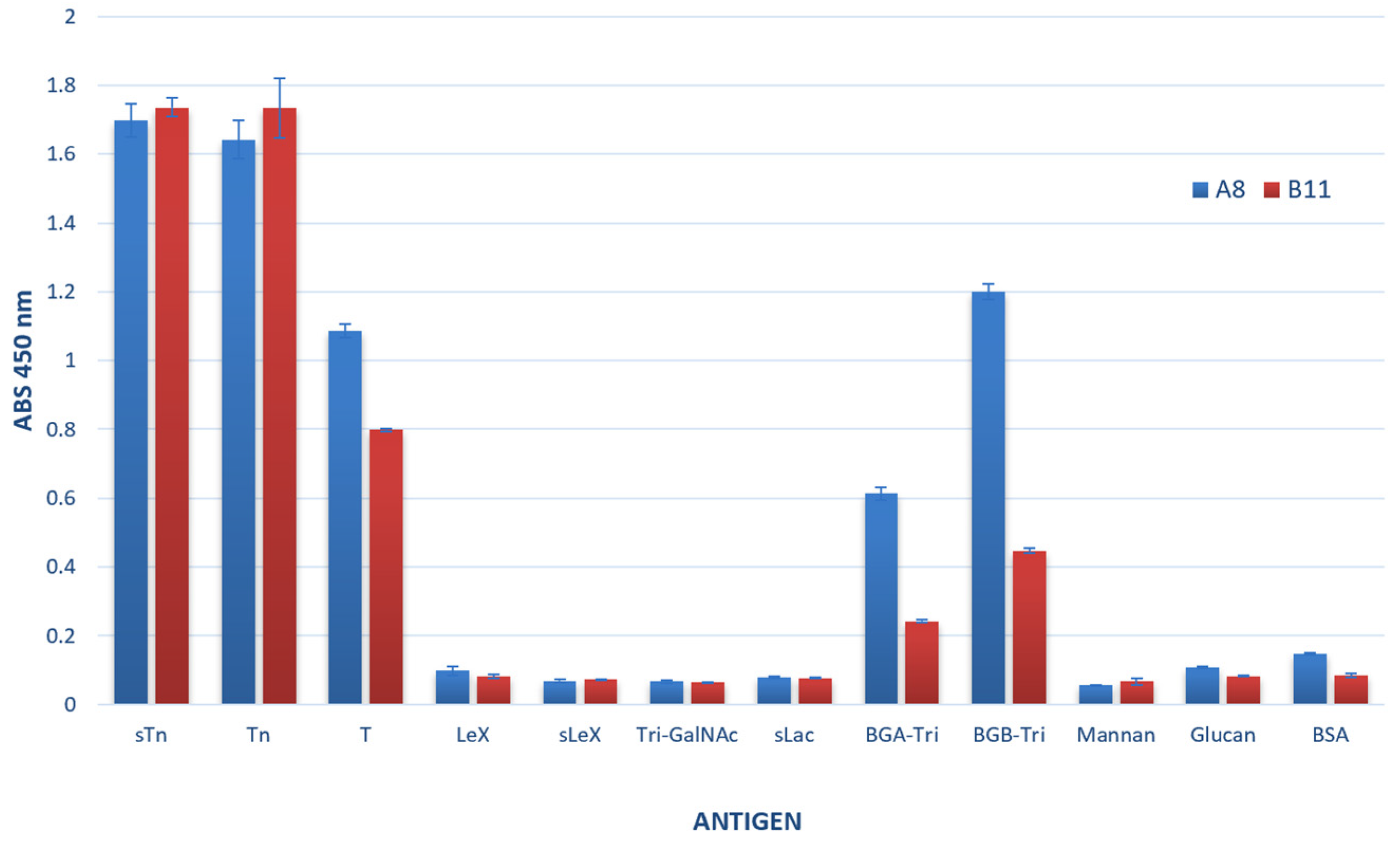
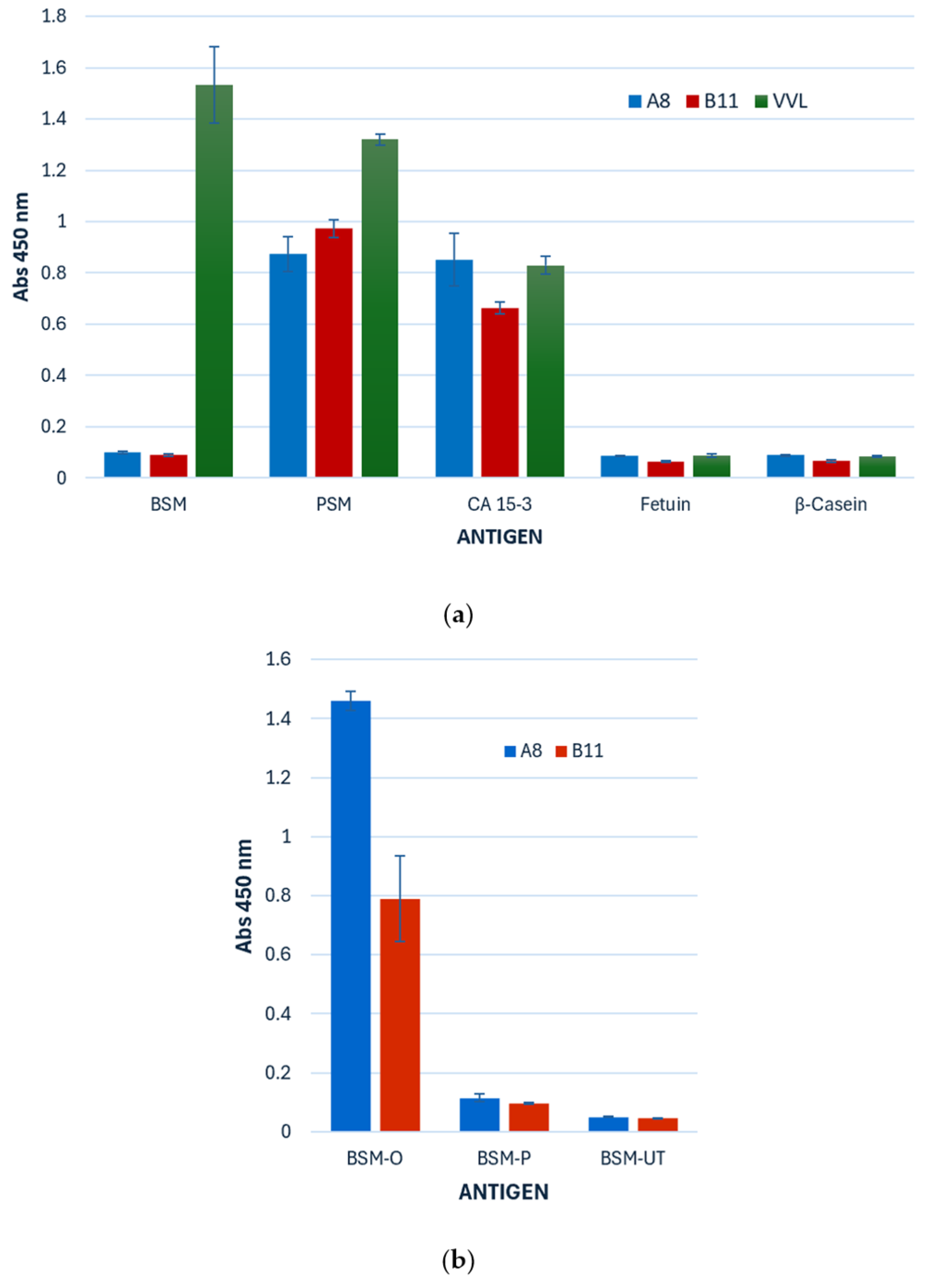
3.3.1. Ccombody Specificity via ELISA
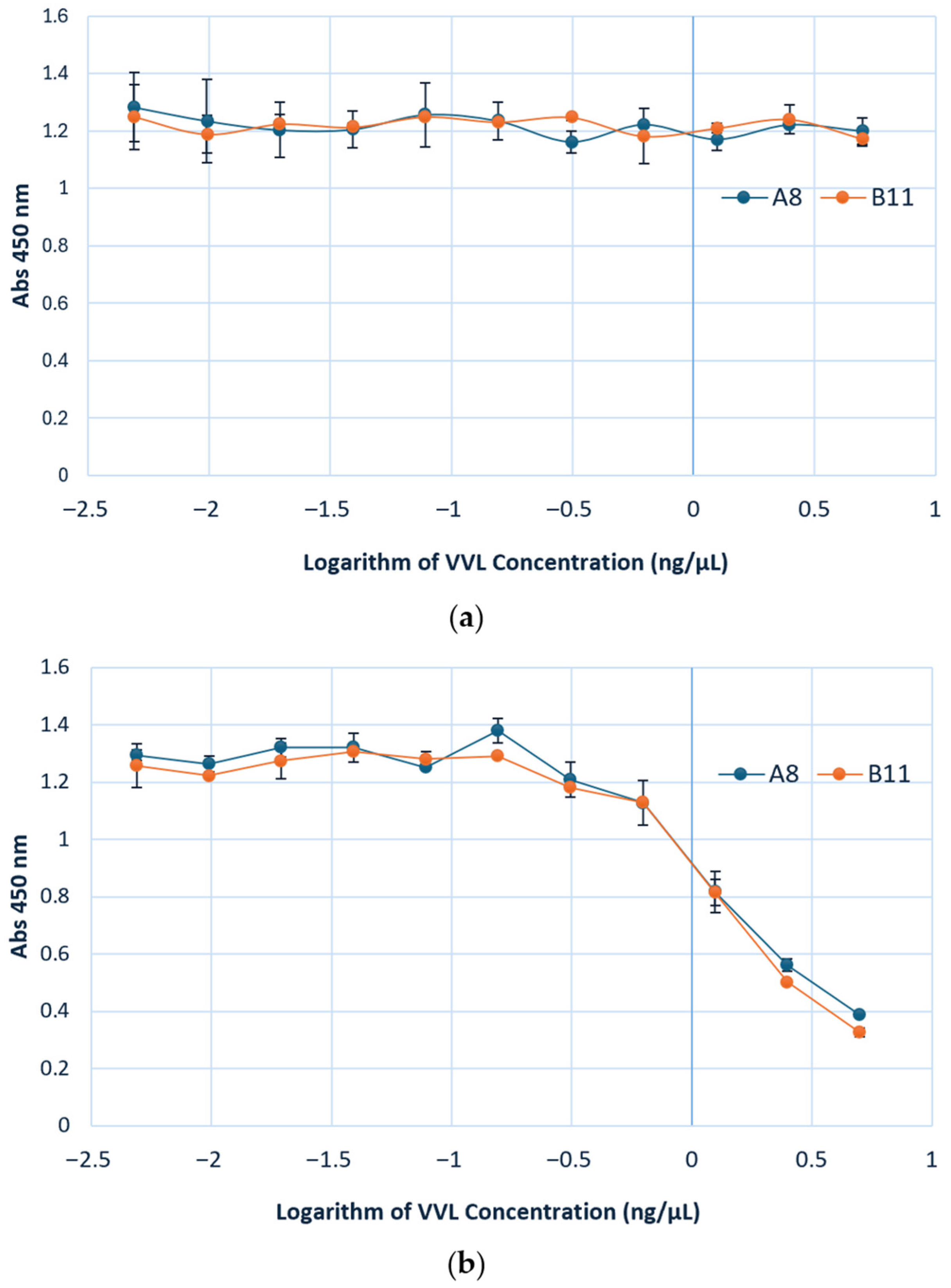
3.3.2. VVL and Ccombody Competition Assay
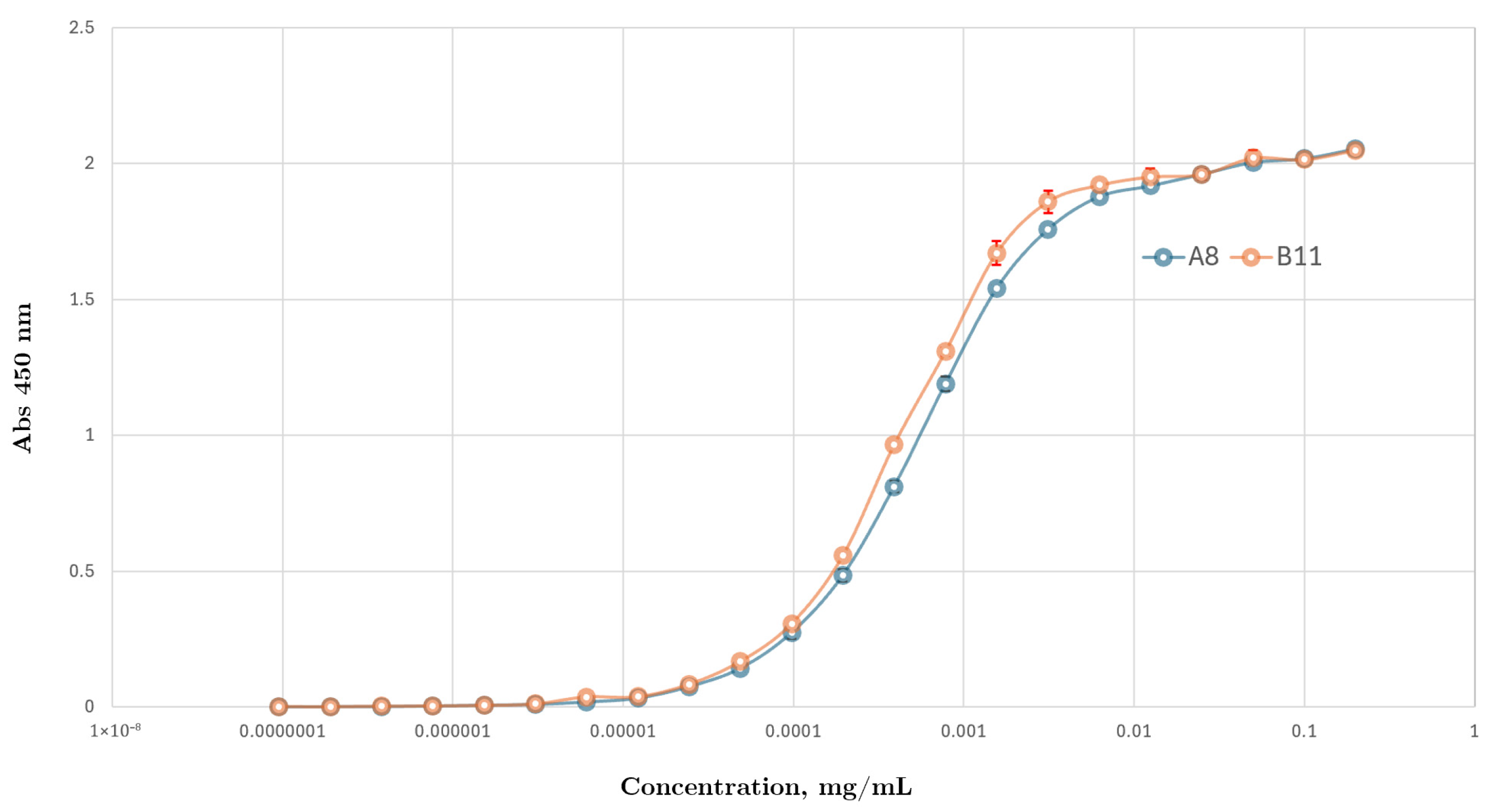
3.3.3. Ccombody Relative Affinity Expressed as EC50
3.3.4. Binding with STn Expressed on Cells and Glycoproteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| BSM | Bovine submaxillary mucin |
| COSMC | core1-specific-molecular-chaperone |
| ELISA | Enzyme-linked immunosorbent assay |
| FITC | Fluorescein isothiocyanate |
| FPLC | Fast protein liquid chromatography |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| LRR | Leucine-rich repeats |
| PCR | Polymerase chain reaction |
| PSM | Porcine stomach mucin |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TACA | Tumor-associated carbohydrate antigen |
| TLR | Toll-like receptor |
| VLR | Variable lymphocyte receptor |
| VVL | Vicia villosa lectin |
References
- Diniz, F.; Coelho, P.; Duarte, H.O.; Sarmento, B.; Reis, C.A.; Gomes, J. Glycans as Targets for Drug Delivery in Cancer. Cancers 2022, 14, 911. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. Aberrant Glycosylation in Cancer: A Novel Molecular Mechanism Controlling Metastasis. Cancer Cell 2017, 31, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Mantuano, N.; Natoli, M.; Zippelius, A.; Läubli, H. Tumor-Associated Carbohydrates and Immunomodulatory Lectins as Targets for Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, e001222. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-Associated Carbohydrate Antigens (TACAs)-Based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-Tn in Cancer: (How) Did We Miss the Target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef]
- Xu, F.; Fan, C.; Fan, S.; Liu, F.; Wen, T.; An, G.; Feng, G. Expression Profile of Mucin-Associated Sialyl-Tn Antigen in Chinese Patients with Different Colorectal Lesions (Adenomas, Carcinomas). Int. J. Clin. Exp. Pathol. 2015, 8, 11549–11554. [Google Scholar]
- Rajesh, C.; Radhakrishnan, P. The (Sialyl) Tn Antigen: Contributions to Immunosuppression in Gastrointestinal Cancers. Front. Oncol. 2023, 12, 1093496. [Google Scholar] [CrossRef]
- Al-Alem, L.; Prendergast, J.M.; Clark, J.; Zarrella, B.; Zarrella, D.T.; Hill, S.J.; Growdon, W.B.; Pooladanda, V.; Spriggs, D.R.; Cramer, D.; et al. Sialyl-Tn Serves as a Potential Therapeutic Target for Ovarian Cancer. J. Ovarian Res. 2024, 17, 71. [Google Scholar] [CrossRef]
- Eavarone, D.A.; Al-Alem, L.; Lugovskoy, A.; Prendergast, J.M.; Nazer, R.I.; Stein, J.N.; Dransfield, D.T.; Behrens, J.; Rueda, B.R. Humanized Anti-Sialyl-Tn Antibodies for the Treatment of Ovarian Carcinoma. PLoS ONE 2018, 13, e0201314. [Google Scholar] [CrossRef]
- Prendergast, J.M.; Paula, A.; Eavarone, D.A.; Ghaderi, D.; Zhang, M.; Brady, D.; Wicks, J.; DeSander, J.; Behrens, J.; Rueda, B.R. Novel Anti-Sialyl-Tn Monoclonal Antibodies and Antibody-Drug Conjugates Demonstrate Tumor Specificity and Anti-Tumor Activity. MAbs 2017, 9, 615–627. [Google Scholar] [CrossRef]
- Soares, C.O.; Laugieri, M.E.; Grosso, A.S.; Natale, M.; Coelho, H.; Behren, S.; Yu, J.; Cai, H.; Franconetti, A.; Oyenarte, I.; et al. Decoding the Molecular Basis of the Specificity of an Anti-STn Antibody. JACS Au 2024, 5, 225–236. [Google Scholar] [CrossRef]
- Loureiro, L.; Carrascal, M.; Barbas, A.; Ramalho, J.; Novo, C.; Delannoy, P.; Videira, P. Challenges in Antibody Development against Tn and Sialyl-Tn Antigens. Biomolecules 2015, 5, 1783–1809. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, Z. Improving the Antigenicity of STn Antigen by Modification of Its Sialic Acid Residue for Development of Glycoconjugate Cancer Vaccines. Bioconjugate Chem. 2006, 17, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.G.; Ehrhardt, G.R.A.; Grunebaum, E. Variable Lymphocyte Receptor B Technologies—Are They Ready for Prime Time? Immunol. Investig. 2025, 54, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Han, B.W.; Herrin, B.R.; Cooper, M.D.; Wilson, I.A. Antigen Recognition by Variable Lymphocyte Receptors. Science 2008, 321, 1834–1837. [Google Scholar] [CrossRef]
- Velikovsky, C.A.; Deng, L.; Tasumi, S.; Iyer, L.M.; Kerzic, M.C.; Aravind, L.; Pancer, Z.; Mariuzza, R.A. Structure of a Lamprey Variable Lymphocyte Receptor in Complex with a Protein Antigen. Nat. Struct. Mol. Biol. 2009, 16, 725–730. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Herrin, B.; Han, B.; Turnbough, C.; Cooper, M.; Wilson, I. Variable Lymphocyte Receptor Recognition of the Immunodominant Glycoprotein of Bacillus Anthracis Spores. Structure 2012, 20, 479–486. [Google Scholar] [CrossRef]
- Nakahara, H.; Herrin, B.R.; Alder, M.N.; Catera, R.; Yan, X.-J.; Chiorazzi, N.; Cooper, M.D. Chronic Lymphocytic Leukemia Monitoring with a Lamprey Idiotope-Specific Antibody. Cancer Immunol. Res. 2013, 1, 223–228. [Google Scholar] [CrossRef]
- Bommakanti, G. Lamprey Variable Lymphocyte Receptor Monoclonal Antibodies for Whole-Cell Surface Antigens. Methods Mol. Biol. 2021, 2421, 115–125. [Google Scholar] [CrossRef]
- Umlauf, B.J.; Clark, P.A.; Lajoie, J.M.; Georgieva, J.V.; Bremner, S.; Herrin, B.R.; Kuo, J.S.; Shusta, E.V. Identification of Variable Lymphocyte Receptors That Can Target Therapeutics to Pathologically Exposed Brain Extracellular Matrix. Sci. Adv. 2019, 5, eaau4245. [Google Scholar] [CrossRef]
- Bela-ong, D.B.; Kim, J.; Thompson, K.D.; Jung, T.S. Leveraging the Biotechnological Promise of the Hagfish Variable Lymphocyte Receptors: Tools for Aquatic Microbial Diseases. Fish Shellfish Immunol. 2024, 150, 109565. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Nomura, K.; Cooper, M.D.; Herrin, B.R.; He, S.Y. Leucine-Rich-Repeat-Containing Variable Lymphocyte Receptors as Modules to Target Plant-Expressed Proteins. Plant Methods 2017, 13, 29. [Google Scholar] [CrossRef]
- Boehm, T.; Cooper, M.D.; Hirano, M.; Das, S.; Morimoto, R.; Rast, J.P. The Variable Lymphocyte Receptor B System of the Jawless Vertebrates. In Molecular Biology of B Cells; Academic Press: Cambridge, MA, USA, 2024; pp. 77–91. [Google Scholar]
- Sirimanapong, W.; Thaijongrak, P.; Sudpraseart, C.; Bela-ong, D.B.; Rodelas-Angelia, A.J.D.; Angelia, M.R.N.; Hong, S.; Kim, J.; Thompson, K.D.; Jung, T.S. Passive Immunoprophylaxis with Ccombodies against Vibrio Parahaemolyticus in Pacific White Shrimp (Penaeus Vannamei). Fish Shellfish Immunol. 2024, 154, 109973. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ho, M. Isolation of Antibodies to Heparan Sulfate on Glypicans by Phage Display. Curr. Protoc. Protein Sci. 2018, 94, e66. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, Inc. Quest Graph™ Four Parameter Logistic (4PL) Curve Calculator; AAT Bioquest: Pleasanton, CA, USA, 2024. [Google Scholar]
- McConnell, S.J.; Dinh, T.; Le, M.-H.; Spinella, D.G. Biopanning Phage Display Libraries Using Magnetic Beads vs. Polystyrene Plates. BioTechniques 1999, 26, 208–214. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Benbrook, D.M.; Deng, W.; Gold, M.A.; Rai, R.; Conrad, R.; van der Wel, H.; Husain, S.; Moore, K.; Spirtos, N.; Jackson, A.L.; et al. Association of Sialyl Tn Antigen with Cervical Cancer Lymph Node Status: An NRG Oncology/GOG Study. Gynecol. Oncol. 2023, 171, 67–75. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human Tumor Antigens Tn and Sialyl Tn Arise from Mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef]
- Masters, J.R. HeLa Cells 50 Years On: The Good, the Bad and the Ugly. Nat. Rev. Cancer 2002, 2, 315–319. [Google Scholar] [CrossRef]
- Bu, X.-D.; Li, N.; Tian, X.-Q.; Huang, P.-L. Caco-2 and LS174T Cell Lines Provide Different Models for Studying Mucin Expression in Colon Cancer. Tissue Cell 2011, 43, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Smart, T.G. HEK293 Cell Line: A Vehicle for the Expression of Recombinant Proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Mateu Ferrando, R.; Lay, L.; Polito, L. Gold Nanoparticle-Based Platforms for Vaccine Development. Drug Discov. Today: Technol. 2021, 38, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Khilji, S.K.; Goerdeler, F.; Frensemeier, K.; Warschkau, D.; Lühle, J.; Fandi, Z.; Schirmeister, F.; Chen, Z.A.; Turak, O.; Mallagaray, A.; et al. Generation of Glycan-Specific Nanobodies. Cell Chem. Biol. 2022, 29, 1353–1361.e6. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Redman, R.L.; Horiya, S.; Bailey, J.K.; Xu, B.; Stanfield, R.L.; Temme, J.S.; LaBranche, C.C.; Wang, S.; Rodal, A.A.; et al. The Impact of Sustained Immunization Regimens on the Antibody Response to Oligomannose Glycans. ACS Chem. Biol. 2020, 15, 789–798. [Google Scholar] [CrossRef]
- Polonskaya, Z.; Savage, P.B.; Finn, M.G.; Teyton, L. High-Affinity Anti-Glycan Antibodies: Challenges and Strategies. Curr. Opin. Immunol. 2019, 59, 65–71. [Google Scholar] [CrossRef]
- Ward, E.M.; Kizer, M.E.; Imperiali, B. Strategies and Tactics for the Development of Selective Glycan-Binding Proteins. ACS Chem. Biol. 2021, 16, 1795–1813. [Google Scholar] [CrossRef]
- Rogals, M.J.; Eletsky, A.; Huang, C.; Morris, L.C.; Moremen, K.W.; Prestegard, J.H. Glycan Conformation in the Heavily Glycosylated Protein, CEACAM1. ACS Chem. Biol. 2022, 17, 3527–3534. [Google Scholar] [CrossRef]
- Akhtar, A.; Lata, M.; Sunsunwal, S.; Yadav, A.; Lnu, K.; Subramanian, S.; Ramya, T.N.C. New Carbohydrate Binding Domains Identified by Phage Display Based Functional Metagenomic Screens of Human Gut Microbiota. Commun. Biol. 2023, 6, 371. [Google Scholar] [CrossRef]
- Cunningham, S.; Starr, E.; Shaw, I.; Glavin, J.; Kane, M.; Joshi, L. Development of a Convenient Competitive ELISA for the Detection of the Free and Protein-Bound Nonhuman Galactosyl-α-(1,3)-Galactose Epitope Based on Highly Specific Chicken Single-Chain Antibody Variable-Region Fragments. Anal. Chem. 2012, 85, 949–955. [Google Scholar] [CrossRef]
- Fukuda, M.N. Peptide-Displaying Phage Technology in Glycobiology. Glycobiology 2011, 22, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, R.; Kwan, D.H. Resources and Methods for Engineering “Designer” Glycan-Binding Proteins. Molecules 2021, 26, 380. [Google Scholar] [CrossRef] [PubMed]
- Diehl, R.C.; Chorghade, R.S.; Keys, A.M.; Alam, M.M.; Early, S.A.; Dugan, A.E.; Miri, K.; Ribbeck, K.; Kulik, H.J.; Kiessling, L.L. CH−π Interactions Are Required for Human Galectin-3 Function. JACS Au 2024, 4, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.O.; Grosso, A.S.; Ereño-Orbea, J.; Coelho, H.; Marcelo, F. Molecular Recognition Insights of Sialic Acid Glycans by Distinct Receptors Unveiled by NMR and Molecular Modeling. Front. Mol. Biosci. 2021, 8, 727847. [Google Scholar] [CrossRef]
- Kiessling, L.L.; Diehl, R.C. CH−π Interactions in Glycan Recognition. ACS Chem. Biol. 2021, 16, 1884–1893. [Google Scholar] [CrossRef]
- Sanz-Martinez, I.; Pereira, S.; Merino, P.; Corzana, F.; Hurtado-Guerrero, R. Molecular Recognition of GalNAc in Mucin-Type O-Glycosylation. Acc. Chem. Res. 2023, 56, 548–560. [Google Scholar] [CrossRef]
- Luo, M.; Velikovsky, C.A.; Yang, X.; Siddiqui, M.A.; Hong, X.; Barchi, J.J.; Gildersleeve, J.C.; Pancer, Z.; Mariuzza, R.A. Recognition of the Thomsen-Friedenreich Pancarcinoma Carbohydrate Antigen by a Lamprey Variable Lymphocyte Receptor. J. Biol. Chem. 2013, 288, 23597–23606. [Google Scholar] [CrossRef]
- Tasumi, S.; Velikovsky, C.A.; Xu, G.; Gai, S.A.; Wittrup, K.D.; Flajnik, M.F.; Mariuzza, R.A.; Pancer, Z. High-Affinity Lamprey VLRA and VLRB Monoclonal Antibodies. Proc. Natl. Acad. Sci. 2009, 106, 12891–12896. [Google Scholar] [CrossRef]
- Amon, R.; Grant, O.C.; Leviatan Ben-Arye, S.; Makeneni, S.; Nivedha, A.K.; Marshanski, T.; Norn, C.; Yu, H.; Glushka, J.N.; Fleishman, S.J.; et al. A Combined Computational-Experimental Approach to Define the Structural Origin of Antibody Recognition of Sialyl-Tn, a Tumor-Associated Carbohydrate Antigen. Sci. Rep. 2018, 8, 10786. [Google Scholar] [CrossRef]
- Pinczower, G.D.; Williams, R.P.W.; Gianello, R.D.; Robinson, H.C.; Preston, B.N.; Linnane, A.W. Characterisation of the Tumour-Associated Carbohydrate Epitope Recognised by Monoclonal Antibody 4D3. Int. J. Cancer 1996, 66, 636–644. [Google Scholar] [CrossRef]
- Zhang, S.; Walberg, L.A.; Ogata, S.; Itzkowitz, S.H.; Koganty, R.R.; Reddish, M.; Gandhi, S.S.; Longenecker, B.M.; Lloyd, K.O.; Livingston, P.O. Immune Sera and Monoclonal Antibodies Define Two Configurations for the Sialyl Tn Tumor Antigen. Cancer Res. 1995, 55, 3364–3368. [Google Scholar]
- Reddish, M.A.; Jackson, L.; Rao Koganty, R.; Qiu, D.; Hong, W.; Longenecker, B.M. Specificities of Anti-Sialyl-Tn and Anti-Tn Monoclonal Antibodies Generated Using Novel Clustered Synthetic Glycopeptide Epitopes. Glycoconj. J. 1997, 14, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, D.; Martínez-Sáez, N.; Somovilla, V.J.; Coelho, H.; Valero-González, J.; Castro-López, J.; Asensio, J.L.; Jiménez-Barbero, J.; Busto, J.H.; Avenoza, A.; et al. Detection of Tumor-Associated Glycopeptides by Lectins: The Peptide Context Modulates Carbohydrate Recognition. ACS Chem. Biol. 2014, 10, 747–756. [Google Scholar] [CrossRef] [PubMed]
- McKitrick, T.R.; Goth, C.K.; Rosenberg, C.S.; Nakahara, H.; Heimburg-Molinaro, J.; McQuillan, A.M.; Falco, R.; Rivers, N.J.; Herrin, B.R.; Cooper, M.D.; et al. Development of Smart Anti-Glycan Reagents Using Immunized Lampreys. Commun. Biol. 2020, 3, 91. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, C.; Ha, J.; Lee, J.; Kim, D.; Ji, M.; Park, C.; Lee, J.; Kim, D.; Kim, H. Structural and Quantitative Characterization of Mucin-Type O-Glycans and the Identification of O-Glycosylation Sites in Bovine Submaxillary Mucin. Biomolecules 2020, 10, 636. [Google Scholar] [CrossRef]
- Angelia, M.R.N.; Rodelas-Angelia, A.J.D.; Yang, C.; Park, S.; Jeong, S.; Jang, H.; Bela-ong, D.B.; Jang, H.; Thompson, K.D.; Jung, T. Screening and Characterization of Sialic Acid-Binding Variable Lymphocyte Receptors from Hagfish. BioTech 2024, 13, 46. [Google Scholar] [CrossRef]
- Collins, B.C.; Gunn, R.J.; McKitrick, T.R.; Cummings, R.D.; Cooper, M.D.; Herrin, B.R.; Wilson, I.A. Structural Insights into VLR Fine Specificity for Blood Group Carbohydrates. Structure 2017, 25, 1667–1678. [Google Scholar] [CrossRef]
- McKitrick, T.R.; Hanes, M.S.; Rosenberg, C.S.; Heimburg-Molinaro, J.; Cooper, M.D.; Herrin, B.R.; Cummings, R.D. Identification of Glycan-Specific Variable Lymphocyte Receptors Using Yeast Surface Display and Glycan Microarrays. Methods Mol. Biol. 2022, 2421, 73–89. [Google Scholar] [CrossRef]
- Tulin, E.K.C.; Muerner, L.; McKitrick, T.R.; Tilton, C.A.; Pepi, L.E.; Jia, T.; Noel, M.; Zheng, L.Y.; Stowell, S.R.; von Gunten, S.; et al. Blood Group O Expression in Normal Tissues and Tumors. FEBS J. 2025. [Google Scholar] [CrossRef]
- Xu, G.; Tasumi, S.; Pancer, Z. Yeast Surface Display of Lamprey Variable Lymphocyte Receptors. Methods Mol. Biol. 2011, 21–33. [Google Scholar] [CrossRef]
- Hong, X.-R.; Mark, Z.; Gildersleeve, J.C.; Chowdhury, S.; Barchi, J.J.; Mariuzza, R.A.; Murphy, M.; Mao, L.; Pancer, Z. Sugar-Binding Proteins from Fish: Selection of High Affinity “Lambodies” That Recognize Biomedically Relevant Glycans. ACS Chem. Biol. 2012, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.A.; Shusta, E.V. The Variable Lymphocyte Receptor as an Antibody Alternative. Curr. Opin. Biotechnol. 2018, 52, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Ponnusamy, M.P.; Lakshmanan, I.; Jain, M.; Batra, S.K. Mucins in the Pathogenesis of Breast Cancer: Implications in Diagnosis, Prognosis and Therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2011, 1815, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-Associated Antigens: Tn Antigen, STn Antigen, and T Antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Li, W.; Zhang, J.; Zhang, Y. Mucin Glycans: A Target for Cancer Therapy. Molecules 2023, 28, 7033. [Google Scholar] [CrossRef]
- Duffy, M.J.; Shering, S.; Sherry, F.; McDermott, E.; O’Higgins, N. CA 15–3: A Prognostic Marker in Breast Cancer. Int. J. Biol. Markers 2000, 15, 330–333. [Google Scholar] [CrossRef]
- Mirabelli, P.; Incoronato, M. Usefulness of Traditional Serum Biomarkers for Management of Breast Cancer Patients. BioMed Res. Int. 2013, 2013, 685641. [Google Scholar] [CrossRef]
- Beckwith, D.M.; Cudic, M. Tumor-Associated O-Glycans of MUC1: Carriers of the Glyco-Code and Targets for Cancer Vaccine Design. Semin. Immunol. 2020, 47, 101389. [Google Scholar] [CrossRef]
- Radziejewska, I. Tumor-Associated Carbohydrate Antigens of MUC1–Implication in Cancer Development. Biomed. Pharmacother. 2024, 174, 116619. [Google Scholar] [CrossRef]
- Chik, J.H.L.; Zhou, J.; Moh, E.S.X.; Christopherson, R.; Clarke, S.J.; Molloy, M.P.; Packer, N.H. Comprehensive Glycomics Comparison between Colon Cancer Cell Cultures and Tumours: Implications for Biomarker Studies. J. Proteom. 2014, 108, 146–162. [Google Scholar] [CrossRef]
- Cox, K.E.; Liu, S.; Lwin, T.M.; Hoffman, R.M.; Batra, S.K.; Bouvet, M. The Mucin Family of Proteins: Candidates as Potential Biomarkers for Colon Cancer. Cancers 2023, 15, 1491. [Google Scholar] [CrossRef]
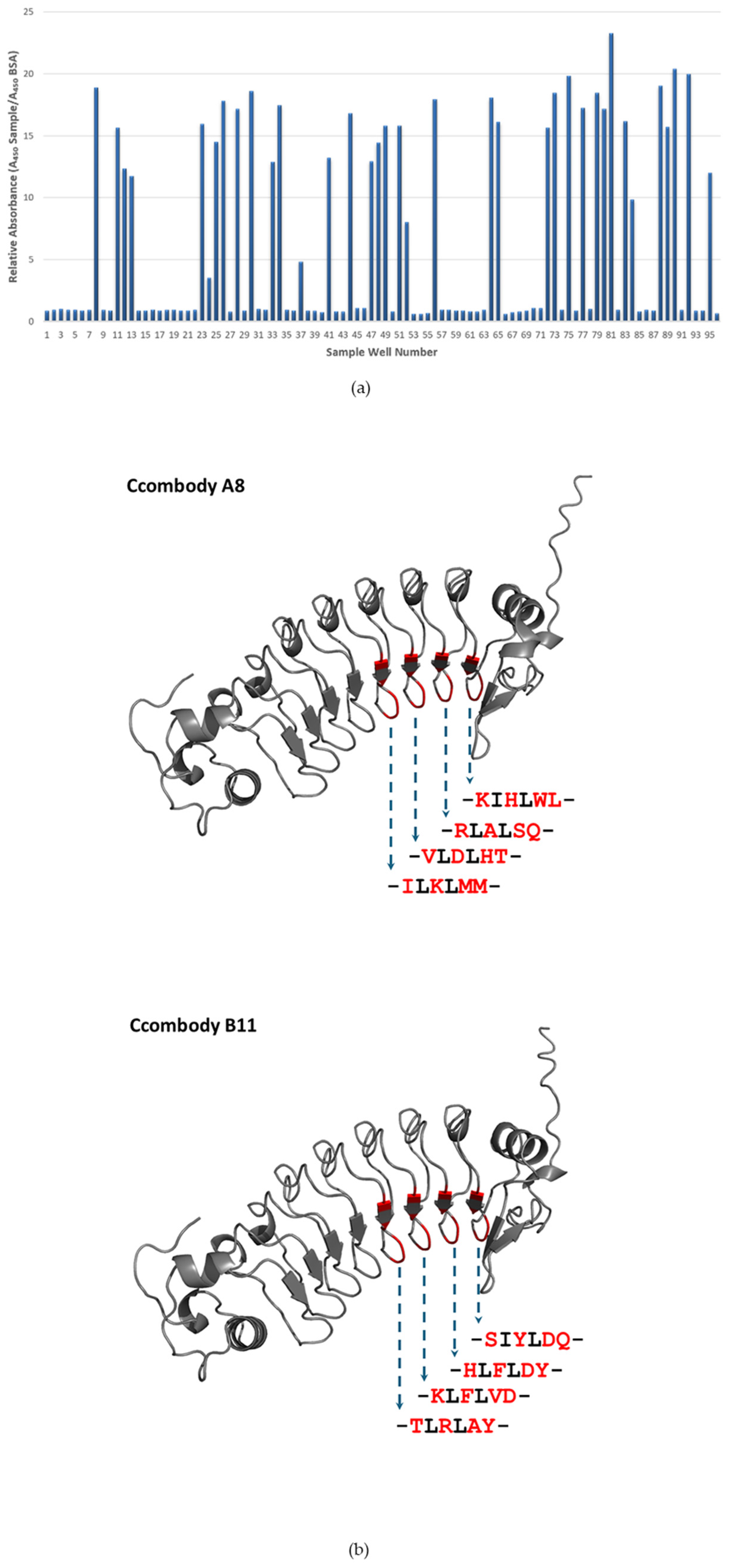
| Ccombody Segment | Sequence |
|---|---|
| LRR1 | QIIANN |
| LRRV1 | YLALGG |
| LRRV2 | YLILTG |
| LRRV3 | ELVLVE |
| LRRV4 | XLXLXX |
| LRRV5 | XLXLXX |
| LRRVe | XLXLXX |
| CP | XIXLXX |
| Classification | Frequency | |
|---|---|---|
| A8 | B11 | |
| Acidic | 1 | 3 |
| Basic | 5 | 3 |
| Aromatic | 1 | 5 |
| Polar, Uncharged | 3 | 3 |
| Non-polar, Aliphatic | 6 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelia, M.R.N.; Rodelas-Angelia, A.J.D.; Kim, Y.; Yang, C.; Jang, H.; Jeong, S.; Mun, J.; Thompson, K.D.; Jung, T. Phage Display Reveals VLRB-Mediated Recognition of Minimal Tumor Glycan Antigen Sialyl-Tn. Curr. Issues Mol. Biol. 2025, 47, 802. https://doi.org/10.3390/cimb47100802
Angelia MRN, Rodelas-Angelia AJD, Kim Y, Yang C, Jang H, Jeong S, Mun J, Thompson KD, Jung T. Phage Display Reveals VLRB-Mediated Recognition of Minimal Tumor Glycan Antigen Sialyl-Tn. Current Issues in Molecular Biology. 2025; 47(10):802. https://doi.org/10.3390/cimb47100802
Chicago/Turabian StyleAngelia, Mark Rickard N., Abigail Joy D. Rodelas-Angelia, Youngrim Kim, Cheolung Yang, Hyeok Jang, Seungpyo Jeong, Jihyun Mun, Kim D. Thompson, and Taesung Jung. 2025. "Phage Display Reveals VLRB-Mediated Recognition of Minimal Tumor Glycan Antigen Sialyl-Tn" Current Issues in Molecular Biology 47, no. 10: 802. https://doi.org/10.3390/cimb47100802
APA StyleAngelia, M. R. N., Rodelas-Angelia, A. J. D., Kim, Y., Yang, C., Jang, H., Jeong, S., Mun, J., Thompson, K. D., & Jung, T. (2025). Phage Display Reveals VLRB-Mediated Recognition of Minimal Tumor Glycan Antigen Sialyl-Tn. Current Issues in Molecular Biology, 47(10), 802. https://doi.org/10.3390/cimb47100802






