Chemopreventive Agents from Nature: A Review of Apigenin, Rosmarinic Acid, and Thymoquinone
Abstract
1. Introduction
2. Rosmarinic Acid (RA)
2.1. Source and Chemical Structure
2.2. In Vivo and In Vitro Studies of RA
2.2.1. In Vitro Studies of RA
2.2.2. In Vivo Studies of RA
| Study Focus | Animal Model | RA Dose and Route of Administration | Findings | Reference |
|---|---|---|---|---|
| Prevention of colon cancer induced by 1,2-dimethylhydrazine (DMH) | Rats | Oral, up to 16 mg/kg/day | RA significantly reduced DNA damage and tumor formation | [17] |
| Prevention of colon cancer induced by DMH | Rats | Oral at 5 mg/kg/day for 30 weeks | RA prevented tumor formation and induced apoptosis | [18] |
| Prevention of skin cancer induced by DMBA | Swiss Albino mice | Oral at 100 mg/kg/day for a week | RA prevented skin cancer and induced apoptosis | [19] |
| Mechanisms of RA anti-cancer activity in colorectal cancer | Male BALB/c mice | 30 mg/kg/day for a week | RA suppressed tumor progression by inhibiting TLR4-mediated NF-κB and STAT3 activation | [20] |
| Enhancement of doxorubicin’s efficacy in breast cancer | Female BALB/C mice | IV 8 mg/kg combined with doxorubicin | Combination showed better pharmacokinetics and anti-cancer efficacy than doxorubicin alone | [21] |
| Enhancement of cisplatin’s efficacy in cisplatin-resistant NSCLC lung cancer | Xenograft in nude female BALB mice | Intraperitoneal, combined with cisplatinvolume of administration of 10 μL/g | Significant inhibition of tumor growth with cisplatin combined with RA when compared to cisplatin alone | [23] |
2.3. In Silico Studies of RA
3. Apigenin
3.1. Source and Chemical Structure
3.2. In Vitro and In Vivo Studies of Apigenin
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.3. In Silico Studies of Apigenin
4. Thymoquinone (TQ)
4.1. Source and Chemical Structure
4.2. In Vivo and In Vitro Studies of TQ
4.2.1. In Vitro Studies of TQ
4.2.2. In Vivo Studies of TQ
| Cancer Type | Cell Lines | Animal Model | TQ Dosage | Mechanism of TQ Action | Overall Outcome | References |
|---|---|---|---|---|---|---|
| Bladder cancer | T-24 and 253 J cell lines | Xenograft mouse | 10 mg/kg/3 days | ↑ E-cadherin, ↓ N-cadherin, vimentin, Wnt/β-catenin, MYC, axin-2, MMP7, cyclin D1 | Augmentation of gemcitabine anti-cancer activities through the upregulation of apoptosis and autophagy processes | [93] |
| Breast cancer | MDA-MB-231 and MDA-MB-436 | Xenograft mouse | 5 mg/kg/day | ↑ miR-361, ↓ Rac, RhoA, VEGF-A | Angiogenesis and metastasis suppression and a decrease in tumor weight | [94] |
| Cervical cancer | SiHa cell lines | - | - | ↑ p53, ↓ Bcl-2 | Cell cycle arrest at the sub-G1 phase, induction of apoptosis and necrosis | [95] |
| Glioblastoma | S6 cell lines | - | - | ↓ ERK, FAK, MMP-2, MMP-9 | Reduced cell survival, migration, adhesion, and metastasis processes | [96] |
| Liver cancer | SNU-7721 and HepG2 cell lines | Xenograft rats | 20 mg/kg/day | ↑ Bax, caspase-8, ↓ Bcl-2, VEGF | Cell cycle arrest at G2/M phase and induction of apoptosis | [97] |
| Prostate cancer | DU-145, PC-3 | Xenograft mouse | 5–30 mg/kg/2 days | ↑ E-cadherin, ↓ Slug, TGF-β, Smad-2, Smad-3, vimentin | Reduced cell survival, migration, and invasion | [98] |
| Prostate cancer | PC-3, LNCaP | Xenograft mouse | 10–20 mg/kg/day | ↑ p21, p27, caspases; ↓ Bcl-2, Cyclin D1, CDK4 | Induction of apoptosis, cell cycle arrest, inhibition of tumor growth | [99] |
| Gastric cancer | BGC-823, HGC-27, MGC-803, SGC-7901 | Xenograft mouse | 20 mg/kg/day | ↑ Bax, caspase-3, caspase-9, cytochrome c, ↓ Bcl-2 | Increased sensitivity to 5-FU, induction of apoptosis, decrease in tumor weight | [100] |
| Gastric cancer | BGC-823, HGC-27, SGC-7901 | Xenograft mouse | 10–30 mg/kg/2 days | ↑: Bax, caspase-3, caspase-7, caspase-9 ↓: Bcl-2, cyclin D, c-Src, JAK2, STAT3, survivin, VEGF | Inhibition of cell growth and angiogenesis, apoptosis induction, and reduction in tumor weight | [72] |
| HGC-27, MGC-803, and SGC-7901 | Xenograft mouse | 10 mg/kg/2 days | ↑: AIF, Bax, caspase-3, caspase-9, cytochrome c, PTEN ↓: Bcl-2, cyclin D1, p-gp | Increased sensitivity to cisplatin, induction of apoptosis, decrease in tumor weight | [101] | |
| Colorectal cancer | HCT 116wt, DLD-1, HT29 | - | 25 mg/kg/day | ↓ ERK1/2, MEK, PAK1 | Decreased cell viability, induction of apoptosis and necrosis, decrease in tumor weight | [102] |
| Colorectal cancer | 5FU-resistant HCT116 | Xenograft mouse | 20 mg/kg/2 days | ↑ p21, p53, γH2AX, ↓ CD44, EpCAM, ki67, NF-κB, MEK | Induction of apoptosis, reduced cell invasion and migration, decrease in tumor weight | [103] |
| Irinotecan (CPT-11)-resistant LoVo cell lines | ↓: IKKα/β, NF-κB, Snail, Twist, vimentin, MMP-2, MMP-9, ERK1/2, PI3K | Increased cell rate, mitochondrial mem- brane permeability, induction of apoptosis and autophagy | [104] | |||
| Pancreatic cancer | PANC-1, BxPC-3 | Xenograft mouse | 5 mg/kg/day | ↑ Bax, caspase-3, p53; ↓ Bcl-2, NF-κB | Induction of apoptosis, suppression of tumor growth | [105] |
| Lung cancer | A549 | - | - | ↓: cyclin D1, ERK1/2, MMP-2, MMP-9, PCNA | Decreased rate of cancer cell proliferation, migration, invasion, and metastasis, cell cycle arrest at the G0/ G1 phase | [106] |
| Lung cancer | A549 | - | - | ↓: Bcl-2 | Decreased cell viability and induction of Apoptosis, as well as necrosis Depolymerization of microtubules and disruption of mitotic spindle organization, promotion of apoptosis, and decrease in cell viability | [107,108] |
| Ovarian cancer | ID8_NGL, NCI/ADR, and OVCAR-3 | Xenograft mouse | 20 mg/kg/2 days | ↓: Bcl-2, PCNA | Increased cell death, sensitivity of cancer cells to cisplatin, induced apoptosis | [109] |
| Ovarian cancer | SK-OV-3 cell lines | - | - | ↓: Bcl-2 | Induced apoptosis, cell cycle arrest at the S phase, and reduced anti-cancer impact of cisplatin | [110] |
4.3. In Silico Studies of TQ
5. Comparison of RA, Apigenin, and TQ: Chemopreventive Mechanisms and Extraction Methods
6. Clinical Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akhtar, M.S.; Swamy, M.K. Anticancer Plants: Mechanisms and Molecular Interactions; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2018; Volume 4. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Phytochemicals in Cancer Prevention and Therapy: Truth or Dare? Toxins 2010, 2, 517–551. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of Cancer: Current Evidence and Future Prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Dmitrovsky, E.; Sporn, M.B. Chemoprevention, Pharmacology of. In Encyclopedia of Cancer, 2nd ed.; Bertino, J.R., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 449–455. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; DiGiovanni, J. Environmental Carcinogenesis. In The Molecular Basis of Cancer, 4th ed.; Mendelsohn, J., Gray, J.W., Howley, P.M., Israel, M.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 103–128. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic Acid: Modes of Action, Medicinal Values and Health Benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of Rosmarinic Acid and Caffeic Acid in Aromatic Herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic Acid: New Aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative Study of Rosmarinic Acid Content in Some Plants of Labiatae Family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shen, Z.; Zou, Y.; Gao, K. Rosmarinic Acid Inhibits Migration, Invasion, and p38/AP-1 Signaling via miR-1225-5p in Colorectal Cancer Cells. J. Recept. Signal Transduct. Res. 2021, 41, 284–293. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic Acid Inhibits Proliferation and Invasion of Hepatocellular Carcinoma Cells SMMC 7721 via PI3K/AKT/mTOR Signal Pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Liu, L.; Cheng, X.L.; Cai, J.; Zhou, J.; Wang, T. Cytotoxic Effects of Rosmarinic Acid in OVCAR-3 Ovarian Cancer Cells Are Mediated via Induction of Apoptosis, Suppression of Cell Migration and Modulation of lncRNA MALAT-1 Expression. J. BUON 2018, 23, 763–768. [Google Scholar] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic Acid-Induced Apoptosis and Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, S.; Cai, Z.; Pan, D.; Li, Z.; Huang, Z.; Zhang, P.; Zhu, H.; Lei, L.; Wang, W. Anti-Warburg Effect of Rosmarinic Acid via miR-155 in Gastric Cancer Cells. Drug Des. Devel. Ther. 2015, 9, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; Oliveira, B.R.; Silva, L.R.; Cleto, S.S.; Munari, C.C.; Cunha, W.R.; Tavares, D.C. Chemopreventive Effects of Rosmarinic Acid on Rat Colon Carcinogenesis. Eur. J. Cancer Prev. 2015, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Namasivayam, N. Biochemical and Molecular Mechanisms Underlying the Chemopreventive Efficacy of Rosmarinic Acid in a Rat Colon Cancer. Eur. J. Pharmacol. 2016, 791, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, R.; Manoharan, S. Anti-Tumor Activity of Rosmarinic Acid in 7,12-Dimethylbenz(a)Anthracene (DMBA) Induced Skin Carcinogenesis in Swiss Albino Mice. Indian. J. Exp. Biol. 2012, 50, 187–194. [Google Scholar] [PubMed]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic Acid Represses Colitis-Associated Colon Cancer: A Pivotal Involvement of the TLR4-Mediated NF-κB-STAT3 Axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef]
- Xue, X.; Ricci, M.; Qu, H.; Lindstrom, A.; Zhang, D.; Wu, H.; Lin, T.Y.; Li, Y. Iron-Crosslinked Rososome with Robust Stability and High Drug Loading for Synergistic Cancer Therapy. J. Control. Release 2021, 329, 794–804. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, Y.; Huang, R.; Zheng, X. Rosmarinic Acid Combined with Adriamycin Induces Apoptosis by Triggering Mitochondria-Mediated Signaling Pathway in HepG2 and Bel-7402 Cells. Med. Sci. Monit. 2018, 24, 7898–7908. [Google Scholar] [CrossRef]
- Liao, X.Z.; Gao, Y.; Sun, L.L.; Liu, J.H.; Chen, H.R.; Yu, L.; Chen, Z.Z.; Chen, W.H.; Lin, L.Z. Rosmarinic Acid Reverses Non-Small Cell Lung Cancer Cisplatin Resistance by Activating the MAPK Signaling Pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef]
- Chou, S.T.; Ho, B.Y.; Tai, Y.T.; Huang, C.J.; Chao, W.W. Bidirect Effects from Cisplatin Combined with Rosmarinic Acid (RA) or Hot Water Extracts of Glechoma hederacea (HWG) on Renal Cancer Cells. Chin. Med. 2020, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Cytotoxic Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef]
- Jelić, D.; Mildner, B.; Koštrun, S.; Nujić, K.; Verbanac, D.; Čulić, O.; Antolović, R.; Brandt, W. Homology Modeling of Human Fyn Kinase Structure: Discovery of Rosmarinic Acid as a New Fyn Kinase Inhibitor and in Silico Study of Its Possible Binding Modes. J. Med. Chem. 2007, 50, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health Functionality of Apigenin: A Review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Mozafarian, V. Flora of Iran; Forest & Ranglands Research Institute Press: Tehran, Iran, 2007; Volume 1, p. 54. [Google Scholar]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to MicroRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective Growth-Inhibitory, Cell-Cycle Deregulatory and Apoptotic Response of Apigenin in Normal Versus Human Prostate Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin Induces Apoptosis by Targeting Inhibitor of Apoptosis Proteins and Ku70-Bax Interaction in Prostate Cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of Nuclear Factor-Kappa B, Bax, and Bcl-2 in Induction of Cell Cycle Arrest and Apoptosis by Apigenin in Human Prostate Carcinoma Cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The Flavonoid Apigenin Reduces Prostate Cancer CD44(+) Stem Cell Survival and Migration Through PI3K/Akt/NF-κB Signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin Inhibits Prostate Cancer Progression in TRAMP Mice via Targeting PI3K/Akt/FoxO Pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, S.; Franzen, C.A.; Pelling, J.C. Apigenin Inhibits TGF-β-Induced VEGF Expression in Human Prostate Carcinoma Cells via a Smad2/3- and Src-Dependent Mechanism. Mol. Carcinog. 2014, 53, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Ku Mo, J.; Choi, H.; Woo, J.; Jang, B.; Go, H.; Ko, S. Apigenin Induces Caspase-Dependent Apoptosis by Inhibiting Signal Transducer and Activator of Transcription 3 Signaling in HER2-Overexpressing SKBR3 Breast Cancer Cells. Mol. Med. Rep. 2015, 12, 2977–2984. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-H.; Chien, M.-H.; Lin, W.-L.; Wen, Y.-C.; Chow, J.-M.; Chen, C.-K.; Lee, W.-J. Inhibition of MDA-MB-231 Breast Cancer Cell Proliferation and Tumor Growth by Apigenin Through Induction of G2/M Arrest and Histone H3 Acetylation-Mediated p21(WAF1/CIP1) Expression. Environ. Toxicol. 2017, 32, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy Inhibition Enhances Apigenin-Induced Apoptosis in Human Breast Cancer Cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar] [CrossRef]
- Seo, H.-S.; Ku, J.M.; Choi, H.-S.; Woo, J.-K.; Jang, B.-H.; Shin, Y.C.; Ko, S.-G. Induction of Caspase-Dependent Apoptosis by Apigenin by Inhibiting STAT3 Signaling in HER2-Overexpressing MDA-MB-453 Breast Cancer Cells. Anticancer. Res. 2014, 34, 2869–2882. [Google Scholar]
- Seo, H.-S.; Jo, J.K.; Ku, J.M.; Choi, H.-S.; Choi, Y.K.; Woo, J.-K.; Ko, S.-G. Induction of Caspase-Dependent Extrinsic Apoptosis by Apigenin Through Inhibition of Signal Transducer and Activator of Transcription 3 (STAT3) Signalling in HER2-Overexpressing BT-474 Breast Cancer Cells. Biosci. Rep. 2015, 35, e00276. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.-Y.; Hwang, S.Y.; Kim, N.D. Apigenin-Induced Apoptosis is Enhanced by Inhibition of Autophagy Formation in HCT116 Human Colon Cancer Cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Hua, Z.-C. Apigenin Potentiates TRAIL Therapy of Non-Small Cell Lung Cancer via Upregulating DR4/DR5 Expression in a p53-Dependent Manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, M.; Liu, Y.; Zhang, Z.; Lu, R.; Lu, J. Apigenin Inhibits Cell Proliferation, Migration, and Invasion by Targeting Akt in the A549 Human Lung Cancer Cell Line. Anti-Cancer Drugs 2017, 28, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Giuliano, A.E.; Van Herle, A.J. Signal Transduction Pathways Involved in Apigenin-Induced Apoptosis and Cell-Cycle Arrest in Human Breast Cancer Cells. Nutr. Cancer 2001, 39, 114–126. [Google Scholar]
- Fang, J.; Xia, C.; Cao, Z.; Zheng, J.Z.; Reed, E.; Jiang, B.H. Apigenin Inhibits VEGF and HIF-1 Expression via PI3K/AKT/p70S6K1 and HDM2/p53 Pathways. FASEB J. 2005, 19, 342–353. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural Product Apigenin Inhibits Angiogenesis: Implication in Its Anti-Inflammatory and Anti-Oxidative Therapeutic Effects. Cell. Mol. Biol. 2017, 63, 14–21. [Google Scholar]
- Tong, X.; Pelling, J.C. Targeting the PI3K/Akt/mTOR Axis by Apigenin for Cancer Prevention. Anti-Cancer Agents Med. Chem. 2013, 13, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Táborský, J.; Kunt, M.; Klouček, P.; Lachman, J.; Zelený, V.; Kokoška, L. Identification of potential sources of thymoquinone and related compounds in asteraceae, cupressaceae, lamiaceae, and ranunculaceae families. Open Chem. 2012, 10, 1899–1906. [Google Scholar] [CrossRef]
- Long, X.; Fan, M.; Bigsby, R.M.; Nephew, K.P. Apigenin Inhibits Antiestrogen-Resistant Breast Cancer Cell Growth through Estrogen Receptor-α-Dependent and Estrogen Receptor-α-Independent Mechanisms. Mol. Cancer Ther. 2008, 7, 2096–2108. [Google Scholar] [CrossRef]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of Apoptosis by Apigenin and Related Flavonoids through Cytochrome c Release and Activation of Caspase-9 and Caspase-3 in Leukemia HL-60 Cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhu, Y.; Li, J.F.; Wang, X.; Liang, Z.; Li, S.Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.Y.; et al. Apigenin Inhibits Renal Cell Carcinoma Cell Proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S. Apigenin and Naringenin Suppress Colon Carcinogenesis through the Aberrant Crypt Stage in Azoxymethane-Treated Rats. Exp. Biol. Med. 2010, 235, 710–717. [Google Scholar] [CrossRef]
- King, J.C.; Lu, Q.Y.; Li, G.; Moro, A.; Takahashi, H.; Chen, M. Evidence for Activation of Mutated p53 by Apigenin in Human Pancreatic Cancer. Biochim. Biophys. Acta 2012, 1823, 593–604. [Google Scholar] [CrossRef]
- Silvan, S.; Manoharan, S. Apigenin Prevents Deregulation in the Expression Pattern of Cell-Proliferative, Apoptotic, Inflammatory, and Angiogenic Markers During 7,12-Dimethylbenz[a]anthracene-Induced Hamster Buccal Pouch Carcinogenesis. Arch. Oral. Biol. 2013, 58, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Silvan, S.; Manoharan, S.; Baskaran, N.; Anusuya, C.; Karthikeyan, S.; Prabhakar, M.M. Chemo-Preventive Potential of Apigenin in 7,12-Dimethylbenz[a]anthracene-Induced Experimental Oral Carcinogenesis. Eur. J. Pharmacol. 2011, 670, 571–577. [Google Scholar] [CrossRef]
- Liu, L.Z.; Fang, J.; Zhou, Q.; Hu, X.; Shi, X.; Jiang, B.H. Apigenin Inhibits Expression of Vascular Endothelial Growth Factor and Angiogenesis in Human Lung Cancer Cells: Implication of Chemoprevention of Lung Cancer. Mol. Pharmacol. 2005, 68, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Krisanapun, C.; Lee, S.H.; Nualsanit, T.; Sams, C.; Peungvicha, P. Molecular Targets of Apigenin in Colorectal Cancer Cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar] [CrossRef]
- Wei, H.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory Effect of Apigenin, a Plant Flavonoid, on Epidermal Ornithine Decarboxylase and Skin Tumor Promotion in Mice. Cancer Res. 1990, 50, 499–502. [Google Scholar] [PubMed]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of mTOR by Apigenin in UVB-Irradiated Keratinocytes: A New Implication of Skin Cancer Prevention. Cell. Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef]
- Kasilingam, T.; Elengoe, A. In Silico Molecular Modeling and Docking of Apigenin Against the Lung Cancer Cell Proteins. Asian J. Pharm. Clin. Res. 2018, 11, 246–252. [Google Scholar] [CrossRef]
- Ganai, S.A.; Farooq, Z.; Banday, S.; Altaf, M. In Silico Approaches for Investigating the Binding Propensity of Apigenin and Luteolin Against Class I HDAC Isoforms. Future Med. Chem. 2018, 10, 1925–1945. [Google Scholar] [CrossRef]
- Wirries, A.; Breyer, S.; Quint, K.; Schobert, R.; Ocker, M. Thymoquinone Hydrazone Derivatives Cause Cell Cycle Arrest in p53-Competent Colorectal Cancer Cells. Exp. Ther. Med. 2010, 1, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Kuester, D.; Mawrin, C.; Bajbouj, K.; Diestel, A.; Ocker, M.; Schneider-Stock, R. Thymoquinone Triggers Inactivation of the Stress Response Pathway Sensor CHEK1 and Contributes to Apoptosis in Colorectal Cancer Cells. Cancer Res. 2008, 68, 5609–5618. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Choi, B.Y.; Jeong, C.-H.; Kundu, J.K.; Chun, K.-S. Thymoquinone Induces Apoptosis in Human Colon Cancer HCT116 Cells Through Inactivation of STAT3 by Blocking JAK2- and Src-Mediated Phosphorylation of EGF Receptor Tyrosine Kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Motaghed, M.; Al-Hassan, F.M.; Hamid, S.S. Cellular responses with thymoquinone treatment in human breast cancer cell line MCF-7. Pharmacogn. Res. 2013, 5, 200–206. [Google Scholar] [CrossRef]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.-S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. 2011, 706, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chehl, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 2009, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149–4159. [Google Scholar] [CrossRef]
- Ahmad, I.; Muneer, K.M.; Tamimi, I.A.; Chang, M.E.; Ata, M.O.; Yusuf, N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013, 270, 70–76. [Google Scholar] [CrossRef]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef]
- Roepke, M.; Diestel, A.; Bajbouj, K.; Walluscheck, D.; Schonfeld, P.; Roessner, A.; Gali-Muhtasib, H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in doxorubicin-resistant liver cancer cells. Oncogene 2007, 26, 3493–3503. [Google Scholar]
- Gali-Muhtasib, H.; Diab-Assaf, M.; Boltze, C.; Al-Hmaira, J.; Hartig, R.; Roessner, A.; Schneider-Stock, R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int. J. Oncol. 2004, 25, 857–866. [Google Scholar]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Wani, A.A. Thymoquinone induces apoptosis through activation of caspase-3 and caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer 2005, 117, 409–417. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Targeting nuclear factor-κB activation pathway by thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res. 2008, 6, 1059–1070. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.N.; Al-Shabanah, O.A. The potential role of thymoquinone (TQ) in the treatment of the oxidative stress in STZ diabetic rats. Res. Commun. Mol. Pathol. Pharmacol. 1999, 104, 211–224. [Google Scholar]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- Mu, L.; Fang, Q.; Chen, X. Thymoquinone induces apoptosis in PC-3 prostate cancer cells through mitochondrial-dependent apoptosis pathway. Nat. Prod. Commun. 2014, 9, 325–328. [Google Scholar]
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef]
- Ravindran, J.; Nair, H.B.; Aggarwal, B.B. Thymoquinone inhibits NF-κB signaling in human multiple myeloma cells and suppresses osteoclastogenesis. Clin. Cancer Res. 2009, 15, 273–282. [Google Scholar]
- Meral, I.; Pala, M.; Akbas, F.; Ustunova, S.; Yildiz, C.; Demirel, M. Effects of thymoquinone on liver miRNAs and oxidative stress in Ehrlich acid mouse solid tumor model. Biotech. Histochem. 2018, 93, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Helmy, S.A.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El Gayar, A.M. Thymoquinone upregulates TRAIL/TRAILR2 expression and attenuates hepatocellular carcinoma in vivo model. Life Sci. 2019, 233, 116673. [Google Scholar] [CrossRef] [PubMed]
- Shabani, H.; Karami, M.H.; Kolour, J.; Sayyahi, Z.; Parvin, M.A.; Soghala, S.; Baghini, S.S.; Mardasi, M.; Chopani, A.; Moulavi, P.; et al. Anticancer activity of thymoquinone against breast cancer cells: Mechanisms of action and delivery approaches. Biomed. Pharmacother. 2023, 165, 114972. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Hashemi, M.; Entezari, M.; Mohsenifar, A. The comparison of anticancer activity of thymoquinone and nanothymoquinone on human breast adenocarcinoma. Iran. J. Pharm. Res. 2015, 14, 539. [Google Scholar] [PubMed]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 1407840. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.Z.; Yang, S.Z.; Ying, X.Z.; Liao, W.; Liu, H.X.; Lin, Z.Q.; Chen, Q.Y.; et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, H.; Huang, Z.; Zhang, P.; Yue, Y.; Wang, W.; Liu, W.; Zeng, J.; Ma, J.; Chen, G.; et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018, 292, 65–75. [Google Scholar] [CrossRef]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer. 2014, 66, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.K.; Yazan, L.S.; Ismail, M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol. Vitro. 2011, 25, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Krylova, N.G.; Drobysh, M.S.; Semenkova, G.N.; Kulahava, T.A.; Pinchuk, S.V.; Shadyro, O.I. Cytotoxic and antiproliferative efects of thymoquinone on rat C6 glioma cells depend on oxidative stress. Mol. Cell Biochem. 2019, 462, 195–206. [Google Scholar] [CrossRef]
- ElKhoely, A.; Hafez, H.F.; Ashmawy, A.M.; Badary, O.; Abdelaziz, A.; Mostafa, A.; Shouman, S.A. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: Mechanistic perspectives. J. Nat. Med. 2015, 69, 313–323. [Google Scholar] [CrossRef]
- Kou, B.; Liu, W.; Zhao, W.; Duan, P.; Yang, Y.; Yi, Q.; Guo, F.; Li, J.; Zhou, J.; Kou, Q. Thymoquinone inhibits epithelial–mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol. Rep. 2017, 38, 3592–3598. [Google Scholar] [CrossRef] [PubMed]
- Alshyarba, M.; Otifi, H.; Al Fayi, M.; A Dera, A.; Rajagopalan, P. Thymoquinone inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol. Appl. Biochem. 2021, 68, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lv, X.; Liu, M.; Yang, Z.; Ji, M.; Guo, X.; Dong, W. Thymoquinone inhibits growth and augments 5-fuorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem. Biophys. Res. Commun. 2012, 417, 864–868. [Google Scholar] [CrossRef]
- Ma, J.; Hu, X.; Li, J.; Wu, D.; Lan, Q.; Wang, Q.; Tian, S.; Dong, W. Enhancing conventional chemotherapy drug cisplatin-induced antitumor efects on human gastric cancer cells both in vitro and in vivo by thymoquinone targeting PTEN gene. Oncotarget 2017, 8, 85926. [Google Scholar] [CrossRef] [PubMed]
- El-Baba, C.; Mahadevan, V.; Fahlbusch, F.B.; Rau, T.T.; Gali-Muhtasib, H.; Schneider-Stock, R. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK–ERK signaling in colorectal cancer. Mol. Cancer. 2014, 13, 201. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, N.-H.; Hsu, H.-H.; Ho, T.-J.; Tu, C.-C.; Hsieh, D.J.-Y.; Lin, Y.-M.; Chen, L.-M.; Kuo, W.-W.; Huang, C.-Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540. [Google Scholar] [CrossRef]
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor efect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Digest Dis. Sci. 2015, 60, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J. Cell Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Chatterjee, A.; Ganguli, A.; Bhattacharya, S.; Chakrabarti, G. Thymoquinone inhibits microtubule polymerization by tubulin binding and causes mitotic arrest following apoptosis in A549 cells. Biochimie 2014, 97, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tumor efects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, J.; Cai, W.; Pan, Y.; Li, R.; Li, B. The efect of thymoquinone on apoptosis of SK-OV-3 ovarian cancer cell by regulation of Bcl-2 and Bax. Int. J. Gynecol. Cancer. 2017, 27, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Sundaravadivelu, S.; Raj, S.K.; Kumar, B.S.; Arumugamand, P.; Ragunathan, P.P. Reverse Screening Bioinformatics Approach to Identify Potential Anti Breast Cancer Targets Using Thymoquinone from Neutraceuticals Black Cumin Oil. Anti-Cancer Agents Med. Chem. 2019, 19, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Nithya, G.; Ilakkia, A.; Sakthisekaran, D. In silico docking studies on the anti-cancer effect of thymoquinone on interaction with phosphatase and tensin homolog located on chromosome 10q23: A regulator of PI3K/AKT pathway. Asian J. Pharm. Clin. Res. 2015, 8, 192–195. [Google Scholar]
- Durga, B.; Julius, A. In-Silico Docking studies of thymoquinone as potential anti-cancer drug target on Lung Cancer Cells. Eur. J. Mol. Clin. Med. 2020, 7, 1706–1716. [Google Scholar]
- Choi, E.J.; Kim, G.H. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J. Clin. Biochem. Nutr. 2009, 44, 260–265. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.V.; Mohan, M.E.; Prabhakaran, P.; Maliakel, B.; Krishnakumar, I.M. A phase I clinical trial to evaluate the safety of thymoquinone-rich black cumin oil (BlaQmax®) on healthy subjects: Randomized, double-blinded, placebo-controlled prospective study. Toxicol. Rep. 2022, 9, 999–1007. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar] [CrossRef]
- Clinical and Immunohisochemical Evaluation of Chemopreventive Effect of Thymoquinone on Oral Potenti. Available online: https://ctv.veeva.com/study/clinical-and-immunohisochemical-evaluation-of-chemopreventive-effect-of-thymoquinone-on-oral-potenti (accessed on 16 May 2024).
- ClinicalTrials.gov Identifier: NCT03208790. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03208790 (accessed on 16 May 2024).
- ClinicalTrials.gov Study: NCT03208790. Available online: https://clinicaltrials.gov/study/NCT03208790?intr=Thymoquinone&cond=Cancer&limit=100&page=1&rank=1 (accessed on 16 May 2024).

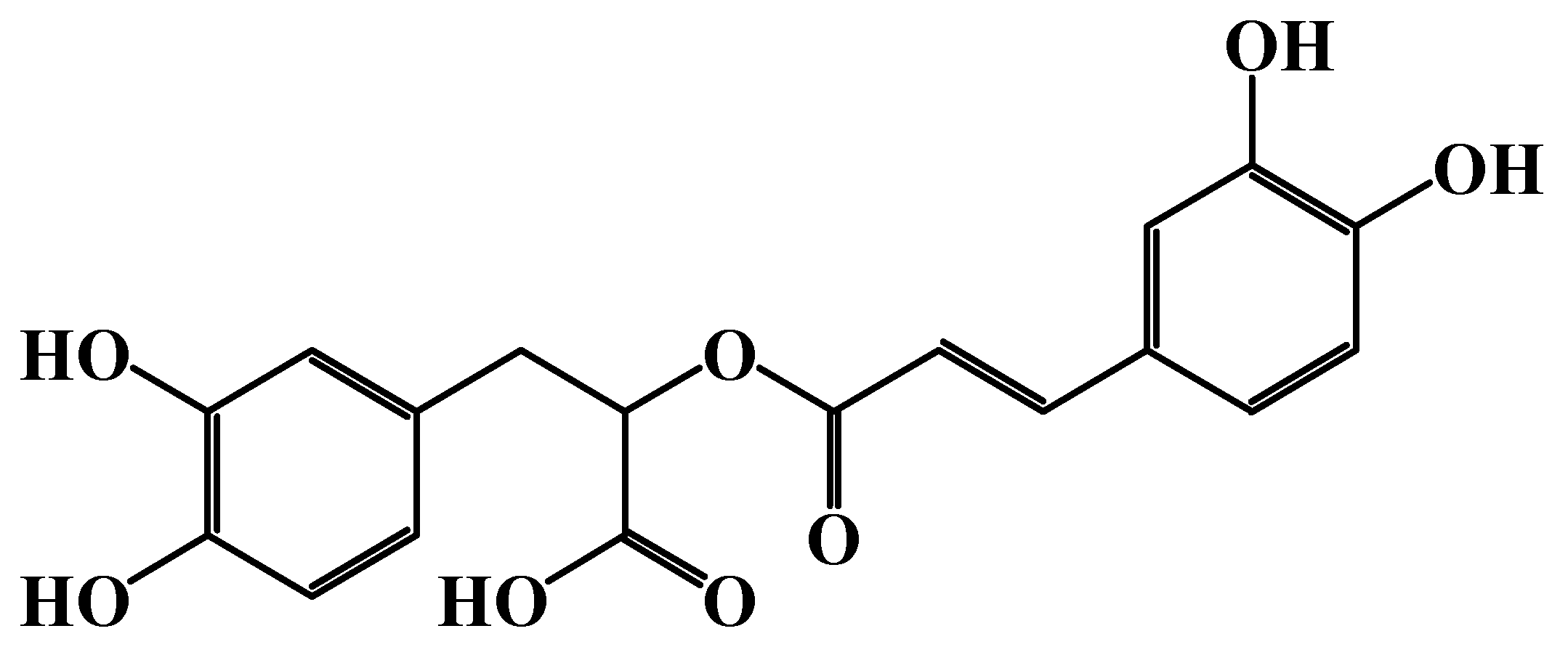
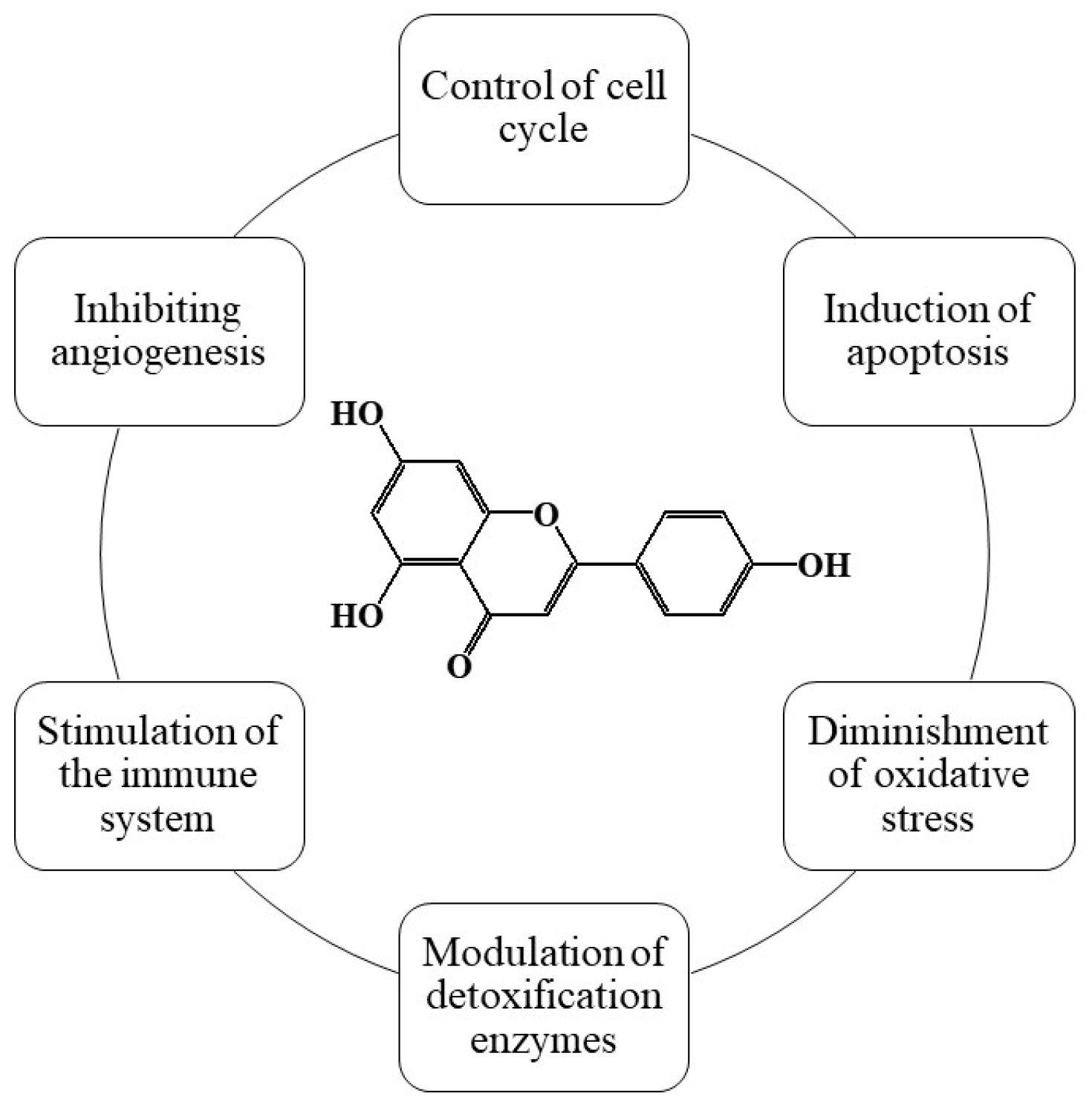

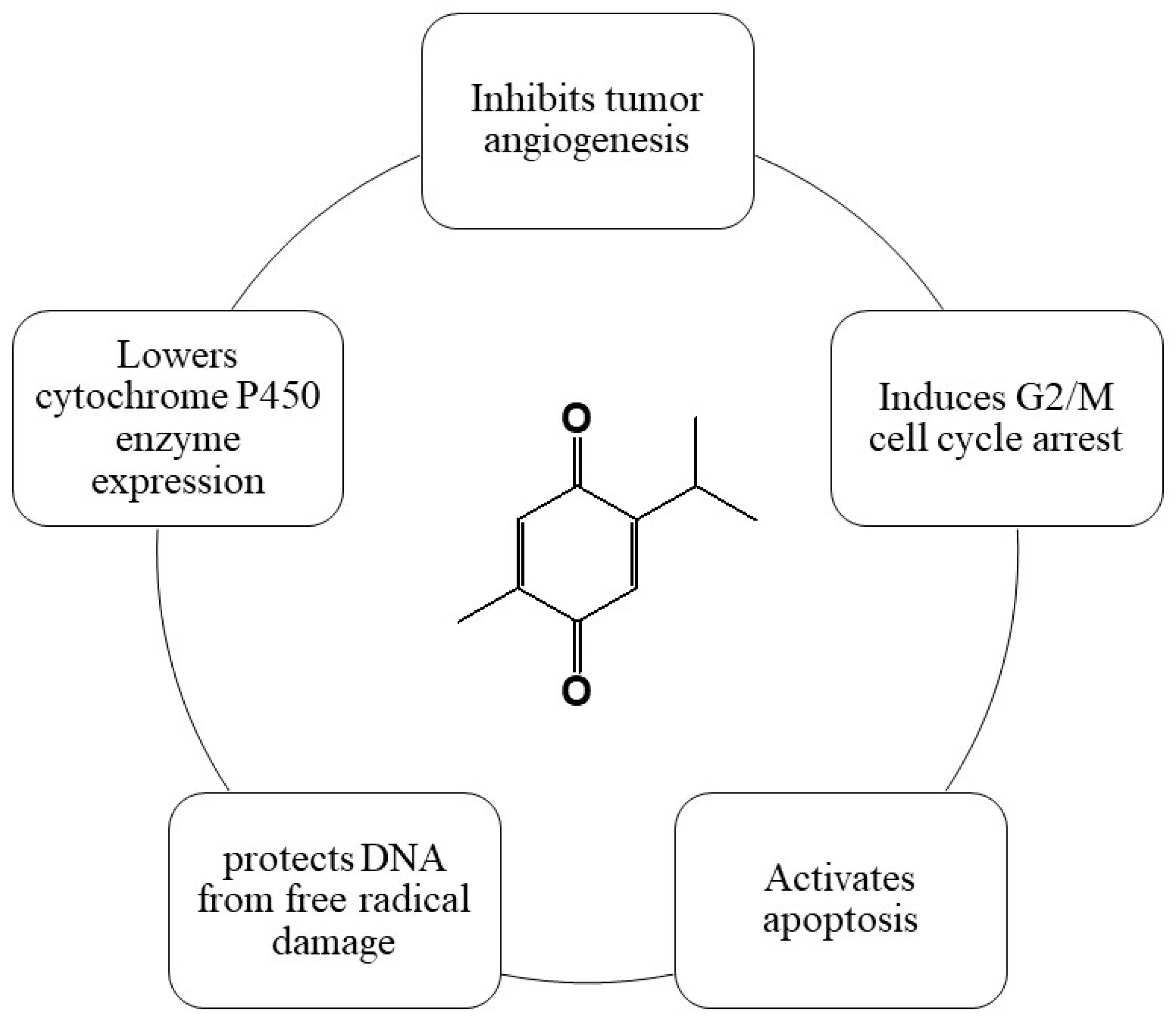

| Source | Quantity of Apigenin (mg/kg) | Reference |
|---|---|---|
| Chinese cabbage | 187.0 | [29] |
| Bell pepper | 272.0 | [29] |
| Garlic | 217.0 | [29] |
| Bilimbi fruit | 458.0 | [29] |
| French peas | 176.0 | [29] |
| Guava | 579.0 | [29] |
| Wolfberry leaves | 547.0 | [29] |
| Daun turi | 39.5 | [29] |
| Kadok | 34.5 | [29] |
| Celery seeds | 786.5 | [30] |
| Spinach | 620 | [30] |
| Parsley | 450.4 | [30] |
| Marjoram | 44.0 | [30] |
| Oregano | 35.0 | [30] |
| Sage | 24.0 | [30] |
| Chamomile | 30–50 | [30] |
| Rosemary | 5.5 | [30] |
| Pistachio | 0.3 | [30] |
| Study Model | Apigenin Dosage and Administration | Key Findings | Reference |
|---|---|---|---|
| Colon carcinogenesis in rats | Dietary intake of 0.1% apigenin | Triggered apoptosis of luminal surface colonocytes, reduced aberrant crypt foci, decreased peritoneal metastasis | [55] |
| Lung cancer xenografts in nude mice | Dietary intake of 0.2% apigenin for 6 weeks | Reduced tumor volume, suppressed HIF-1α-VEGF pathway | [56] |
| Prostate cancer in TRAMP mice | Oral administration of 20 and 50 μg/mice for 20 weeks | Reduced tumor volumes and distant organ metastasis by suppressing PI3K/Akt/FoxO pathway | [35] |
| DMBA-induced oral carcinogenesis in hamsters | Oral administration of 2.5 mg/kg for 15 weeks | Reduced tumor volume and incidence, modulated cell proliferation, apoptosis, inflammation, and angiogenesis markers | [57] |
| Lung cancer xenografts in nude mice | Oral administration of 3 mg/kg | Decreased tumor volume and wet weight, reduced serum IGF-I levels, induced apoptosis and cell cycle arrest | [59] |
| APCMin/+ mice model | Oral administration of apigenin | Reduction in polyp number by activation of p53 | [60] |
| Murine skin tumorigenesis in SENCAR mice | Topical application of 5 and 20 μmol | Marked reduction in the incidence and number of papillomas and carcinomas | [61] |
| UVB-induced skin inflammation in SKH-1 mice | Topical application of 5 μM prior to UVB exposure | Reduced UVB-induced ear edema and COX-2 expression, modulated HIF-1α, and suppressed mTOR signaling | [62] |
| Source | Quantity of TQ (mg/kg) | Reference |
|---|---|---|
| Eupatorium cannabinum L. | 8 | [51] |
| Juniperus communis L. | 6 free TQ, 15 glycosidically bound TQ | [51] |
| Monarda didyma L. | 3029 | [51] |
| Monarda didyma L. | 3425 | [51] |
| Monarda media Willd. | 2995 | [51] |
| Monarda menthifolia Graham | 1381 | [51] |
| Satureja hortensis L. | 217 | [51] |
| Satureja montana L. | 1052 | [51] |
| Thymus pulegioides L. | 233 | [51] |
| Thymus serpyllum L. | 233 | [51] |
| Thymus vulgaris L. | 300 | [51] |
| Nigella sativa L. | 1881 | [51] |
| Compound | Key Mechanisms | Specific Targets | References |
|---|---|---|---|
| Apigenin | Inhibits proliferation, induces apoptosis, modulates cell cycle | Cell cycle regulatory proteins, PI3K/AKT, MAPK, caspases, Bcl-2 family, mitochondrial membrane potential | [33,53] |
| Rosmarinic Acid (RA) | Induces apoptosis, inhibits metastasis, affects glucose metabolism (anti-Warburg effect) [13,16] | PI3K/AKT/mTOR, epithelial–mesenchymal transition, apoptosis-related genes, glucose uptake, and lactate production [13,16] | [13,16] |
| Thymoquinone (TQ) | Induces cell cycle arrest and apoptosis, modulates oxidative stress, anti-inflammatory, inhibits metastasis and angiogenesis | Cyclins, CDKs, p53, Bcl-2, Bax, NF-κB, ROS, MMPs, VEGF | [69,75,77,78,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutayeh, R.F.; Altah, M.; Mehdawi, A.; Al-Ataby, I.; Ardakani, A. Chemopreventive Agents from Nature: A Review of Apigenin, Rosmarinic Acid, and Thymoquinone. Curr. Issues Mol. Biol. 2024, 46, 6600-6619. https://doi.org/10.3390/cimb46070393
Abutayeh RF, Altah M, Mehdawi A, Al-Ataby I, Ardakani A. Chemopreventive Agents from Nature: A Review of Apigenin, Rosmarinic Acid, and Thymoquinone. Current Issues in Molecular Biology. 2024; 46(7):6600-6619. https://doi.org/10.3390/cimb46070393
Chicago/Turabian StyleAbutayeh, Reem Fawaz, Maram Altah, Amani Mehdawi, Israa Al-Ataby, and Adel Ardakani. 2024. "Chemopreventive Agents from Nature: A Review of Apigenin, Rosmarinic Acid, and Thymoquinone" Current Issues in Molecular Biology 46, no. 7: 6600-6619. https://doi.org/10.3390/cimb46070393
APA StyleAbutayeh, R. F., Altah, M., Mehdawi, A., Al-Ataby, I., & Ardakani, A. (2024). Chemopreventive Agents from Nature: A Review of Apigenin, Rosmarinic Acid, and Thymoquinone. Current Issues in Molecular Biology, 46(7), 6600-6619. https://doi.org/10.3390/cimb46070393





