HDAC9 and miR-512 Regulate CAGE-Promoted Anti-Cancer Drug Resistance and Cellular Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Cell Culture
2.3. Colony Formation
2.4. Cell Viability Determination
2.5. Invasion Assays
2.6. ChIP Assays
2.7. Tumor Spheroid Forming Potential
2.8. Transfection
2.9. miRNA Extraction and qRT-PCR
2.10. TargetScan Analysis
2.11. Immunofluorescence Staining
2.12. Immunoblot and Immunoprecipitation
2.13. Luciferase Activity Assays
2.14. Expression and Purification of CAGE Proteins
2.15. In Vivo Tumorigenic Potential
2.16. TCGA Dataset Analysis
2.17. Statistical Analysis
3. Results
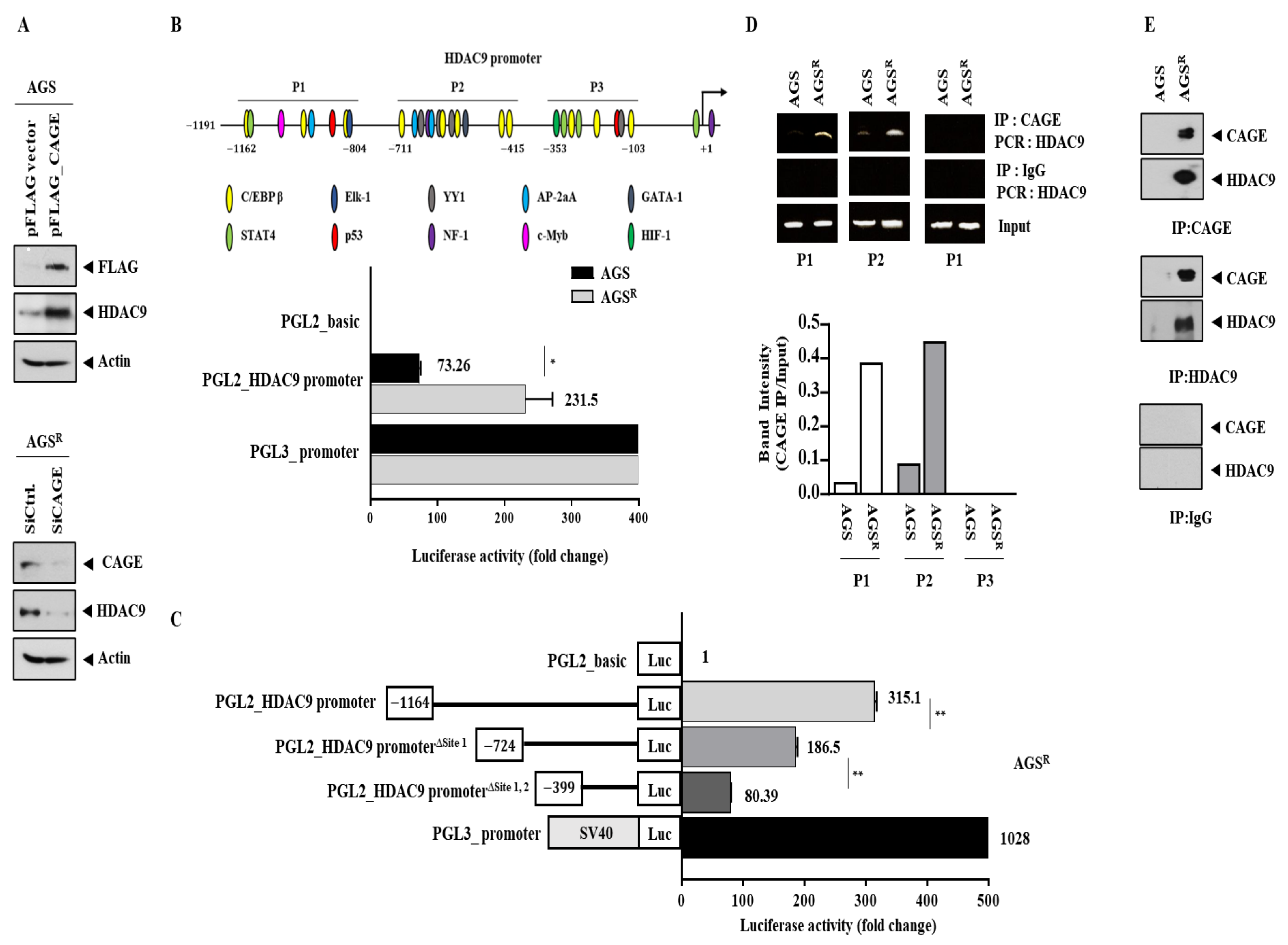
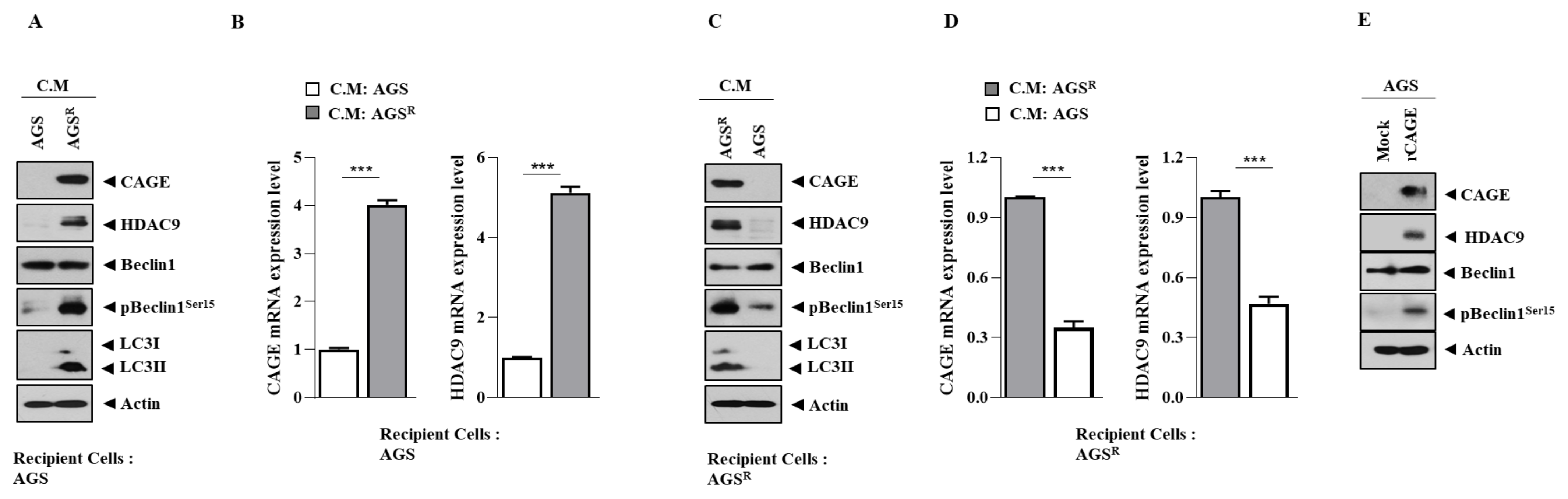
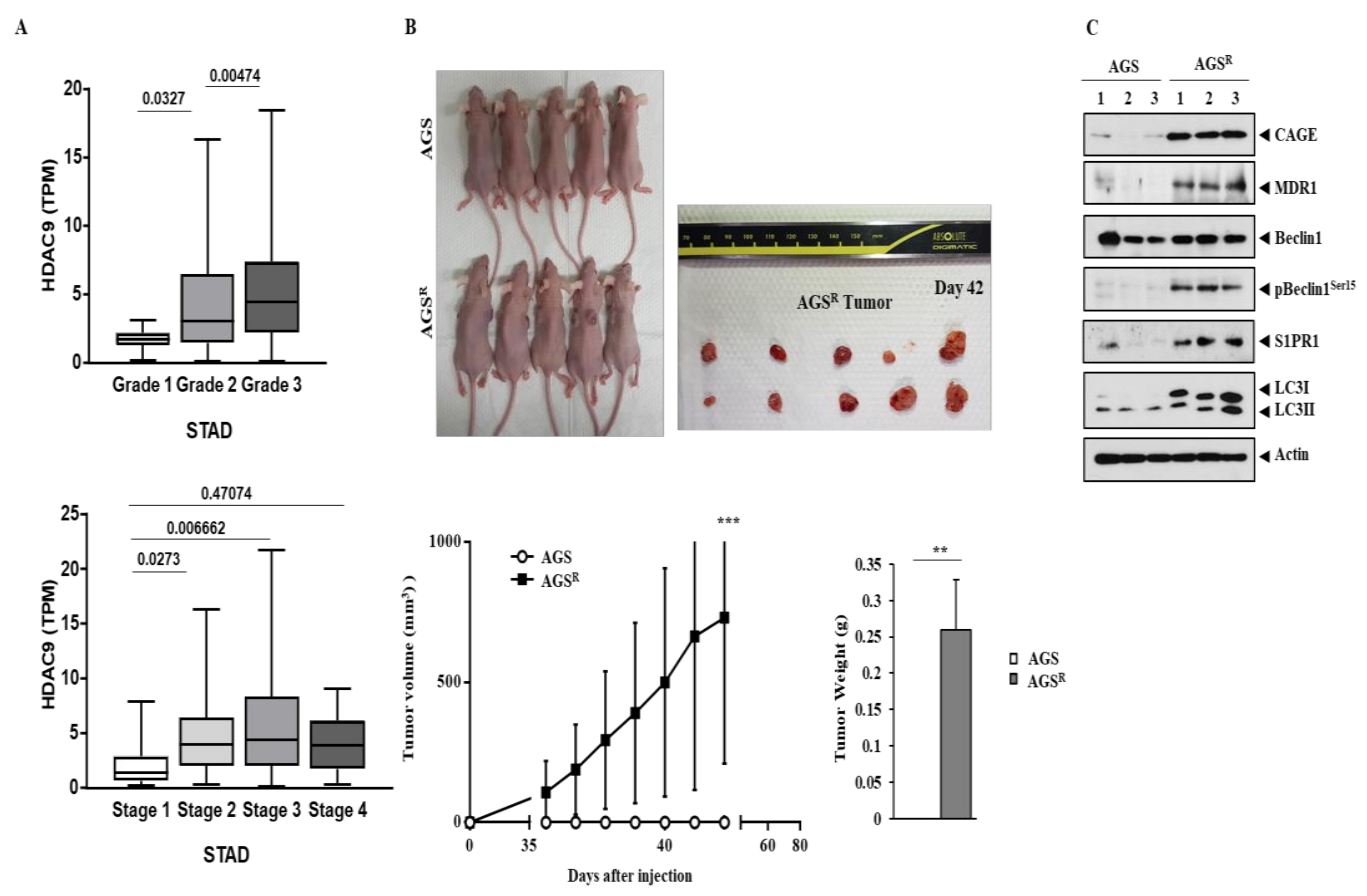
3.1. Anti-Cancer Drug-Resistant Gastric Cancer Cells Show Increased Expression of CAGEs and HDAC9
3.2. CAGEs Regulate the Expression of HDAC9
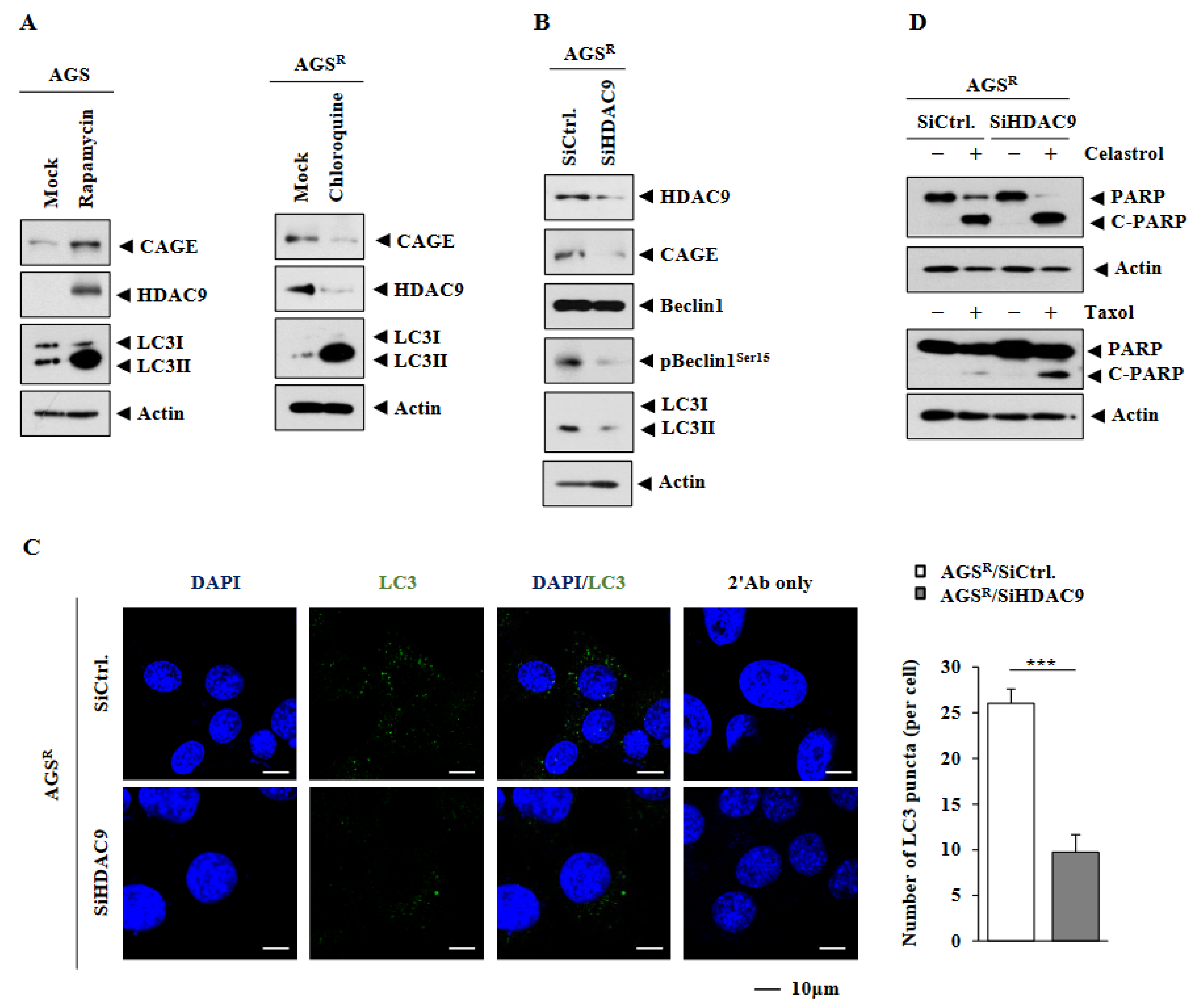
3.3. HDAC9 Is Necessary for Increased Autophagic Flux in AGSR Cells
3.4. Downregulation of HDAC9 Decreases Invasion, Migration, and Tumor Spheroid Forming Potential of AGSR Cells
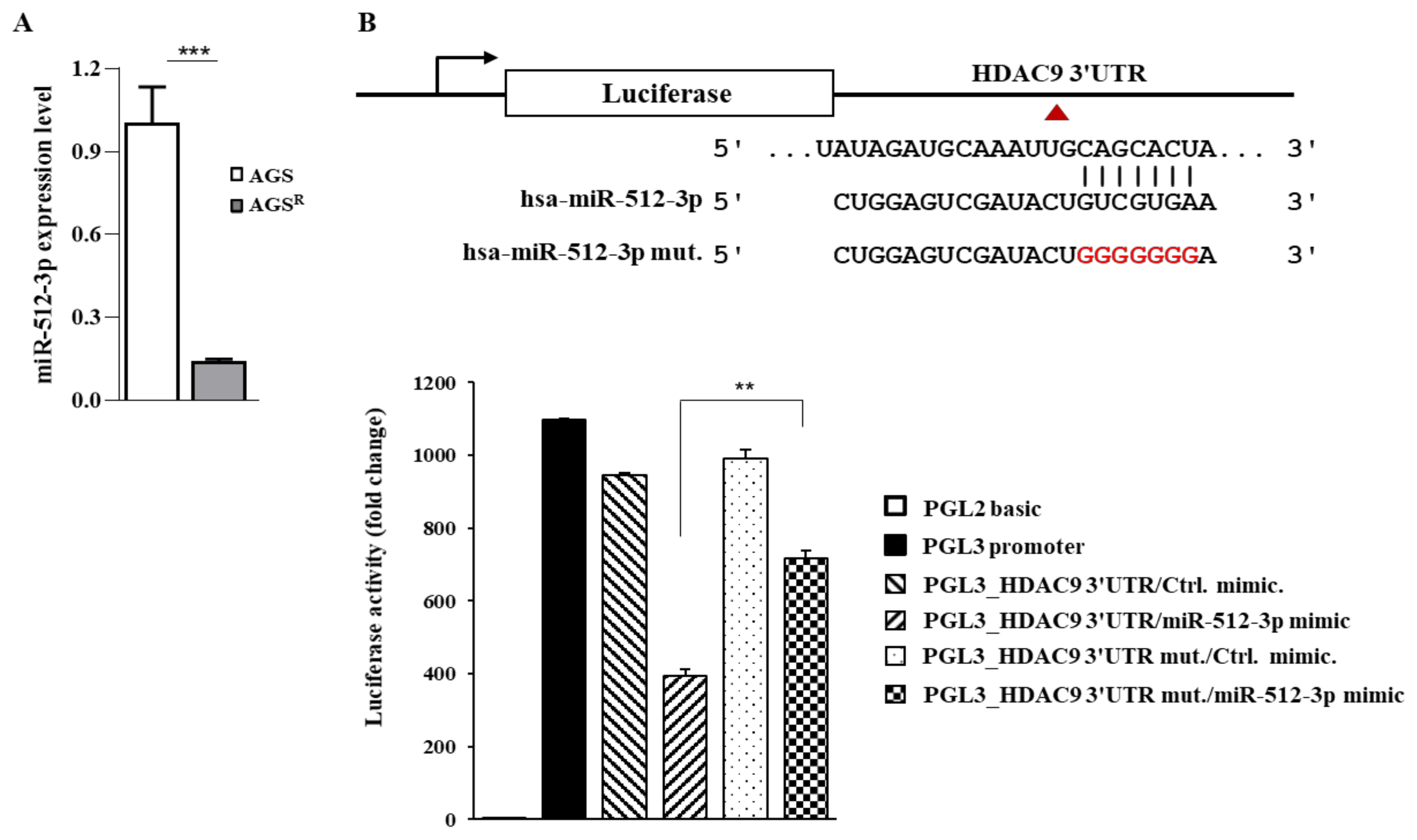
3.5. miR-512 Directly Regulates the Expression of HDAC9
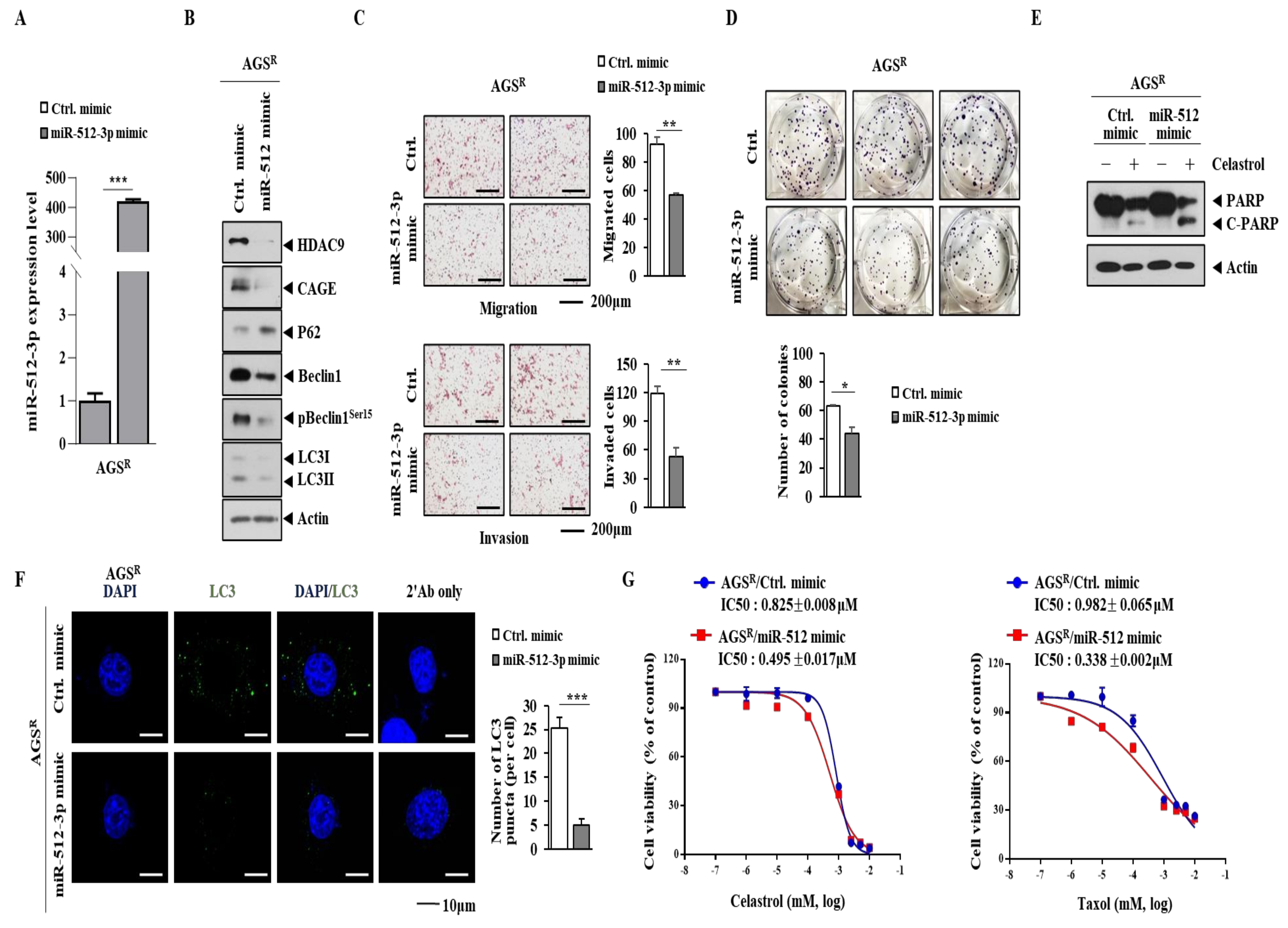
3.6. miR-512 Decreases Autophagic Flux, Invasion/Migration, and Cellular Proliferation but Enhances Sensitivity to Anti-Cancer Drugs
3.7. Soluble Factors Regulate HDAC9 Expression
3.8. AGSR Cells Display Tumorigenic Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, B.; Lim, Y.; Lee, D.Y.; Park, S.Y.; Lee, H.; Kim, W.H.; Yang, H.; Bang, Y.J.; Jeoung, D.I. Identification and characterization of a novel cancer/testis antigen gene CAGE. Biochem. Biophys. Res. Commun. 2002, 292, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Fujita, T.; Hirao, N.; Matsuzaki, Y.; Okada, T.; Mochimaru, H.; Susumu, N.; Matsumoto, E.; Sugano, K.; Yamashita, N.; et al. Frequent immune responses to a cancer/testis antigen, CAGE, in patients with microsatellite instability-positive endometrial cancer. Clin. Cancer Res. 2005, 11, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Meistere, I.; Werner, S.; Zayakin, P.; Siliņa, K.; Rulle, U.; Pismennaja, A.; Šantare, D.; Kikuste, I.; Isajevs, S.; Leja, M.; et al. The Prevalence of Cancer-Associated Autoantibodies in Patients with Gastric Cancer and Progressive Grades of Premalignant Lesions. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Liggins, A.P.; Lim, S.H.; Soilleux, E.J.; Pulford, K.; Banham, A.H. A panel of cancer-testis genes exhibiting broad-spectrum expression in haematological malignancies. Cancer Immun. 2010, 10, 8. [Google Scholar] [PubMed]
- Lee, T.S.; Kim, J.W.; Kang, G.H.; Park, N.H.; Song, Y.S.; Kang, S.B.; Lee, H.P. DNA hypomethylation of CAGE promotors in squamous cell carcinoma of uterine cervix. Ann. N. Y. Acad. Sci. 2006, 1091, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Por, E.; Byun, H.J.; Lee, E.J.; Lim, J.H.; Jung, S.Y.; Park, I.; Kim, Y.M.; Jeoung, D.I.; Lee, H. The cancer/testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1- and E2F-dependent manner. J. Biol. Chem. 2010, 285, 14475–14485. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, H.; Park, D.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Kim, Y.M.; Jeoung, D. Cancer/testis antigen CAGE exerts negative regulation on p53 expression through HDAC2 and confers resistance to anti-cancer drugs. J. Biol. Chem. 2010, 285, 25957–25968. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, D.; Kim, H.; Choi, M.; Lee, H.; Lee, Y.S.; Choe, J.; Kim, Y.M.; Jeoung, D. miR-200b and cancer/testis antigen CAGE form a feedback loop to regulate the invasion and tumorigenic and angiogenic responses of a cancer cell line to microtubule-targeting drugs. J. Biol. Chem. 2013, 288, 36502–36518. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Park, D.; Lee, H.; Lee, Y.S.; Choe, J.; Kim, Y.M.; Jeon, D.; Jeoung, D. The pentapeptide Gly-Thr-Gly-Lys-Thr confers sensitivity to anti-cancer drugs by inhibition of CAGE binding to GSK3β and decreasing the expression of cyclinD1. Oncotarget 2017, 8, 13632–13651. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Jeoung, D. DDX53 Promotes Cancer Stem Cell-Like Properties and Autophagy. Mol. Cells 2017, 40, 54–65. [Google Scholar] [CrossRef]
- Yeon, M.; Kim, Y.; Pathak, D.; Kwon, E.; Kim, D.Y.; Jeong, M.S.; Jung, H.S.; Jeoung, D. The CAGE-MiR-181b-5p-S1PR1 Axis Regulates Anticancer Drug Resistance and Autophagy in Gastric Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 666387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhou, Z.F. Exosomes derived from Taxol-resistant nasopharyngeal carcinoma (NPC) cells transferred DDX53 to NPC cells and promoted cancer resistance to Taxol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 127–138. [Google Scholar] [PubMed]
- Yang, C.; Croteau, S.; Hardy, P. Histone deacetylase (HDAC) 9: Versatile biological functions and emerging roles in human cancer. Cell. Oncol. 2021, 44, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Wang, Y.S.; Cai, Q.Q.; Wang, J.Q.; Yao, W.T. Up-regulation of HDAC9 promotes cell proliferation through suppressing p53 transcription in osteosarcoma. Int. J. Clin. Exp. Med. 2015, 8, 11818–11823. [Google Scholar] [PubMed]
- Gil, V.S.; Bhagat, G.; Howell, L.; Zhang, J.; Kim, C.H.; Stengel, S.; Vega, F.; Zelent, A.; Petrie, K. Deregulated expression of HDAC9 in B cells promotes development of lymphoproliferative disease and lymphoma in mice. Dis. Models Mech. 2016, 9, 1483–1495. [Google Scholar]
- Xiong, K.; Zhang, H.; Du, Y.; Tian, J.; Ding, S. Identification of HDAC9 as a viable therapeutic target for the treatment of gastric cancer. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hao, F. Knockdown of long non-coding RNA HCP5 suppresses the malignant behavior of retinoblastoma by sponging miR-3619-5p to target HDAC9. Int. J. Mol. Med. 2021, 47, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yan, J.; Li, H.; Meng, L. HDAC9 Silencing Exerts Neuroprotection Against Ischemic Brain Injury via miR-20a-Dependent Downregulation of NeuroD1. Front. Cell. Neurosci. 2021, 14, 544285. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, R.; Saleh, E.; Hamoudi, R.; Ramadan, W.S.; Mazitschek, R.; Nael, M.A.; Elokely, K.M.; Abou-Gharbia, M.; Childers, W.E.; Srinivasulu, V.; et al. Discovery of novel class of histone deacetylase inhibitors as potential anticancer agents. Bioorg. Med. Chem. 2021, 42, 116251. [Google Scholar] [CrossRef]
- Lecce, L.; Xu, Y.; V’Gangula, B.; Chandel, N.; Pothula, V.; Caudrillier, A.; Santini, M.P.; d’Escamard, V.; Ceholski, D.K.; Gorski, P.A.; et al. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J. Clin. Investig. 2021, 131, e131178. [Google Scholar] [CrossRef]
- Kim, B.; Sohn, H.M.; Hyun, H.; Lim, W. Effect of HDAC9 inhibition on epithelial-mesenchymal transition in CD133+ prostate cancer cell lines. J. Chemother. 2022, 34, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Zhou, Y.; Li, L.; Zhao, J.; Qin, W.; Jin, Z.; Liu, W. Increase in HDAC9 suppresses myoblast differentiation via epigenetic regulation of autophagy in hypoxia. Cell Death Dis. 2019, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Pei, Y.C.; Jiang, Y.Z.; Xue, M.Z.; Li, D.Q.; Li, X.G.; Zheng, Y.Z.; Liu, X.Y.; Qiao, F.; Sun, W.L. Truncated HDAC9 identified by integrated genome-wide screen as the key modulator for paclitaxel resistance in triple-negative breast cancer. Theranostics 2020, 10, 11092–11109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cong, X.; Jiang, S.; Dong, Y.; Zhao, L.; Zang, Y.; Tan, M.; Li, J. Phosphoproteomics Reveals AMPK Substrate Network in Response to DNA Damage and Histone Acetylation. Genom. Proteom. Bioinform. 2021, 20, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yang, J.; Liu, X.; Chu, L. Long noncoding RNA CBR3 antisense RNA 1 promotes the aggressive phenotypes of non-small-cell lung cancer by sponging microRNA-509-3p and competitively upregulating HDAC9 expression. Oncol. Rep. 2020, 44, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Liu, J.; Yang, L.; Xue, H.; Bai, S.; Wang, K. PD-L1 combined with HDAC9 is a useful prognostic predictor in hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fei, Y.; Zhao, Y.; Chen, Q.; Chen, P.; Pan, L. Expression and prognostic analyses of HDACs in human gastric cancer based on bioinformatic analysis. Medicine 2021, 100, e26554. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Shi, Q.; Wang, M.; Li, H.; Yang, X.; Zhang, J. MicroRNA-936 inhibits the malignant phenotype of retinoblastoma by directly targeting HDAC9 and deactivating the PI3K/AKT pathway. Oncol. Rep. 2020, 43, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Kanki, K.; Watanabe, R.; Thai, L.N.; Zhao, C.H.; Naito, K. HDAC9 Is Preferentially Expressed in Dedifferentiated Hepatocellular Carcinoma Cells and Is Involved in an Anchorage-Independent Growth. Cancers 2020, 12, 2734. [Google Scholar] [CrossRef]
- Huang, N.; Xu, C.; Deng, L.; Li, X.; Bian, Z.; Zhang, Y.; Long, S.; Chen, Y.; Zhen, N.; Li, G. PAICS contributes to gastric carcinogenesis and participates in DNA damage response by interacting with histone deacetylase 1/2. Cell Death Dis. 2020, 11, 507. [Google Scholar] [CrossRef]
- Sun, J.; Piao, J.; Li, N.; Yang, Y.; Kim, K.Y.; Lin, Z. Valproic acid targets HDAC1/2 and HDAC1/PTEN/Akt signalling to inhibit cell proliferation via the induction of autophagy in gastric cancer. FEBS J. 2020, 287, 2118–2133. [Google Scholar] [CrossRef] [PubMed]
- Orenay-Boyacioglu, S.; Kasap, E.; Gerceker, E.; Yuceyar, H.; Demirci, U.; Bilgic, F.; Korkmaz, M. Expression profiles of histone modification genes in gastric cancer progression. Mol. Biol. Rep. 2018, 45, 2275–2282. [Google Scholar] [CrossRef]
- Dong, J.; Zheng, N.; Wang, X.; Tang, C.; Yan, P.; Zhou, H.B.; Huang, J. A novel HDAC6 inhibitor exerts an anti-cancereffect by triggering cell cycle arrest and apoptosis in gastric cancer. Eur. J. Pharmacol. 2018, 828, 67–79. [Google Scholar] [CrossRef]
- Jo, H.; Shim, K.; Jeoung, D. Targeting HDAC6 to Overcome Autophagy-Promoted Anti-Cancer Drug Resistance. Int. J. Mol. Sci. 2022, 23, 9592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, W.; Ji, C.; Wang, X.; Yu, Y.; Deng, X.; Zhou, X.; Fang, L. Circular RNA circRPPH1 promotes breast cancer progression via circRPPH1-miR-512-5p-STAT1 axis. Cell Death Discov. 2021, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Shen, C.; Yu, C.; Yang, X.; Shao, J.; Guo, J.; Zhu, X.; Zhou, G. Long non-coding RNA LINC00997 silencing inhibits the progression and metastasis of colorectal cancer by sponging miR-512-3p. Bioengineered 2021, 12, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Zhu, L.; Yang, Y.; Yin, X. YY1-Induced lncRNA PART1 Enhanced Resistance of Ovarian Cancer Cells to Cisplatin by Regulating miR-512-3p/CHRAC1 Axis. DNA Cell Biol. 2021, 40, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Suzuki, H.; Tsugawa, H.; Nakagawa, I.; Matsuzaki, J.; Kanai, Y.; Hibi, T. Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene 2009, 28, 2738–2744. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liao, J.; Hu, D.; Su, Y.; Lin, H.; Lin, K.; Luo, X.; Zheng, X.; Zhang, L.; Huang, T. SNHG8 Promotes the Progression of Epstein-Barr Virus-Associated Gastric Cancer via SpongingmiR-512-5p and Targeting TRIM28. Front. Oncol. 2021, 11, 734694. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wu, M.; Lv, S.; Hu, Q.; Xu, W.; Zeng, A.; Huang, K.; Zhu, X. Exosomes derived from microRNA-512-5p-transfected bone mesenchymal stem cells inhibit glioblastoma progression by targeting JAG1. Aging 2021, 13, 9911–9926. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Han, Y.; Zhao, Y.; Zhang, H. miR-512-3p Overcomes Resistance to Cisplatin in Retinoblastoma by Promoting Apoptosis Induced by Endoplasmic Reticulum Stress. Med. Sci. Monit. 2020, 26, e923817. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Gong, K.K.; Yang, L.J.; Dai, J.J.; Zhang, Q.; Wang, F.; Li, X.L.; Xi, S.C.; Du, J.; Scutellariabarbata, D. Don extraction selectively targets stemness-prone NSCLC cells by attenuating SOX2/SMO/GLI1 network loop. J. Ethnopharmacol. 2021, 265, 113295. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.L.P.A.; Bakthavachalam, V.; Vudutha, M.; Vinjamuri, S.; Chinnapaka, S.; Munirathinam, G. Eprinomectin, a novel semi-synthetic macrocylic lactone is cytotoxic to PC3 metastatic prostate cancer cells via inducing apoptosis. Toxicol. Appl. Pharmacol. 2020, 401, 115071. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, H.; Liang, W.; Jiang, E.; Zhou, X.; Shao, Z.; Liu, K.; Shang, Z. Autophagy regulates the cancer stem cell phenotype of head and neck squamous cell carcinoma through the noncanonical FOXO3/SOX2 axis. Oncogene 2022, 41, 634–646. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mukherjee, S.; Khan, P.; Banerjee, S.; Dutta, A.; Banerjee, N.; Sengupta, D.; Basak, U.; Chakraborty, S.; Dutta, A.; et al. SMAR1 repression by pluripotency factors and consequent chemoresistance in breast cancer stem-like cells is reversed by aspirin. Sci. Signal 2020, 13, eaay6077. [Google Scholar] [CrossRef]
- Wu, R.; Wang, C.; Li, Z.; Xiao, J.; Li, C.; Wang, X.; Kong, P.; Cao, J.; Huang, F.; Li, Z. SOX2 promotes resistance of melanoma with PD-L1 high expression to T-cell-mediated cytotoxicity that can be reversed by SAHA. J. Immunother. Cancer 2020, 8, e001037. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Y.; Yang, Y.; Zhang, Y.; Yue, Z.; Pan, Z.; Ren, X. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed. Pharmacother. 2017, 96, 378–383. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeon, M.; Kwon, N.; Jeoung, J.; Jeoung, D. HDAC9 and miR-512 Regulate CAGE-Promoted Anti-Cancer Drug Resistance and Cellular Proliferation. Curr. Issues Mol. Biol. 2024, 46, 5178-5193. https://doi.org/10.3390/cimb46060311
Yeon M, Kwon N, Jeoung J, Jeoung D. HDAC9 and miR-512 Regulate CAGE-Promoted Anti-Cancer Drug Resistance and Cellular Proliferation. Current Issues in Molecular Biology. 2024; 46(6):5178-5193. https://doi.org/10.3390/cimb46060311
Chicago/Turabian StyleYeon, Minjeong, Nayeon Kwon, Jaewhoon Jeoung, and Dooil Jeoung. 2024. "HDAC9 and miR-512 Regulate CAGE-Promoted Anti-Cancer Drug Resistance and Cellular Proliferation" Current Issues in Molecular Biology 46, no. 6: 5178-5193. https://doi.org/10.3390/cimb46060311
APA StyleYeon, M., Kwon, N., Jeoung, J., & Jeoung, D. (2024). HDAC9 and miR-512 Regulate CAGE-Promoted Anti-Cancer Drug Resistance and Cellular Proliferation. Current Issues in Molecular Biology, 46(6), 5178-5193. https://doi.org/10.3390/cimb46060311






