Unraveling the Multifaceted Role of the miR-17-92 Cluster in Colorectal Cancer: From Mechanisms to Biomarker Potential
Abstract
1. Introduction
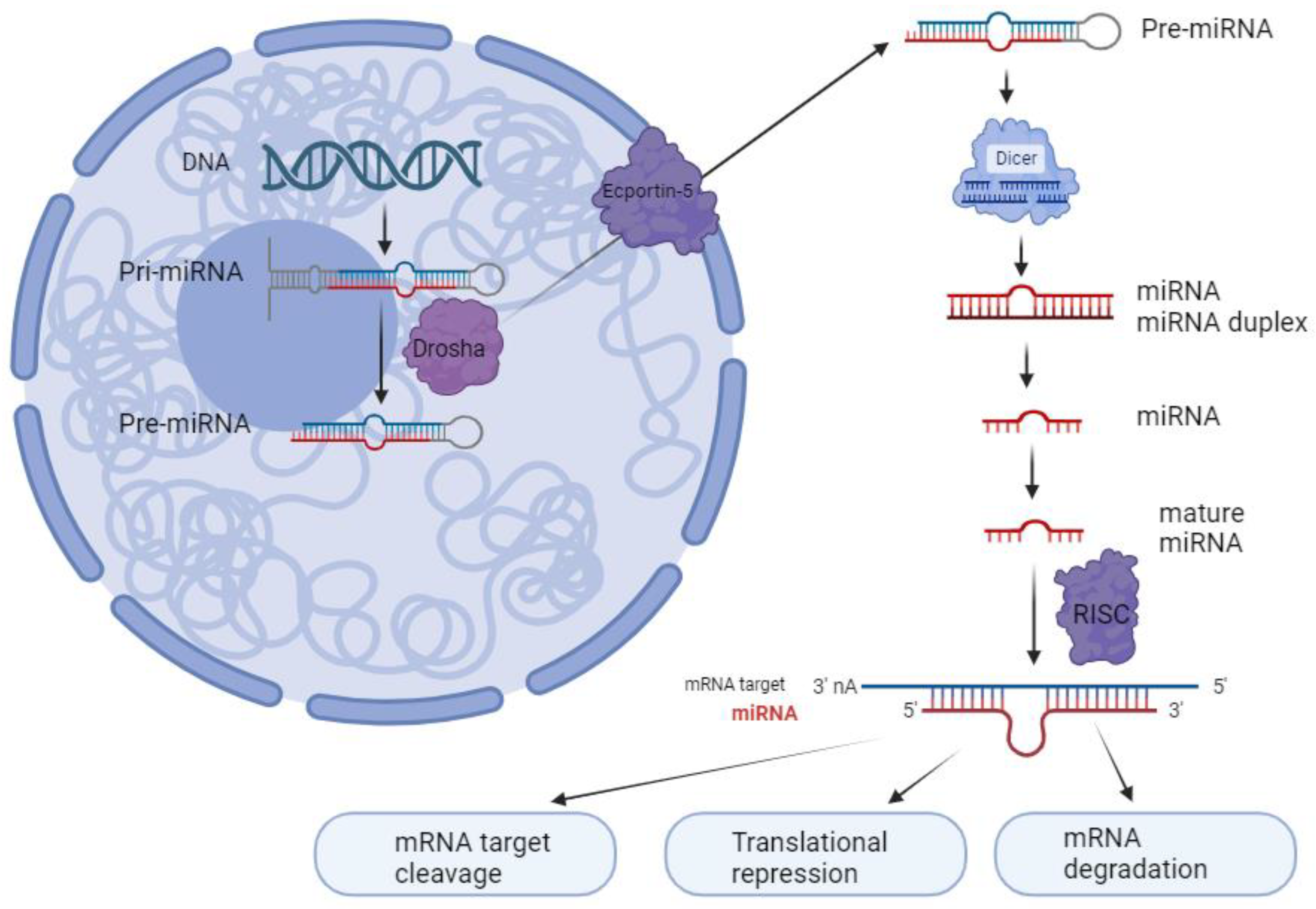
2. miRNA Biology and Biogenesis
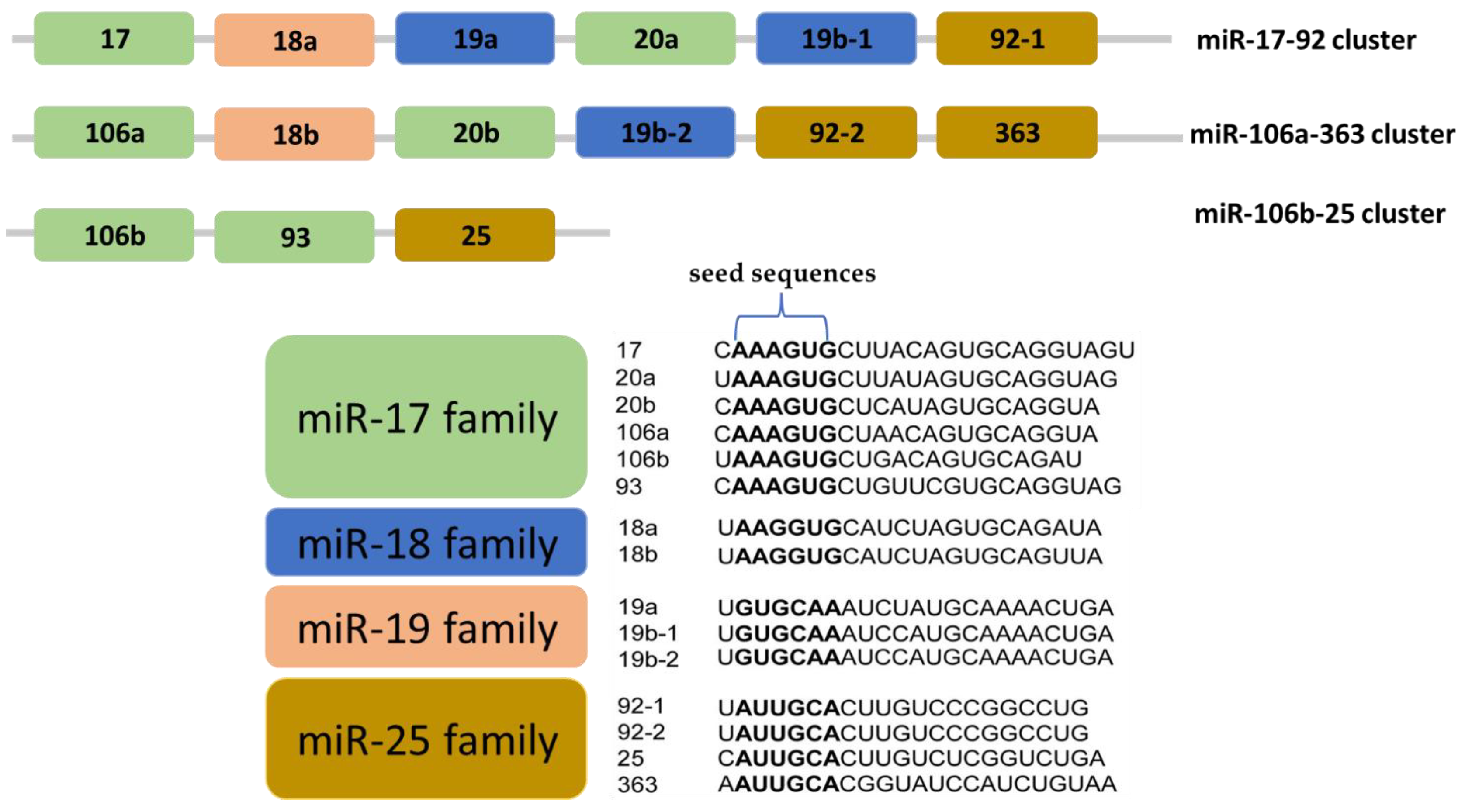
3. Structure and Organization of the miR-17-92 Cluster Families
4. Mechanisms Underlying Altered miR-17-92 Expression in Colorectal Cancer
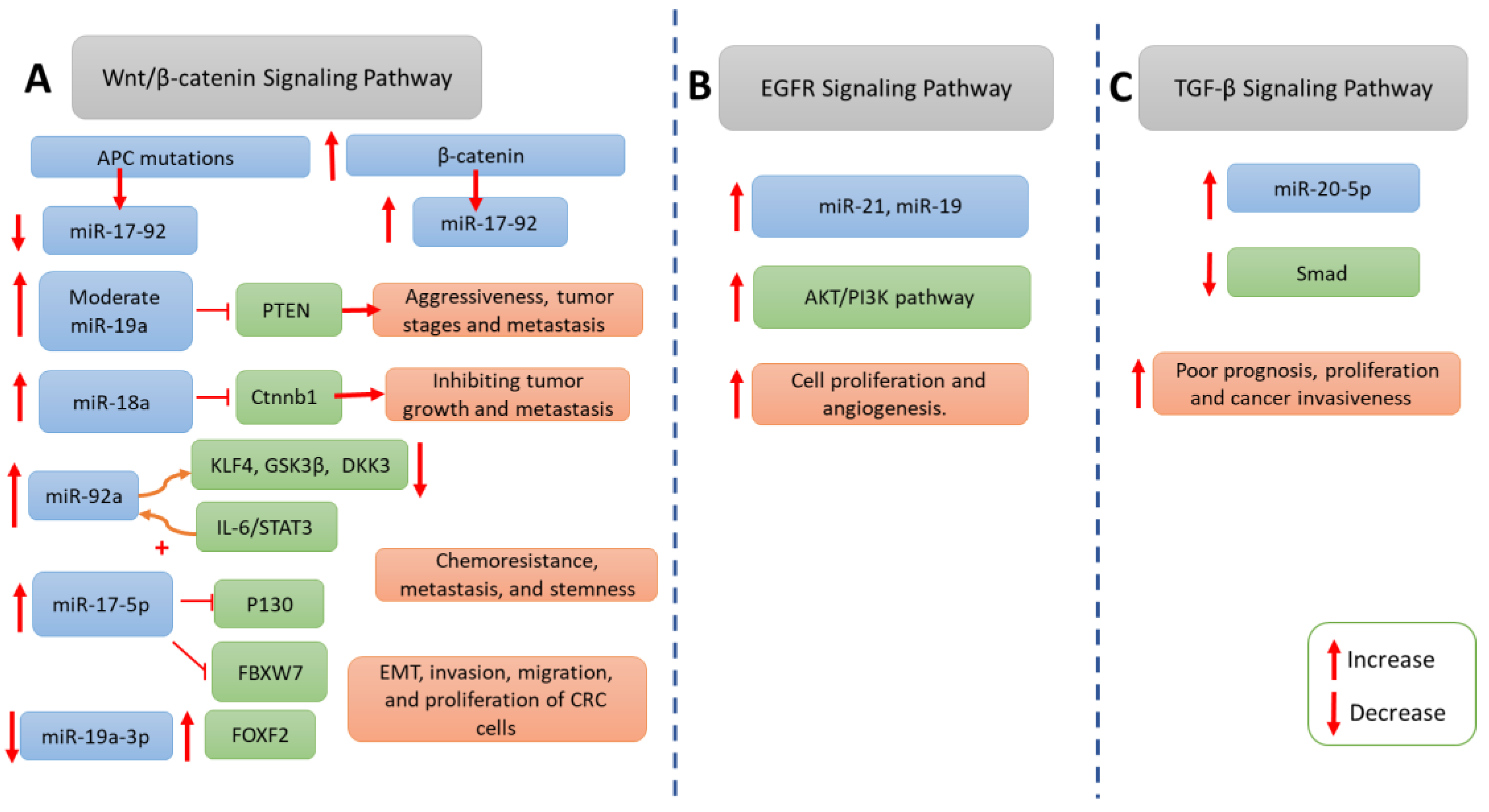
5. Involvement of the miR-17-92 Cluster in Signaling Pathways in Colorectal Cancer Pathogenesis
5.1. Wnt/β-catenin Pathway
5.2. Epidermal Growth Factor Receptor Signaling Pathway
5.3. Extracellular Matrix Breakdown and Epithelial–Mesenchymal Transition
5.4. TGF-β Signaling Pathway
6. The Role of miR-17-92 in Colorectal Tumorigenesis
6.1. Tumor Initiation and Growth
6.2. Stimulation of Angiogenesis
6.3. Metastasis
6.4. Involvement in Epithelial–Mesenchymal Transition (EMT)
6.5. Role of miR-17-92 in Responsiveness and Resistance to Therapy
| miRNA | Dysregulation | Targets | Pathway/Effects | Experimental Validation | References |
|---|---|---|---|---|---|
| miR-19a | Upregulation | β-catenin/APC mutation | Wnt/β-catenin pathway | Luciferase reporter vectors and protein expression changes | [31] |
| miR-19a | Moderate | PTEN | Luciferase assays | [32] | |
| miR-92a | Upregulation | KLF4, GSK3β, and DKK3 | Negative regulators of the Wnt/β-catenin pathway | Luciferase assays | [28] |
| miR-17-5p | P130 | Activating the Wnt/β-catenin pathway | Luciferase assays | [34] | |
| miR-19a-3p | Upregulation | FOXF2 | Increase EMT, invasion, migration, and proliferation of CRC cells Activating the Wnt/β-catenin pathway | Dual luciferase reporter assay | [35] |
| miR-20a | Upregulation | - | metabolic CRC Activating the Wnt/β-catenin pathway | - | [36] |
| miR-19b | Upregulation | FBXW7 | Radio-resistance and stemness properties | Dual luciferase reporter assay | [37] |
| miR-21, miR-19, and miR-96 | Upregulation | - | AKT/PIK3 pathway | - | [43] |
| microRNA-20–5p | Upregulation | Smad-4 | TGF-b signaling pathway | Luciferase reporter assay | [47] |
| miR-92a | Upregulation | BCL-2-interacting mediator of cell death (BIM) | Promotion of cell proliferation and the inhibition of apoptosis | Protein expression changes | [7] |
| miR-92a | Upregulation | KLF4, p21, | Increase in the proliferation and migration capacity | Bioinformatics and luciferase reporter analysis and protein expression changes | [48] |
| miR-17-92 | Upregulation | TGFBR2, HIF1α, and VEGFA | Angiogenesis | - | [50] |
| miR-17-92 | Upregulation | suppress Tsp1 and CTGF | Angiogenesis | Protein expression changes | [5] |
| miR-92a-3p | Upregulation | DKK3 and claudin-11 | Promote angiogenesis | Luciferase reporter assay | [51] |
| miR-92a-3p | Upregulation | - | RECK-MMP signaling pathway increase invasion and migration | - | [59] |
| miR-17-3p and miR-92a | Upregulation | - | Metastasis | - | [61,62] |
| miRNA-17 -5p | Down-regulation | vimentin | EMT | Luciferase reporter vectors and protein expression changes | [67] |
| miR-92a-3p | Upregulation | FBXW7 and MOAP1 | Enhanced cell stemness, EMT, metastasis and resistance against 5 FU/L-OHP treatment | Luciferase report assay, real-time qPCR, Western blot | [68] |
| miR-17-5p | Upregulation | MFN2 METTL14 l | 5-FU chemotherapy resistance reduced apoptosis and decreased drug sensitivity | Luciferase reporter as-say | [69] |
| miRNA20a-5p, miRNA17-5p, miRNA19a-3p | Upregulation | PTEN, ERK, and EGFR | 5-FU chemotherapy responsive | Protein expression level changes | [71] |
7. miR-17-92 as a Diagnostic and Prognostic Biomarker
7.1. Utility of miR-17-92 as an Early Diagnostic Tool for CRC
7.2. Predictive Value of miR-17-92 Expression in CRC Prognosis
8. The Role of Circulating the miR-17-92 Cluster in Plasma and Serum for CRC Diagnosis
9. Fecal miR-17-92 Cluster for CRC Diagnosis
10. Conclusions
11. Future Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rattray, N.J.W.; Charkoftaki, G.; Rattray, Z.; Hansen, J.E.; Vasiliou, V.; Johnson, C.H. Environmental influences in the etiology of colorectal cancer: The premise of metabolomics. Curr. Pharmacol. Rep. 2017, 3, 114–125. [Google Scholar] [CrossRef]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Dews, M.; Homayouni, A.; Yu, D.; Murphy, D.; Sevignani, C.; Wentzel, E.; Furth, E.E.; Lee, W.M.; Enders, G.H.; Mendell, J.T.; et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat. Genet. 2006, 38, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Inoue, H.; Takatsuno, Y.; Tanaka, F.; Mimori, K.; Uetake, H.; Sugihara, K.; Mori, M. Over- and under-expressed microRNAs in human colorectal cancer. Int. J. Oncol. 2009, 34, 1069–1075. [Google Scholar] [CrossRef][Green Version]
- Tsuchida, A.; Ohno, S.; Wu, W.; Borjigin, N.; Fujita, K.; Aoki, T.; Ueda, S.; Takanashi, M.; Kuroda, M. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011, 102, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Tang, J.-Q.; Tian, M.-L.; Li, H.; Wang, X.; Wu, T.; Zhu, J.; Huang, S.-J.; Wan, Y.-L. Prognostic values of the miR-17-92 cluster and its paralogs in colon cancer. J. Surg. Oncol. 2012, 106, 232–237. [Google Scholar] [CrossRef]
- Ng, E.K.; Chong, W.W.; Jin, H.; Lam, E.K.; Shin, V.Y.; Yu, J.; Poon, T.C.; Ng, S.S.; Sung, J.J. Differential expression of microRNAs in plasma of patients with colorectal cancer: A potential marker for colorectal cancer screening. Gut 2009, 58, 1375–1381. [Google Scholar] [CrossRef]
- Reid, J.F.; Sokolova, V.; Zoni, E.; Lampis, A.; Pizzamiglio, S.; Bertan, C.; Zanutto, S.; Perrone, F.; Camerini, T.; Gallino, G. miRNA profiling in colorectal cancer highlights miR-1 involvement in MET-dependent proliferation. Mol. Cancer Res. MCR 2012, 10, 504–515. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.J.; Plasterk, R.H.A.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Adachi, Y.; Taniguchi, H.; Kunimoto, H.; Nosho, K.; Suzuki, H.; Shinomura, Y. Interrelationship between microsatellite instability and microRNA in gastrointestinal cancer. World J. Gastroenterol. 2012, 18, 2745–2755. [Google Scholar] [CrossRef]
- Al-Nakhle, H.; Burns, P.A.; Cummings, M.; Hanby, A.M.; Hughes, T.A.; Satheesha, S.; Shaaban, A.M.; Smith, L.; Speirs, V. Estrogen receptor {beta}1 expression is regulated by miR-92 in breast cancer. Cancer Res. 2010, 70, 4778–4784. [Google Scholar] [CrossRef]
- Wang, C.; Gao, C.; Zhuang, J.-L.; Ding, C.; Wang, Y. A combined approach identifies three mRNAs that are down-regulated by microRNA-29b and promote invasion ability in the breast cancer cell line MCF-7. J. Cancer Res. Clin. Oncol. 2012, 138, 2127–2136. [Google Scholar] [CrossRef]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Diosdado, B.; van de Wiel, M.A.; Terhaar Sive Droste, J.S.; Mongera, S.; Postma, C.; Meijerink, W.J.H.J.; Carvalho, B.; Meijer, G.A. MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br. J. Cancer 2009, 101, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Smith, A.L.; Iwanaga, R.; Drasin, D.J.; Micalizzi, D.S.; Vartuli, R.L.; Tan, A.-C.; Ford, H.L. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene 2012, 31, 5162–5171. [Google Scholar] [CrossRef]
- Li, Y.; Tan, W.; Neo, T.W.L.; Aung, M.O.; Wasser, S.; Lim, S.G.; Tan, T.M.C. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009, 100, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, G.; Zhang, Q.; Wu, Q.; Li, B.; Zhang, Z. MicroRNA-20b promotes cell growth of breast cancer cells partly via targeting phosphatase and tensin homologue (PTEN). Cell Biosci. 2014, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Iijima, T.; Wakaume, R.; Takahashi, K.; Matsumoto, H.; Nakano, D.; Nakayama, Y.; Mori, T.; Horiguchi, S.; Miyaki, M. Underexpression of miR-126 and miR-20b in Hereditary and Nonhereditary Colorectal Tumors. Oncology 2014, 87, 58–66. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Cao, W.; Wang, X.; Xu, Q.; Yan, M.; Wu, X.; Chen, W. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int. J. Biochem. Cell Biol. 2013, 45, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Huang, G.; Zhao, Y.; Zhong, D.; Xu, Z.; Zeng, Y.; Zhang, Y.; Li, S.; He, F. MicroRNA-363-mediated downregulation of S1PR1 suppresses the proliferation of hepatocellular carcinoma cells. Cell. Signal. 2014, 26, 1347–1354. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted Deletion Reveals Essential and Overlapping Functions of the miR-17-92 Family of miRNA Clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef]
- Martens-de Kemp, S.R.; Komor, M.A.; Hegi, R.; Bolijn, A.S.; Tijssen, M.; de Groen, F.L.; Depla, A.; van Leerdam, M.; Meijer, G.A.; Fijneman, R.J.; et al. Overexpression of the miR-17-92 cluster in colorectal adenoma organoids causes a carcinoma-like gene expression signature. Neoplasia 2022, 32, 100820. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Li, L.-F.; Yang, G.-D.; Xia, S.-S.; Wang, R.; Leng, Z.-W.; Liu, Z.-L.; Tian, H.-P.; He, Y.; Meng, C.-Y.; et al. MiR-92a promotes stem cell-like properties by activating Wnt/β-catenin signaling in colorectal cancer. Oncotarget 2017, 8, 101760–101770. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef]
- Disoma, C.; Zhou, Y.; Li, S.; Peng, J.; Xia, Z. Wnt/β-catenin signaling in colorectal cancer: Is therapeutic targeting even possible? Biochimie 2022, 195, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lauriola, M.; Kim, D.; Francesconi, M.; D’Uva, G.; Shibata, D.; Malafa, M.P.; Yeatman, T.J.; Coppola, D.; Solmi, R.; et al. Adenomatous polyposis coli (APC) regulates miR17-92 cluster through β-catenin pathway in colorectal cancer. Oncogene 2016, 35, 4558–4568. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, P.; Wang, Q.; Wang, B.; Mu, J.; Zhuang, X.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Quantitatively Controlling Expression of miR-17-92 Determines Colon Tumor Progression in a Mouse Tumor Model. Am. J. Pathol. 2014, 184, 1355–1368. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, B.; Han, W.; Zhao, D. LncRNA-MIR17HG mediated upregulation of miR-17 and miR-18a promotes colon cancer progression via activating Wnt/β-catenin signaling. Transl. Cancer Res. 2019, 8, 1097–1108. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Zhang, H.; Yang, Y.; Shi, C.; Xia, Y.; Peng, J.; Liu, W.; Yang, Z.; et al. Elevated oncofoetal miR-17-5p expression regulates colorectal cancer progression by repressing its target gene P130. Nat. Commun. 2012, 3, 1291. [Google Scholar] [CrossRef]
- Yu, F.-B.; Sheng, J.; Yu, J.-M.; Liu, J.-H.; Qin, X.-X.; Mou, B. MiR-19a-3p regulates the Forkhead box F2-mediated Wnt/β-catenin signaling pathway and affects the biological functions of colorectal cancer cells. World J. Gastroenterol. 2020, 26, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, C.; Zhang, J.; Yao, Y.; Xiao, H.; Yuan, R.; Li, K.; Yang, J.; Zhao, W.; Zhang, Y. Integrated multi-omics analysis reveals miR-20a as a regulator for metabolic colorectal cancer. Heliyon 2022, 8, e09068. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yin, Y.-F.; Jin, H.-G.; Liu, H.-R.; Tian, W.-C. Exosomal microRNA-19b targets FBXW7 to promote colorectal cancer stem cell stemness and induce resistance to radiotherapy. Kaohsiung J. Med. Sci. 2022, 38, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Hu, W.; Feng, J.; Geng, Y. Promotion or remission: A role of noncoding RNAs in colorectal cancer resistance to anti-EGFR therapy. Cell Commun. Signal. CCS 2022, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Oliveira, C.; Ferreira, A.; Ferreira, A.C.; Suriano, G.; Schwartz, S.J.; Duval, A.; Carneiro, F.; Machado, J.C.; Hamelin, R.; et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur. J. Cancer 2005, 41, 1649–1654. [Google Scholar] [CrossRef]
- Fang, L.; Li, H.; Wang, L.; Hu, J.; Jin, T.; Wang, J.; Yang, B.B. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget 2014, 5, 2974–2987. [Google Scholar] [CrossRef]
- Michas, A.; Michas, V.; Anagnostou, E.; Galanopoulos, M.; Tolia, M.; Tsoukalas, N. The Clinical Significance of MicroRNAs in Colorectal Cancer Signaling Pathways: A Review. Glob. Med. Genet. 2023, 10, 315–323. [Google Scholar] [CrossRef]
- Cellura, D.; Pickard, K.; Quaratino, S.; Parker, H.; Strefford, J.C.; Thomas, G.J.; Mitter, R.; Mirnezami, A.H.; Peake, N.J. miR-19-Mediated Inhibition of Transglutaminase-2 Leads to Enhanced Invasion and Metastasis in Colorectal Cancer. Mol. Cancer Res. MCR 2015, 13, 1095–1105. [Google Scholar] [CrossRef]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Kuo, P.-H.; Liu, Y.-L.; Yang, A.C.; Tsai, S.-J. Transforming growth factor-β signaling pathway-associated genes SMAD2 and TGFBR2 are implicated in metabolic syndrome in a Taiwanese population. Sci. Rep. 2017, 7, 13589. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhao, S.; Tang, H.; Zhang, D.; Sun, H.; Yu, F.; Jiang, W.; Yue, B.; Wang, J.; Zhang, M. MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4. Oncotarget 2016, 7, 45199–45213. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, Z.; Wang, Y.; Li, C.; Gong, W.; Wang, X. MicroRNA-92a Promotes Colorectal Cancer Cell Growth and Migration by Inhibiting KLF4. Oncol. Res. 2016, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Ma, H.; Pan, J.-S.; Jin, L.-X.; Wu, J.; Ren, Y.-D.; Chen, P.; Xiao, C.; Han, J. MicroRNA-17~92 inhibits colorectal cancer progression by targeting angiogenesis. Cancer Lett. 2016, 376, 293–302. [Google Scholar] [CrossRef]
- Yamada, N.O.; Heishima, K.; Akao, Y.; Senda, T. Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4406. [Google Scholar] [CrossRef] [PubMed]
- Lorsy, E.; Topuz, A.S.; Geisler, C.; Stahl, S.; Garczyk, S.; von Stillfried, S.; Hoss, M.; Gluz, O.; Hartmann, A.; Knüchel, R.; et al. Loss of Dickkopf 3 Promotes the Tumorigenesis of Basal Breast Cancer. PLoS ONE 2016, 11, e0160077. [Google Scholar] [CrossRef] [PubMed]
- Al Shareef, Z.; Kardooni, H.; Murillo-Garzón, V.; Domenici, G.; Stylianakis, E.; Steel, J.H.; Rabano, M.; Gorroño-Etxebarria, I.; Zabalza, I.; Vivanco, M.d.M.; et al. Protective effect of stromal Dickkopf-3 in prostate cancer: Opposing roles for TGFBI and ECM-1. Oncogene 2018, 37, 5305–5324. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qin, W. DKK3 blocked translocation of β-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J. Cell. Mol. Med. 2015, 19, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Husted, H.; Moore, T.; Lu, M.; Deng, D.; Liu, Y.; Ramachandran, V.; Arumugam, T.; Niehrs, C.; Wang, H.; et al. Suppression of stromal-derived Dickkopf-3 (DKK3) inhibits tumor progression and prolongs survival in pancreatic ductal adenocarcinoma. Sci. Transl. Med. 2018, 10, eaat3487. [Google Scholar] [CrossRef] [PubMed]
- Busceti, C.L.; Marchitti, S.; Bianchi, F.; Di Pietro, P.; Riozzi, B.; Stanzione, R.; Cannella, M.; Battaglia, G.; Bruno, V.; Volpe, M.; et al. Dickkopf-3 Upregulates VEGF in Cultured Human Endothelial Cells by Activating Activin Receptor-Like Kinase 1 (ALK1) Pathway. Front. Pharmacol. 2017, 8, 111. [Google Scholar] [CrossRef][Green Version]
- Zitt, M.; Untergasser, G.; Amberger, A.; Moser, P.; Stadlmann, S.; Zitt, M.; Müller, H.M.; Mühlmann, G.; Perathoner, A.; Margreiter, R.; et al. Dickkopf-3 as a new potential marker for neoangiogenesis in colorectal cancer: Expression in cancer tissue and adjacent non-cancerous tissue. Dis. Markers 2008, 24, 101–109. [Google Scholar] [CrossRef]
- Safari, E.; Mosayebi, G.; Khorram, S. Dkk-3 as a potential biomarker for diagnosis and prognosis of colorectal cancer. Med. J. Islam. Repub. Iran 2018, 32, 86. [Google Scholar] [CrossRef]
- Wei, Q.-D.; Zheng, W.-B.; Sun, K.; Xue, Q.; Yang, C.-Z.; Li, G.-X. MiR-92a promotes the invasion and migration of colorectal cancer by targeting RECK. Int. J. Clin. Exp. Pathol. 2019, 12, 1565–1577. [Google Scholar]
- Pidíková, P.; Herichová, I. miRNA Clusters with Up-Regulated Expression in Colorectal Cancer. Cancers 2021, 13, 2979. [Google Scholar] [CrossRef]
- Jepsen, R.K.; Novotny, G.W.; Klarskov, L.L.; Bang-Berthelsen, C.H.; Haakansson, I.T.; Hansen, A.; Christensen, I.J.; Riis, L.B.; Høgdall, E. Early metastatic colorectal cancers show increased tissue expression of miR-17/92 cluster members in the invasive tumor front. Hum. Pathol. 2018, 80, 231–238. [Google Scholar] [CrossRef]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, G.; Liu, Z.; Xia, S.; Tian, H. Overexpression of miR-92a correlates with tumor metastasis and poor prognosis in patients with colorectal cancer. Int. J. Color. Dis. 2013, 28, 19–24. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Zhou, H.; Xiao, H.; Zhou, T. miR-20a is an independent prognostic factor in colorectal cancer and is involved in cell metastasis. Mol. Med. Rep. 2014, 10, 283–291. [Google Scholar] [CrossRef]
- Lai, H.; Zhang, J.; Zuo, H.; Liu, H.; Xu, J.; Feng, Y.; Lin, Y.; Mo, X. Overexpression of miR-17 is correlated with liver metastasis in colorectal cancer. Medicine 2020, 99, e19265. [Google Scholar] [CrossRef]
- Ke, T.-W.; Wei, P.-L.; Yeh, K.-T.; Chen, W.T.-L.; Cheng, Y.-W. MiR-92a Promotes Cell Metastasis of Colorectal Cancer Through PTEN-Mediated PI3K/AKT Pathway. Ann. Surg. Oncol. 2015, 22, 2649–2655. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, Y.S.; Yun, N.H.; Shin, C.H.; Hong, H.K.; Kim, H.H.; Cho, Y.B. MicroRNA-17-5p regulates EMT by targeting vimentin in colorectal cancer. Br. J. Cancer 2020, 123, 1123–1130. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, L.; Li, Y.; Huang, B.; Yan, Q.; Wu, C.; Lai, Q.; Fang, Y.; Cai, J.; Liu, Y.; et al. METTL14-dependent maturation of pri-miR-17 regulates mitochondrial homeostasis and induces chemoresistance in colorectal cancer. Cell Death Dis. 2023, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Ooyama, A.; Yamamoto, M.; Ikeda, R.; Haraguchi, M.; Tabata, S.; Furukawa, T.; Che, X.-F.; Iwashita, K.; Oka, T.; et al. Down regulation of c-Myc and induction of an angiogenesis inhibitor, thrombospondin-1, by 5-FU in human colon cancer KM12C cells. Cancer Lett. 2008, 270, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Badr, D.; Fouad, M.A.; Hussein, M.; Salem, S.; Zekri, A.; Shouman, S. Rebound increase in microRNA levels at the end of 5-FU-based therapy in colorectal cancer patients. Sci. Rep. 2023, 13, 14237. [Google Scholar] [CrossRef] [PubMed]
- Khoury, S.; Tran, N. Circulating microRNAs: Potential biomarkers for common malignancies. Biomark. Med. 2015, 9, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.V.; Galbraith, N.J.; Yang, D.; Burton, J.F.; Walker, S.P.; Galandiuk, S. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: A systematic review and meta-analysis. Br. J. Cancer 2017, 116, 762–774. [Google Scholar] [CrossRef]
- Toiyama, Y.; Okugawa, Y.; Fleshman, J.; Boland, C.R.; Goel, A. MicroRNAs as Potential Liquid Biopsy Biomarkers in Colorectal Cancer: A Systematic Review. Biochim. Biophys. Acta Rev. Cancer 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- de Miguel Pérez, D.; Rodriguez Martínez, A.; Ortigosa Palomo, A.; Delgado Ureña, M.; Garcia Puche, J.L.; Robles Remacho, A.; Exposito Hernandez, J.; Lorente Acosta, J.A.; Ortega Sánchez, F.G.; Serrano, M.J. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sun, W.; Liu, R.; Zhou, Z.; Zhang, H.; Chen, X.; Ba, Y. Plasma Exosomal miRNA Expression Profile as Oxaliplatin-Based Chemoresistant Biomarkers in Colorectal Adenocarcinoma. Front. Oncol. 2020, 10, 1495. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, D.; Ni, S.; Peng, Z.; Sheng, W.; Du, X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer 2010, 127, 118–126. [Google Scholar] [CrossRef]
- Liu, G.-H.; Zhou, Z.-G.; Chen, R.; Wang, M.-J.; Zhou, B.; Li, Y.; Sun, X.-F. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2013, 34, 2175–2181. [Google Scholar] [CrossRef]
- Wu, C.W.; Ng, S.S.M.; Dong, Y.J.; Ng, S.C.; Leung, W.W.; Lee, C.W.; Wong, Y.N.; Chan, F.K.L.; Yu, J.; Sung, J.J.Y. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut 2012, 61, 739–745. [Google Scholar] [CrossRef]
- Yau, T.O.; Wu, C.W.; Dong, Y.; Tang, C.-M.; Ng, S.S.M.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. microRNA-221 and microRNA-18a identification in stool as potential biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br. J. Cancer 2014, 111, 1765–1771. [Google Scholar] [CrossRef]
- Yau, T.O.; Wu, C.W.; Tang, C.-M.; Chen, Y.; Fang, J.; Dong, Y.; Liang, Q.; Ng, S.S.M.; Chan, F.K.L.; Sung, J.J.Y.; et al. MicroRNA-20a in human faeces as a non-invasive biomarker for colorectal cancer. Oncotarget 2016, 7, 1559–1568. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Chen, C.-C.; Chang, Y.-S.; Tsai, W.-S.; You, J.-F.; Lin, G.-P.; Chen, T.-W.; Chen, J.-S.; Chan, E.-C. MicroRNA-223 and microRNA-92a in stool and plasma samples act as complementary biomarkers to increase colorectal cancer detection. Oncotarget 2016, 7, 10663–10675. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Yasunaga, M.; Takahashi, A.; Kuroda, J.; Moriya, Y.; Akasu, T.; Fujita, S.; Yamamoto, S.; Baba, H.; Matsumura, Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev. Res. 2010, 3, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.H.; Cho, Y.-S.; Choi, J.H.; Kim, H.-K.; Kim, S.S.; Chae, H.-S. Stool-Based miR-92a and miR-144* as Noninvasive Biomarkers for Colorectal Cancer Screening. Oncology 2019, 97, 173–179. [Google Scholar] [CrossRef]
- Rotelli, M.T.; Di Lena, M.; Cavallini, A.; Lippolis, C.; Bonfrate, L.; Chetta, N.; Portincasa, P.; Altomare, D.F. Fecal microRNA profile in patients with colorectal carcinoma before and after curative surgery. Int. J. Color. Dis. 2015, 30, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Opdenaker, L.; Modarai, S.; Fields, J.Z.; Gonye, G.; Boman, B.M. MicroRNA Expression Profiling of Normal and Malignant Human Colonic Stem Cells Identifies miRNA92a as a Regulator of the LRIG1 Stem Cell Gene. Int. J. Mol. Sci. 2020, 21, 2804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-nakhle, H.H. Unraveling the Multifaceted Role of the miR-17-92 Cluster in Colorectal Cancer: From Mechanisms to Biomarker Potential. Curr. Issues Mol. Biol. 2024, 46, 1832-1850. https://doi.org/10.3390/cimb46030120
Al-nakhle HH. Unraveling the Multifaceted Role of the miR-17-92 Cluster in Colorectal Cancer: From Mechanisms to Biomarker Potential. Current Issues in Molecular Biology. 2024; 46(3):1832-1850. https://doi.org/10.3390/cimb46030120
Chicago/Turabian StyleAl-nakhle, Hakeemah H. 2024. "Unraveling the Multifaceted Role of the miR-17-92 Cluster in Colorectal Cancer: From Mechanisms to Biomarker Potential" Current Issues in Molecular Biology 46, no. 3: 1832-1850. https://doi.org/10.3390/cimb46030120
APA StyleAl-nakhle, H. H. (2024). Unraveling the Multifaceted Role of the miR-17-92 Cluster in Colorectal Cancer: From Mechanisms to Biomarker Potential. Current Issues in Molecular Biology, 46(3), 1832-1850. https://doi.org/10.3390/cimb46030120





