Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104. [Google Scholar] [CrossRef]
- INCA–Instituto Nacional de Câncer. Estimativa 2023: Incidência de Câncer no Brasil. 2023. Available online: https://www.inca.gov.br/publicacoes/livros/estimativa-2023-incidencia-de-cancer-no-brasil (accessed on 15 May 2023).
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef]
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.R.; et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: Variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut 2021, 70, 114–126. [Google Scholar] [CrossRef]
- Lipsyc-Sharf, M.; Zhang, S.; Ou, F.S.; Ma, C.; McCleary, N.J.; Niedzwiecki, D.; Chang, I.W.; Lenz, H.J.; Blanke, C.D.; Piawah, S.; et al. Survival in Young-Onset Metastatic Colorectal Cancer: Findings from Cancer and Leukemia Group B (Alliance)/SWOG 80405. JNCI J. Natl. Cancer Inst. 2021, 114, 427–435. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.M.; Hu, J.; DeSanto, K.; Gao, D.; Singh, S.; Imperiale, T.; Lieberman, D.A.; Boland, C.R.; Patel, S.G. Early-Age Onset Colorectal Neoplasia in Average-Risk Individuals Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1145–1155.e12. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Rachiglio, A.M.; Roma, C.; Fenizia, F.; Esposito, C.; Pasquale, R.; La Porta, M.L.; Iannaccone, A.; Micheli, F.; Santangelo, M.; et al. Molecular diagnostics and personalized medicine in oncology: Challenges and opportunities. J. Cell. Biochem. 2013, 114, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.A.; Malley, R.; Armstrong, S.A. Molecular Profiling in Metastatic Colorectal Cancer. Oncology 2020, 34, 352–355. [Google Scholar] [CrossRef]
- Guerrero, R.M.; Labajos, V.A.; Ballena, S.L.; Macha, C.A.; Lezama, M.S.; Roman, C.P.; Beltran, P.M.; Torrejon, A.F. Targeting BRAF V600E in metastatic colorectal cancer: Where are we today? Ecancermedicalscience 2022, 16, 1489. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.-M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef]
- Harada, S.; Morlote, D. Molecular Pathology of Colorectal Cancer. Adv. Anat. Pathol. 2020, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-H.; Wu, A.-W. Targeting KRAS G12C mutations in colorectal cancer. Gastroenterol. Rep. 2023, 11, goac083. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.C.; Cookson, R. Multiple inequity in health care: An example from Brazil. Soc. Sci. Med. 2019, 228, 1–8. [Google Scholar] [CrossRef]
- Broeders, M.; Elfström, K.M. Importance of International Networking and Comparative Research in Screening to Meet the Global Challenge of Cancer Control. JCO Glob. Oncol. 2020, 6, 180–181. [Google Scholar] [CrossRef]
- RAS Extension Pyro Kit. Available online: https://www.qiagen.com/us/products/diagnosti2c83s-and-clinical-research/oncology/therascreen-solid-tumor/ras-ext-pyro-kit-row (accessed on 23 November 2023).
- BRAF Pyro Kit. Available online: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/oncology/therascreen-solid-tumor/braf-pyro-kit (accessed on 23 November 2023).
- Dedeurwaerdere, F.; Claes, K.B.; Van Dorpe, J.; Rottiers, I.; Van der Meulen, J.; Breyne, J.; Swaerts, K.; Martens, G. Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer. Sci. Rep. 2021, 11, 12880. [Google Scholar] [CrossRef]
- Kazzi, A.I.M.; Passarini, T.D.M.; Duarte, F.A.; Paes, F.R.; Ferrari, B.L.; Jacome, A.A.D.A. 509P A different epidemiological trend in Brazilian early-onset colorectal cancer (EOCRC): Real-world data from a reference cancer center. Ann. Oncol. 2020, 31, S456. [Google Scholar] [CrossRef]
- Pereira, A.A.L.; Fernandes, G.D.S.; Braga, G.T.P.; Marchetti, K.R.; Mascarenhas, C.D.C.; Gumz, B.; Crosara, M.; Dib, L.; Girardi, D.; Barrichello, A.; et al. Differences in Pathology and Mutation Status among Colorectal Cancer Patients Younger Than, Older Than, and of Screening Age. Clin. Color. Cancer 2020, 19, e264–e271. [Google Scholar] [CrossRef]
- Zalis, M.G.; Vieira, F.M.; Zalcberg-Renault, I.; Bonamino, M.H.; Ferreira, C.G.; Oliveira, S. KRAS mutation profile in colorectal cancer patients in Brazil: A cohort of 989 individuals. J. Clin. Oncol. 2009, 27 (Suppl. S15), e15017. [Google Scholar] [CrossRef]
- Chida, K.; Kotani, D.; Masuishi, T.; Kawakami, T.; Kawamoto, Y.; Kato, K.; Fushiki, K.; Sawada, K.; Kumanishi, R.; Shirasu, H.; et al. The Prognostic Impact of KRAS G12C Mutation in Patients with Metastatic Colorectal Cancer: A Multicenter Retrospective Observational Study. Oncologist 2021, 26, 845–853. [Google Scholar] [CrossRef]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Gil Ferreira, C.; Aran, V.; Zalcberg-Renault, I.; Victorino, A.P.; Salem, J.H.; Bonamino, M.H.; Vieira, F.M.; Zalis, M. KRAS mutations: Variable incidences in a Brazilian cohort of 8,234 metastatic colorectal cancer patients. BMC Gastroenterol. 2014, 14, 73. [Google Scholar] [CrossRef]
- Barbier, A.; Mendez, G.; Garino, N.; Dibarbora, D.; Salanova, R.; Bertelli, A.M.; Ruiz, A.; Romero, A.; Cantarella, F.; Colombero, C. Frequency of KRAS, NRAS, and BRAF mutations in colorectal cancer in an Argentinian population. J. Clin. Oncol. 2023, 41, e15604. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Connelly, C.; Frampton, G.; Newberg, J.; Cooke, M.; Miller, V.; Ali, S.; Ross, J.S.; Handorf, E.; Arora, S.; et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat. Commun. 2019, 10, 3722. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.; Sobanski, T.; de Carvalho, A.C.; Evangelista, A.F.; Matsushita, M.; Berardinelli, G.N.; de Oliveira, M.A.; Reis, R.M.; Guimarães, D.P. Mutation profiling of cancer drivers in Brazilian colorectal cancer. Sci. Rep. 2019, 9, 13687. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Bylsma, L.C.; Gillezeau, C.; Garawin, T.A.; Kelsh, M.A.; Fryzek, J.P.; Sangaré, L.; Lowe, K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020, 9, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.E.; Merchan-Hamann, E.; Lima, F.S.D.S.; Laguardia, J.; Gutierrez, M.M.U. Avaliação de desempenho das redes de atenção à saúde: Uma proposta de indicadores. Rev. Eletron. Comun. Inf. Saude 2016, 10, 1–16. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Rossi, L.; Hone, T.; Dornelles, A.Z. Income inequality and non-communicable disease mortality and morbidity in Brazil States: A longitudinal analysis 2002–2017. Lancet Reg. Health 2021, 2, 100042. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.D.; Riechelmann, R.P.; Prolla, G.; Weschenfelder, R.F.; Fernandes, G.d.S.S.; Pereira, G.S.; De-Oliveira, M.d.L.; Rego, J.F.; Rocha-Filho, D.R.; Coutinho, A.K. Treatment choices in metastatic colorectal cancer according to sidedness and RAS/BRAF status: A national survey by the Brazilian Gastrointestinal Tumors Group (GTG). Braz. J. Oncol. 2019, 15, 1–6. [Google Scholar] [CrossRef]
- Weschenfelder, R.F.; Tsuchiya, C.T.; Kim, H.S.J.; Simões, J.A.; La Scala, C.S.K. Sequential biological therapies in metastatic colorectal cancer (mCRC): A cost comparison analysis for wildtype RAS mCRC patients in Brazil. J. Bras. Econ. Saúde 2016, 8, 24–38. [Google Scholar] [CrossRef]
- Fakih, M.; Tu, H.; Hsu, H.; Aggarwal, S.; Chan, E.; Rehn, M.; Chia, V.; Kopetz, S. Real-World Study of Characteristics and Treatment Outcomes Among Patients with KRAS p.G12C-Mutated or Other KRAS Mutated Metastatic Colorectal Cancer. Oncologist 2022, 27, 663–674. [Google Scholar] [CrossRef] [PubMed]
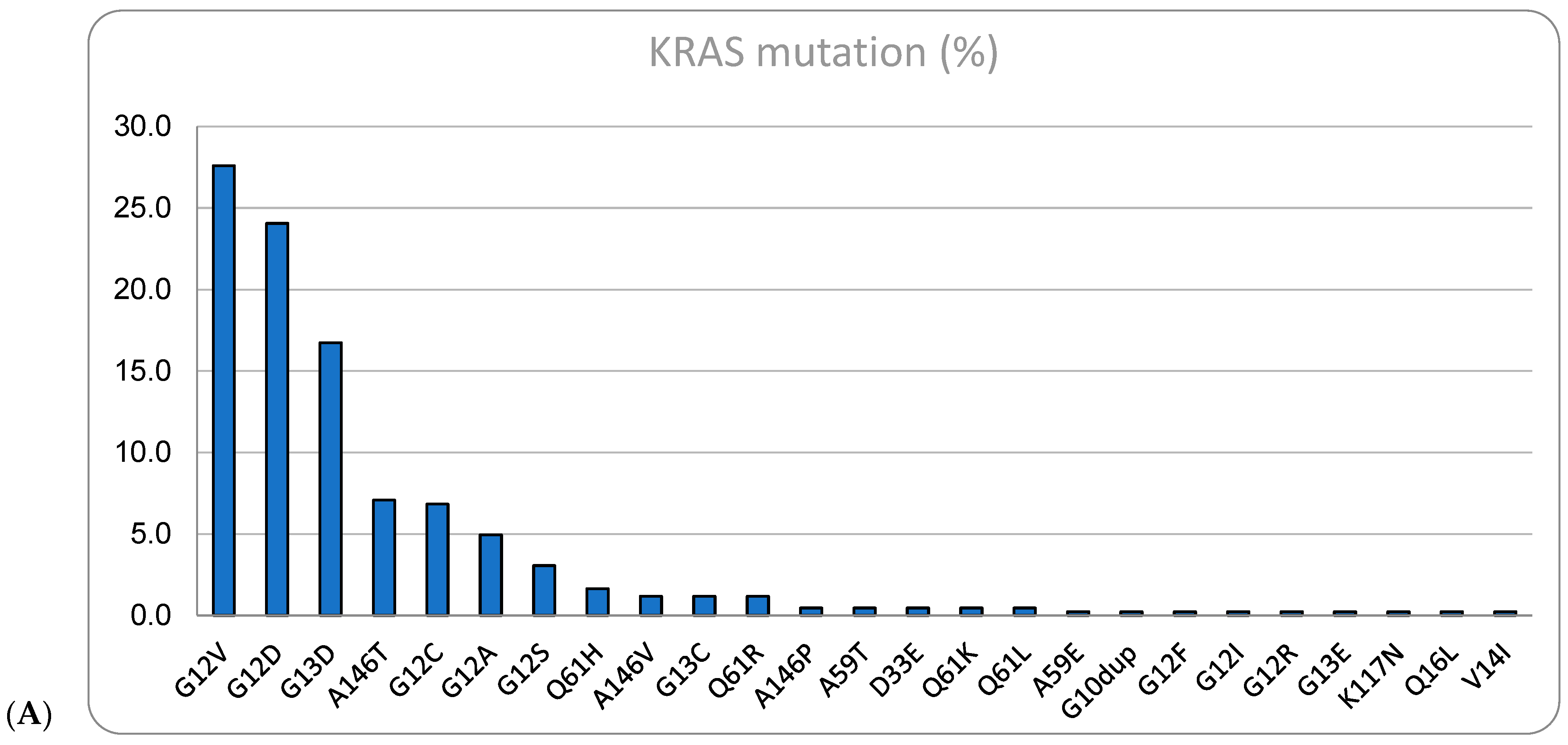
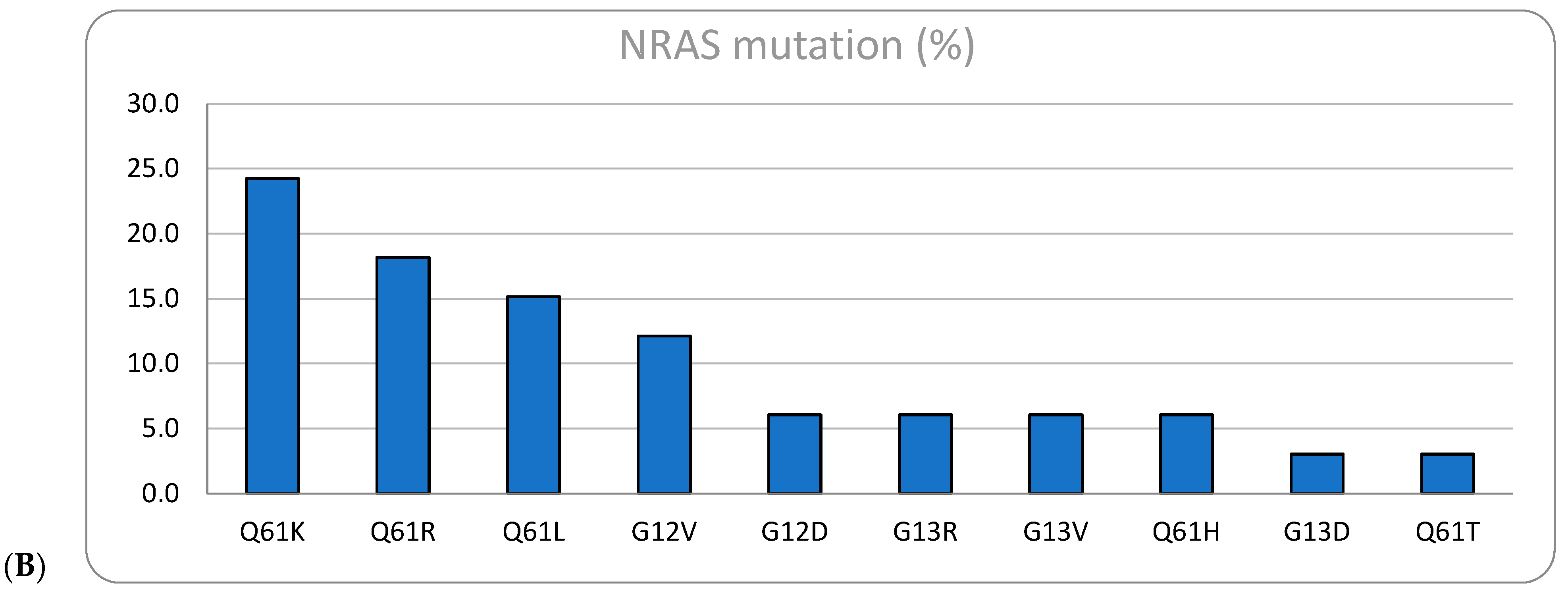
| Variables | Total (n = 858) | Percentage | |
|---|---|---|---|
| Gender | Male | 432 | 50.3% |
| Female | 426 | 49.7% | |
| Region | Northeast | 83 | 9.7% |
| Midwest | 32 | 3.7% | |
| Southeast | 653 | 76.1% | |
| South | 90 | 10.5% | |
| State | Pernambuco | 17 | 2% |
| Paraiba | 19 | 2.2% | |
| Sergipe | 2 | 0.2% | |
| Bahia | 45 | 5.2% | |
| Goais | 6 | 0.7% | |
| Sao Paulo | 75 | 8.7% | |
| Rio de Janeiro | 275 | 32.1% | |
| Espiritu Santo | 31 | 3.6% | |
| Minas Gerais | 272 | 31.7% | |
| Parana | 33 | 3.8% | |
| Rio Grande do Sul | 51 | 5.9% | |
| Santa Catarina | 7 | 0.8% | |
| Distrito Federal | 25 | 2.9% | |
| Age group | <50 years | 149 | 17.4% |
| 50–80 years | 602 | 70.1% | |
| >80 years | 107 | 12.5% | |
| Sidedness | Righ-side | 316 | 36.8% |
| Left-side | 508 | 59.2% | |
| Not specified | 33 | 3.9% | |
| RAS status | RAS wild-type | 401 | 46.7% |
| RAS mutant | 457 | 53.3% | |
| KRAS mutant | 424 | 49.4% | |
| NRAS mutant | 33 | 3.9% | |
| KRAS G12C mutant | 27 | 3.1% | |
| BRAF mutant | 63 | 7.3% | |
| MSI-high/dMMR | 14 | 1.6% |
| Variables | <50 Years (n = 149) | 50–80 Years (n = 602) | >80 Years (n = 107) | p-Value |
|---|---|---|---|---|
| Male | 70 (47.0%) | 307 (51.0%) | 49 (45.8%) | p = 0.473 |
| Female | 79 (53.0%) | 295 (49.0%) | 58 (54.2%) | |
| Right-side | 37 (29.5%) | 223 (38.8%) | 56 (52.8%) | p < 0.001 |
| Left-side | 106 (74.1%) | 352 (61.2%) | 50 (47.2%) | |
| RAS wild-type | 75 (50.3%) | 280 (46.5%) | 46 (43.0%) | p = 0.733 |
| KRAS mutant | 68 (45.6%) | 298 (49.5%) | 58 (54.2%) | |
| NRAS mutant | 6 (4.0%) | 24 (4.0%) | 3 (2.8%) | |
| KRAS G12C mutant | 9 (13.2%) | 17 (5.7%) | 2 (3.4%) | p = 0.046 |
| Other KRAS mutations | 59 (86.8%) | 281 (94.3%) | 56 (96.6%) | |
| BRAF mutant | 12 (8.1%) | 42 (7.0%) | 9 (8.4%) | p = 0.815 |
| BRAF wild-type | 137 (91.9%) | 560 (93.0%) | 98 (91.6%) | |
| MSI-high/dMMR | 1 (0.7%) | 10 (1.7%) | 3 (2.8%) | p = 0.412 |
| MSS/pMMR | 307 (97.2%) | 595 (98.3%) | 104 (97.2%) |
| Variables | Right-Side (n = 316) | Left-Side (n = 316) | p Value |
|---|---|---|---|
| Male | 147 (46.5%) | 261 (51.4%) | p = 0.175 |
| Female | 169 (53.5%) | 247 (48.6%) | |
| <50 years | 37 (11.7%) | 106 (20.9%) | p < 0.001 |
| 50–80 Years | 223 (70.6%) | 352 (69.3%) | |
| >80 years | 56 (17.7%) | 50 (9.8%) | |
| RAS wild-type | 153 (48.4%) | 230 (45.3%) | p = 0.239 |
| KRAS mutant | 155 (49.1%) | 254 (50.0%) | |
| NRAS mutant | 8 (2.5%) | 24 (4.7%) | |
| KRAS G12C mutant | 10 (6.5%) | 17 (6.7%) | p = 0.924 |
| Other KRAS mutations | 145 (93.5%) | 237 (93.3%) | |
| BRAF mutant | 35 (11.1%) | 26 (5.1%) | p = 0.001 |
| BRAF wild-type | 281 (88.9%) | 482 (94.9%) | |
| MSI-high/dMMR | 9 (2.8%) | 3 (0.6%) | p = 0.009 |
| MSS/pMMR | 307 (97.2%) | 505 (99.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunagua Aruquipa, M.; D’Alpino Peixoto, R.; Jacome, A.; Cesar, F.; Lorandi, V.; Dienstmann, R. Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer. Curr. Issues Mol. Biol. 2024, 46, 1374-1382. https://doi.org/10.3390/cimb46020088
Sunagua Aruquipa M, D’Alpino Peixoto R, Jacome A, Cesar F, Lorandi V, Dienstmann R. Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer. Current Issues in Molecular Biology. 2024; 46(2):1374-1382. https://doi.org/10.3390/cimb46020088
Chicago/Turabian StyleSunagua Aruquipa, Marcelo, Renata D’Alpino Peixoto, Alexandre Jacome, Fernanda Cesar, Vinicius Lorandi, and Rodrigo Dienstmann. 2024. "Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer" Current Issues in Molecular Biology 46, no. 2: 1374-1382. https://doi.org/10.3390/cimb46020088
APA StyleSunagua Aruquipa, M., D’Alpino Peixoto, R., Jacome, A., Cesar, F., Lorandi, V., & Dienstmann, R. (2024). Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer. Current Issues in Molecular Biology, 46(2), 1374-1382. https://doi.org/10.3390/cimb46020088






