Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Sample Collection
2.3. CD3+ T-Cell Phenotyping via Flow Cytometry
2.4. CD19+ B-Cell Phenotyping via Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Alterations in Main Circulating Lymphocyte Subsets in Patients with AOSD
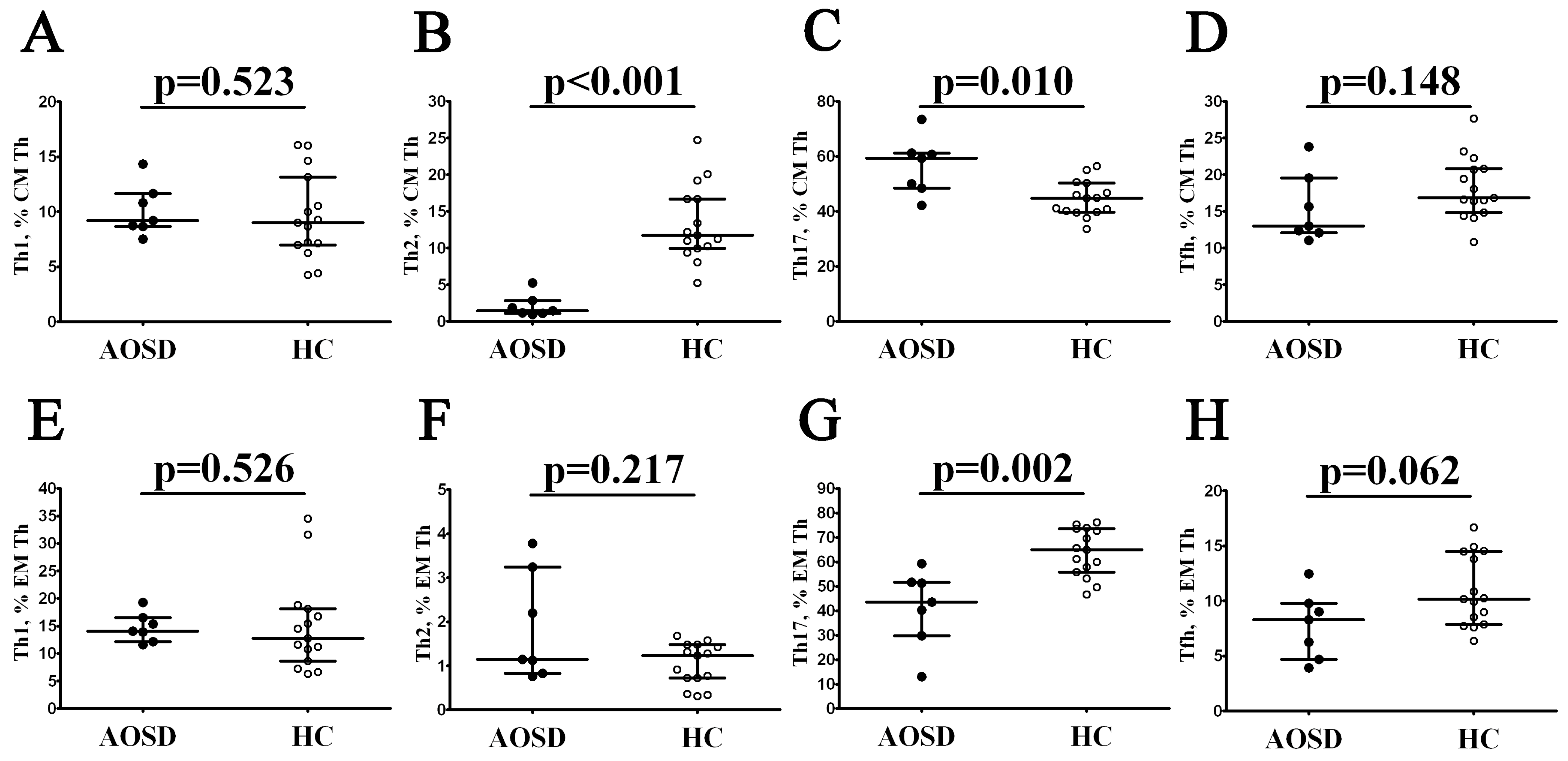
3.2. Alterations in Th Cell Maturation and ‘Polarization’ in Patients with AOSD
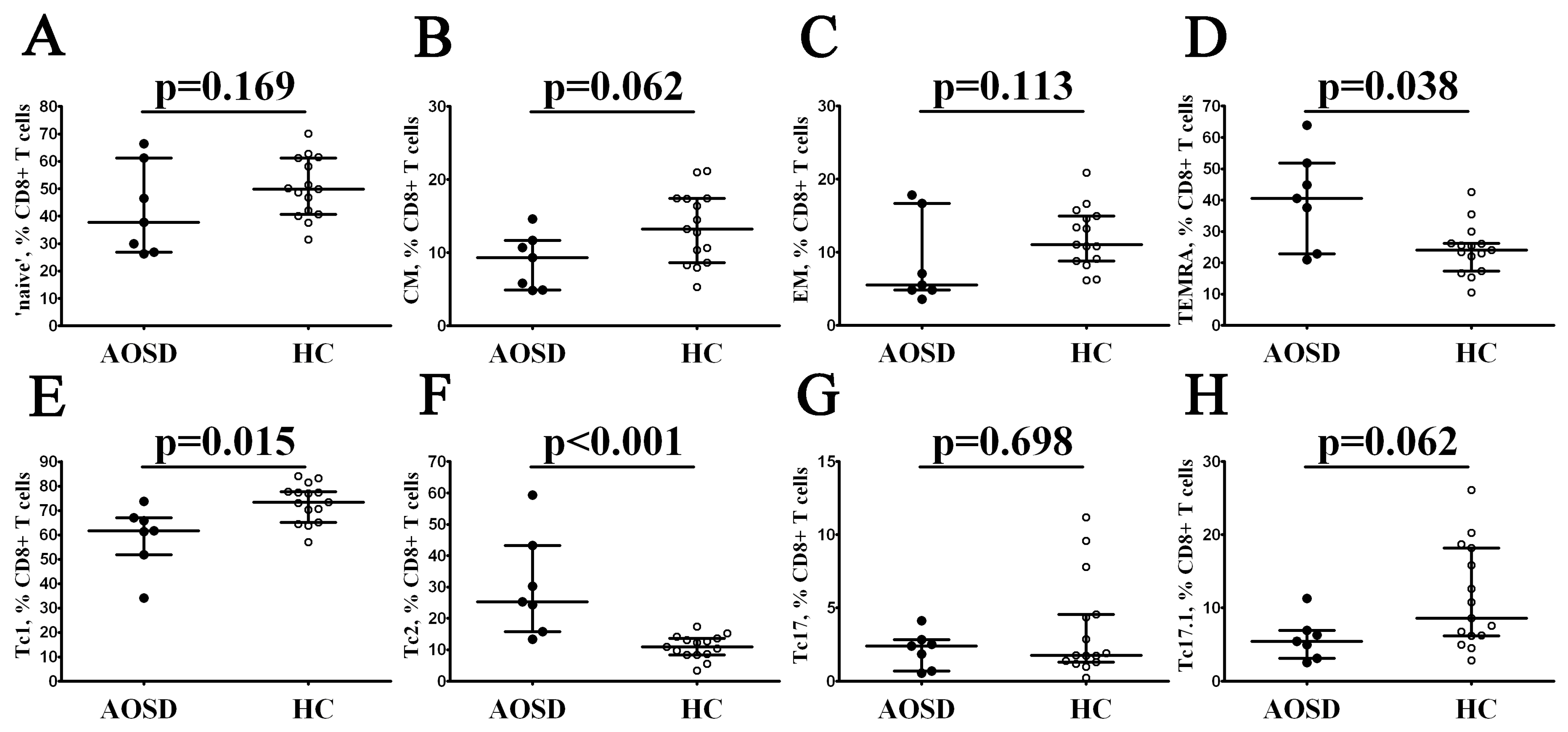
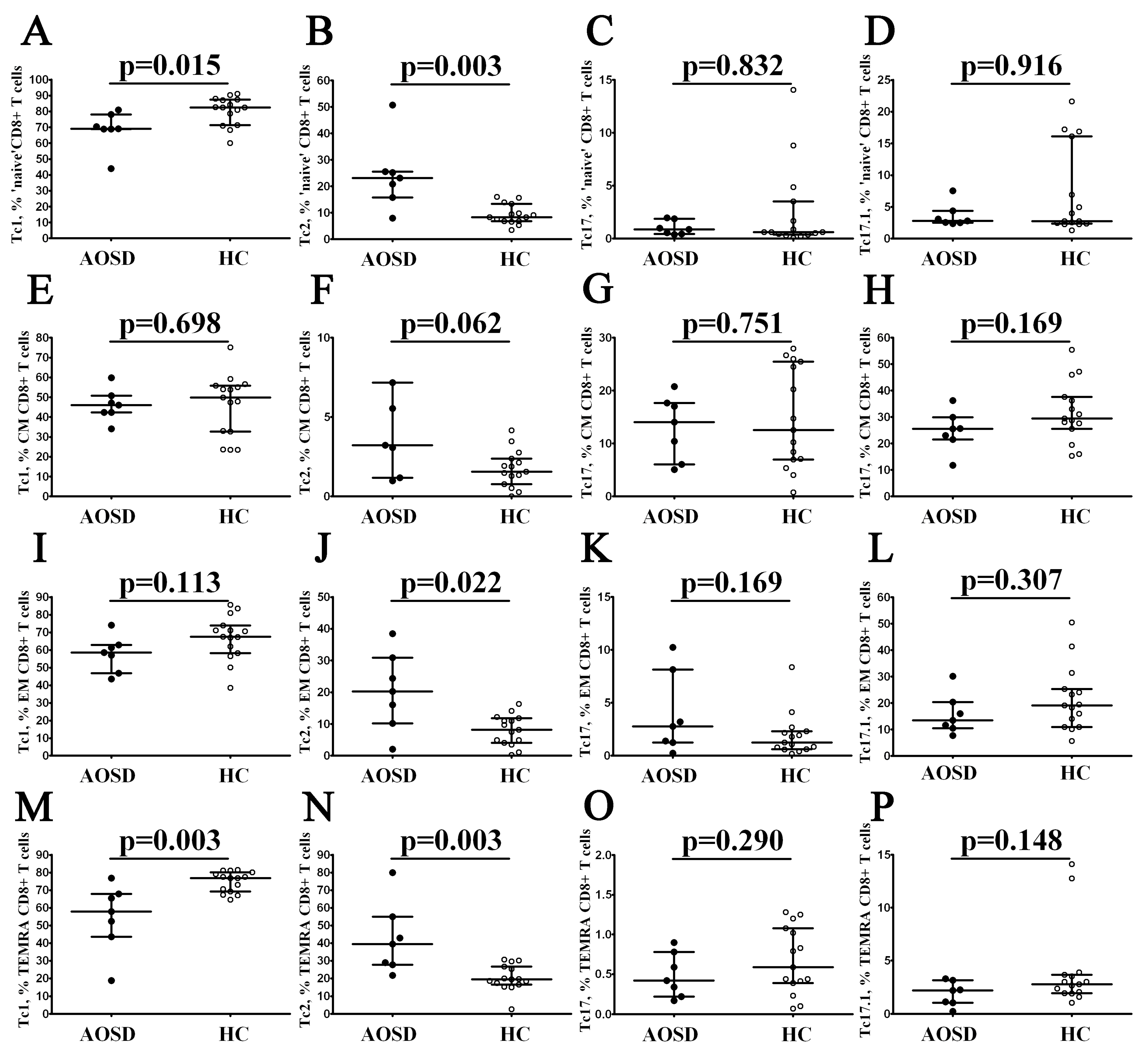
3.3. Alterations in CD8+ T-Cell Maturation and ‘Polarization’ in Patients with AOSD
3.4. Alterations in CD19+ B-Cell Maturation in Patients with AOSD
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Still, G.F. On a Form of Chronic Joint Disease in Children. Arch. Dis. Child. 1941, 16, 156–165. [Google Scholar] [CrossRef][Green Version]
- Bywaters, E.G. Still’s Disease in the Adult. Ann. Rheum. Dis. 1971, 30, 121–133. [Google Scholar] [CrossRef]
- Nirmala, N.; Brachat, A.; Feist, E.; Blank, N.; Specker, C.; Witt, M.; Zernicke, J.; Martini, A.; Junge, G. Gene-expression Analysis of Adult-Onset Still’s Disease and Systemic Juvenile Idiopathic Arthritis is Consistent with a Continuum of a Single disease Entity. Pediatr. Rheumatol. 2015, 13, 50. [Google Scholar] [CrossRef]
- Gerfaud-Valentin, M.; Jamilloux, Y.; Iwaz, J.; Sève, P. Adult-Onset Still’s Disease. Autoimmun. Rev. 2014, 13, 708–722. [Google Scholar] [CrossRef]
- Jia, J.; Wang, M.; Ma, Y.; Meng, J.; Zhu, D.; Chen, X.; Shi, H.; Sun, Y.; Liu, H.; Cheng, X.; et al. Neutrophil Extracellular Trap-Induced Intermediate Monocytes Trigger Macrophage Activation Syndrome in Adult-Onset Still’s Disease. BMC Med. 2023, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Shimizu, M.; Tsunoda, S.; Kawano, M.; Matsumura, M.; Yachie, A. Cytokine Profile in Adult-Onset Still’s Disease: Comparison with Systemic Juvenile Idiopathic Arthritis. Clin. Immunol. 2016, 169, 8–13. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cantarini, L.; Nigrovic, P.A.; McGonagle, D.; Giacomelli, R. Recent Advances and Evolving Concepts in Still’s Disease. Nat. Rev. Rheumatol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Feist, E.; Mitrovic, S.; Fautrel, B. Mechanisms, Biomarkers and Targets for Adult-Onset Still’s Disease. Nat. Rev. Rheumatol. 2018, 14, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-W.; Chen, Y.-M.; Lin, C.-C.; Tang, K.-T.; Chen, H.-H.; Hung, W.-T.; Lai, K.-L.; Chen, D.-Y. Elevated Expression of the NLRP3 Inflammasome and Its Correlation with Disease Activity in Adult-Onset Still Disease. J. Rheumatol. 2017, 44, 1142–1150. [Google Scholar] [CrossRef]
- Hutton, H.L.; Ooi, J.D.; Holdsworth, S.R.; Kitching, A.R. The NLRP3 Inflammasome in Kidney Disease and Autoimmunity. Nephrol. Carlton Vic. 2016, 21, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Wang, K.; Cohen, J.; Paul, W.E. IL-1β Strikingly Enhances Antigen-Driven CD4 and CD8 T-Cell Responses. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 117–124. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ohta, A.; Tsunematsu, T.; Kasukawa, R.; Mizushima, Y.; Kashiwagi, H.; Kashiwazaki, S.; Tanimoto, K.; Matsumoto, Y.; Ota, T. Preliminary Criteria for Classification of Adult Still’s Disease. J. Rheumatol. 1992, 19, 424–430. [Google Scholar] [PubMed]
- Rau, M.; Schiller, M.; Krienke, S.; Heyder, P.; Lorenz, H.; Blank, N. Clinical Manifestations but Not Cytokine Profiles Differentiate Adult-Onset Still’s Disease and Sepsis. J. Rheumatol. 2010, 37, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, A.; Kalinina, O.; Bezrukikh, V.; Aquino, A.; Zaikova, E.; Karonova, T.; Melnik, O.; Vasilieva, E.; Kudryavtsev, I. Imbalanced Immune Response of T-Cell and B-Cell Subsets in Patients with Moderate and Severe COVID-19. Viruses 2021, 13, 1966. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Korobova, Z.R.; Isakov, D.V.; Rubinstein, A.A.; Batsunov, O.K.; Khamitova, I.V.; Kuznetsova, R.N.; Savin, T.V.; Akisheva, T.V.; et al. Heterogenous CD8+ T Cell Maturation and “Polarization” in Acute and Convalescent COVID-19 Patients. Viruses 2022, 14, 1906. [Google Scholar] [CrossRef]
- Dankers, W.; den Braanker, H.; Paulissen, S.M.J.; van Hamburg, J.P.; Davelaar, N.; Colin, E.M.; Lubberts, E. The Heterogeneous Human Memory CCR6+ T Helper-17 Populations Differ in T-Bet and Cytokine Expression but All Activate Synovial Fibroblasts in an IFNγ-Independent Manner. Arthritis Res. Ther. 2021, 23, 157. [Google Scholar] [CrossRef]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef]
- Morita, R.; Schmitt, N.; Bentebibel, S.-E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human Blood CXCR5(+)CD4(+) T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets That Differentially Support Antibody Secretion. Immunity 2011, 34, 108–121. [Google Scholar] [CrossRef]
- Hutloff, A. T Follicular Helper-Like Cells in Inflamed Non-Lymphoid Tissues. Front. Immunol. 2018, 9, 1707. [Google Scholar] [CrossRef]
- Loyal, L.; Warth, S.; Jürchott, K.; Mölder, F.; Nikolaou, C.; Babel, N.; Nienen, M.; Durlanik, S.; Stark, R.; Kruse, B.; et al. SLAMF7 and IL-6R Define Distinct Cytotoxic versus Helper Memory CD8+ T Cells. Nat. Commun. 2020, 11, 6357. [Google Scholar] [CrossRef]
- Trombke, J.; Loyal, L.; Braun, J.; Pleyer, U.; Thiel, A.; Pohlmann, D. Analysis of Peripheral Inflammatory T Cell Subsets and Their Effector Function in Patients with Birdshot Retinochoroiditis. Sci. Rep. 2021, 11, 8604. [Google Scholar] [CrossRef]
- Bohnhorst, J.Ø.; Bjørgan, M.B.; Thoen, J.E.; Natvig, J.B.; Thompson, K.M. Bm1-Bm5 Classification of Peripheral Blood B Cells Reveals Circulating Germinal Center Founder Cells in Healthy Individuals and Disturbance in the B Cell Subpopulations in Patients with Primary Sjögren’s Syndrome. J. Immunol. 2001, 167, 3610–3618. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Lan, J.-L.; Lin, F.-J.; Hsieh, T.-Y. Proinflammatory Cytokine Profiles in Sera and Pathological Tissues of Patients with Active Untreated Adult Onset Still’s Disease. J. Rheumatol. 2004, 31, 2189–2198. [Google Scholar]
- Li, S.; Zheng, S.; Tang, S.; Pan, Y.; Zhang, S.; Fang, H.; Qiao, J. Autoinflammatory Pathogenesis and Targeted Therapy for Adult-Onset Still’s Disease. Clin. Rev. Allergy Immunol. 2020, 58, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sfriso, P.; Bindoli, S.; Galozzi, P. Adult-Onset Still’s Disease: Molecular Pathophysiology and Therapeutic Advances. Drugs 2018, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Y.; Chen, Y.-M.; Lan, J.-L.; Lin, C.-C.; Chen, H.-H.; Hsieh, C.-W. Potential Role of Th17 Cells in the Pathogenesis of Adult-Onset Still’s Disease. Rheumatol. Oxf. Engl. 2010, 49, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Suh, C.-H.; Jung, J.-Y.; Nam, J.-Y.; Kwon, J.E.; Yim, H.; Kim, H.-A. Association of CXCL10 and CXCL13 Levels with Disease Activity and Cutaneous Manifestation in Active Adult-Onset Still’s Disease. Arthritis Res. Ther. 2015, 17, 260. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Suh, C.-H.; Jung, J.-Y.; Ahn, M.-H.; Han, M.H.; Kwon, J.E.; Yim, H.; Kim, H.-A. Elevated Circulating Levels of the Interferon-γ-Induced Chemokines Are Associated with Disease Activity and Cutaneous Manifestations in Adult-Onset Still’s Disease. Sci. Rep. 2017, 7, 46652. [Google Scholar] [CrossRef]
- Saiki, O.; Uda, H.; Nishimoto, N.; Miwa, T.; Mima, T.; Ogawara, T.; Azuma, N.; Katada, Y.; Sawaki, J.; Tsutsui, H.; et al. Adult Still’s Disease Reflects a Th2 Rather than a Th1 Cytokine Profile. Clin. Immunol. Orlando Fla. 2004, 112, 120–125. [Google Scholar] [CrossRef]
- Chen, D.Y.; Lan, J.L.; Lin, F.J.; Hsieh, T.Y.; Wen, M.C. Predominance of Th1 Cytokine in Peripheral Blood and Pathological Tissues of Patients with Active Untreated Adult Onset Still’s Disease. Ann. Rheum. Dis. 2004, 63, 1300–1306. [Google Scholar] [CrossRef]
- Guo, R.; Liu, X.; Li, Y.; Meng, X.; Li, R.; Chen, X.; Lu, L. AOSD Endotypes Based on Immune Cell Profiles: Patient Stratification with Hierarchical Clustering Analysis. Rheumatol. Oxf. Engl. 2023, 62, 1636–1644. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Choi, B.; Sayeed, H.M.; Suh, C.-H.; Kim, Y.W.; Kim, H.-A.; Sohn, S. Characteristic Patterns of HLA Presentation and T Cell Differentiation in Adult-Onset Still’s Disease. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418791284. [Google Scholar] [CrossRef]
- Kim, H.-A.; Kwon, J.E.; Yim, H.; Suh, C.-H.; Jung, J.-Y.; Han, J.H. The Pathologic Findings of Skin, Lymph Node, Liver, and Bone Marrow in Patients with Adult-Onset Still Disease. Medicine 2015, 94, e787. [Google Scholar] [CrossRef]
- Chi, H.; Hong, X.; Dai, N.; Chen, L.; Zhang, H.; Liu, H.; Cheng, X.; Ye, J.; Shi, H.; Hu, Q.; et al. The Landscape of Innate and Adaptive Immune Cell Subsets in Patients with Adult-Onset Still’s Disease. Rheumatol. Oxf. Engl. 2023, kead507. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, H.; Min, Q.; Li, Z.; Bi, J.; Liu, L.; Liang, Y. Adult-Onset Still’s Disease with Multiple Lymphadenopathy: A Case Report and Literature Review. Diagn. Pathol. 2021, 16, 97. [Google Scholar] [CrossRef]
- Omoto, Y.; Yamanaka, K.; Tokime, K.; Kitano, S.; Kakeda, M.; Akeda, T.; Kurokawa, I.; Gabazza, E.C.; Tsutsui, H.; Katayama, N.; et al. Granzyme B Is a Novel Interleukin-18 Converting Enzyme. J. Dermatol. Sci. 2010, 59, 129–135. [Google Scholar] [CrossRef]
- Akeda, T.; Yamanaka, K.; Tsuda, K.; Omoto, Y.; Gabazza, E.C.; Mizutani, H. CD8+ T Cell Granzyme B Activates Keratinocyte Endogenous IL-18. Arch. Dermatol. Res. 2014, 306, 125–130. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Thompson, P.A.; Rosenwasser, L.J.; Dinarello, C.A. The Role of Interleukin 1 in Human B Cell Activation: Inhibition of B Cell Proliferation and the Generation of Immunoglobulin-Secreting Cells by an Antibody against Human Leukocytic Pyrogen. J. Immunol. 1983, 130, 2708–2714. [Google Scholar] [CrossRef]
- Fang, X.; Ye, H.; Xie, Y.; Wei, C.; Liu, S.; Yao, H.; Li, Z.; Jia, Y.; Hu, F. B Cell Subsets in Adult-Onset Still’s Disease: Potential Candidates for Disease Pathogenesis and Immunophenotyping. Arthritis Res. Ther. 2023, 25, 104. [Google Scholar] [CrossRef]
- Burastero, S.E.; Casali, P.; Wilder, R.L.; Notkins, A.L. Monoreactive High Affinity and Polyreactive Low Affinity Rheumatoid Factors Are Produced by CD5+ B Cells from Patients with Rheumatoid Arthritis. J. Exp. Med. 1988, 168, 1979–1992. [Google Scholar] [CrossRef]
- Dauphinée, M.; Tovar, Z.; Talal, N. B Cells Expressing CD5 Are Increased in Sjögren’s Syndrome. Arthritis Rheum. 1988, 31, 642–647. [Google Scholar] [CrossRef]
- Kantor, A.B.; Herzenberg, L.A. Origin of Murine B Cell Lineages. Annu. Rev. Immunol. 1993, 11, 501–538. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B Cells Regulate Autoimmunity by Provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef]
- Sims, G.P.; Ettinger, R.; Shirota, Y.; Yarboro, C.H.; Illei, G.G.; Lipsky, P.E. Identification and Characterization of Circulating Human Transitional B Cells. Blood 2005, 105, 4390–4398. [Google Scholar] [CrossRef]
- Simon, Q.; Pers, J.-O.; Cornec, D.; Le Pottier, L.; Mageed, R.A.; Hillion, S. In-Depth Characterization of CD24(High)CD38(High) Transitional Human B Cells Reveals Different Regulatory Profiles. J. Allergy Clin. Immunol. 2016, 137, 1577–1584.e10. [Google Scholar] [CrossRef]
- Grandemange, S.; Sanchez, E.; Louis-Plence, P.; Tran Mau-Them, F.; Bessis, D.; Coubes, C.; Frouin, E.; Seyger, M.; Girard, M.; Puechberty, J.; et al. A New Autoinflammatory and Autoimmune Syndrome Associated with NLRP1 Mutations: NAIAD (NLRP1-Associated Autoinflammation with Arthritis and Dyskeratosis). Ann. Rheum. Dis. 2017, 76, 1191–1198. [Google Scholar] [CrossRef]
- Brachat, A.H.; Grom, A.A.; Wulffraat, N.; Brunner, H.I.; Quartier, P.; Brik, R.; McCann, L.; Ozdogan, H.; Rutkowska-Sak, L.; Schneider, R.; et al. Early Changes in Gene Expression and Inflammatory Proteins in Systemic Juvenile Idiopathic Arthritis Patients on Canakinumab Therapy. Arthritis Res. Ther. 2017, 19, 13. [Google Scholar] [CrossRef]
- Ghannam, K.; Zernicke, J.; Kedor, C.; Listing, J.; Burmester, G.R.; Foell, D.; Feist, E. Distinct Effects of Interleukin-1β Inhibition upon Cytokine Profile in Patients with Adult-Onset Still’s Disease and Active Articular Manifestation Responding to Canakinumab. J. Clin. Med. 2021, 10, 4400. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; LeClercq, S.A.; Yan, A.; Homik, J.E.; Dinarello, C.A. Rapid Responses to Anakinra in Patients with Refractory Adult-Onset Still’s Disease. Arthritis Rheum. 2005, 52, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Kötter, I.; Wacker, A.; Koch, S.; Henes, J.; Richter, C.; Engel, A.; Günaydin, I.; Kanz, L. Anakinra in Patients with Treatment-Resistant Adult-Onset Still’s Disease: Four Case Reports with Serial Cytokine Measurements and a Review of the Literature. Semin. Arthritis Rheum. 2007, 37, 189–197. [Google Scholar] [CrossRef] [PubMed]


| N | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age, years old | 28 | 26 | 21 | 63 | 30 | 43 | 52 |
| Sex | 2 | 2 | 1 | 1 | 2 | 2 | 2 |
| Years of disease | 10 | 3 | 1 | 2 | <1 | 18 | 6 |
| Course | Polycyclic | Polycyclic | Polycyclic | Polycyclic | Monocyclic | Polycyclic | Polycyclic |
| Modified Pouchot’s score [14] | 5 | 5 | 8 | 4 | 4 | 4 | 2 |
| Laboratory data | |||||||
| White blood cells × 109/L (4.0–9.0) | 9.0 | 13.1 | 12.8 | 17.8 | 12.8 | 13.3 | 6.1 |
| Neutrophils × 109/L (2.0–5.8) | 5.7 | 8.3 | 10.3 | 14.9 | 9.0 | 9.7 | 3.8 |
| Neutrophils (%) | 63.5 | 63.2 | 81.0 | 84.0 | 70.0 | 73.2 | 62.0 |
| Lymphocytes × 109/L (1.2–3.2) | 2.5 | 3.8 | 2.3 | 1.7 | 3.8 | 2.1 | 1.7 |
| NLR | 2.28 | 2.18 | 4.47 | 8.76 | 2.36 | 4.6 | 2.23 |
| Platelets (150–400) | 411 | 430 | 228 | 394 | 428 | 561 | 215 |
| C–reactive protein, mg/L (0.0–5.0) | 117.0 | 8.2 | 45.0 | 51.9 | 22.6 | 231.0 | 6.4 |
| Ferritin, ng/mL (30.0–150) | 512 | 55 | 1800 | 1218 | 311 | 219 | 151 |
| ALT, U/L (0.00–33) | 12 | 16 | 67 | 36 | 12 | 10 | 14 |
| AST, U/L (5.0–32) | 17 | 17 | 49 | 18 | 12 | 11 | 16 |
| Clinical data | |||||||
| Fever | 1 | 1 | 1 | 1 | 1 | 1 | - |
| Sore throat | - | 1 | 1 | 1 | - | 1 | - |
| Pericarditis | - | 1 | 1 | - | - | - | - |
| Pleuritis | - | 1 | 1 | - | - | - | - |
| Artrhitis | 1 | 1 | 1 | - | 1 | 1 | - |
| Arthralgia | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Myalgia | 1 | 1 | 1 | - | 1 | 1 | - |
| Rush | 1 | 1 | 1 | 1 | 1 | - | 1 |
| Lymphadenopathy | 1 | 1 | 1 | 1 | - | - | - |
| Splenomegaly | - | - | 1 | - | - | - | - |
| Hepatomegaly | - | - | 1 | 1 | - | - | - |
| Treatment on relapse | low dose of NSAIDs | prednisolone 6.25 mg, methotrexate 20 mg | low dose of NSAIDs | low dose of NSAIDs | NSAIDs (ibuprofen 2400 mg) pantoprazol 40 mg | Prednis-olone 10 mg | Prednis-olone 5 mg, metho-trexate 15 mg |
| Th17 Cell Subsets | Phenotype | AOSD (n = 7) | Healthy Control (n = 15) | p Value |
|---|---|---|---|---|
| Med (Q25; Q75) | Med (Q25; Q75) | |||
| % within CM CCR6+ Th17: | ||||
| DN Th17 | CCR4–CXCR3– | 26.51 (20.39; 42.77) | 3.27 (2.67; 4.28) | <0.001 |
| class Th17 | CCR4+CXCR3– | 26.31 (19.32; 30.37) | 35.37 (27.72; 42.65) | 0.022 |
| Th17.1 | CCR4–CXCR3+ | 16.60 (15.01; 38.03) | 32.52 (22.66; 35.55) | 0.192 |
| DP Th17 | CCR4+CXCR3+ | 19.11 (10.87; 28.20) | 31.39 (26.61;37.05) | 0.005 |
| % EM within CCR6+ Th17: | ||||
| DN Th17 | CCR4–CXCR3– | 6.55 (2.94; 11.83) | 1.66 (1.53; 3.01) | 0.015 |
| class Th17 | CCR4+CXCR3– | 42.20 (33.62; 46.01) | 21.94 (18.65; 25.66) | 0.008 |
| Th17.1 | CCR4–CXCR3+ | 23.06 (17.63; 31.09) | 47.85 (45.77; 52.17) | <0.001 |
| DP Th17 | CCR4+CXCR3+ | 33.07 (20.28; 34.96) | 27.08 (21.61; 31.60) | 0.275 |
| Tfh Cell Subsets | Phenotype | AOSD (n = 7) | Healthy Control (n = 15) | p Value |
|---|---|---|---|---|
| Med (Q25; Q75) | Med (Q25; Q75) | |||
| % CM CCR6+ Tfh: | ||||
| Tfh1 | CCR6–CXCR3+ | 29.37 (25.38; 31.79) | 27.07 (24.23; 31.27) | 0.503 |
| Tfh2 | CCR6–CXCR3– | 17.00 (11.34; 25.48) | 19.23 (14.12; 22.54) | 0.698 |
| Tfh17 | CCR6+CXCR3– | 24.63 (21.75; 27.81) | 33.64 (27.26; 37.29) | 0.008 |
| DP Tfh | CCR6+CXCR3+ | 27.31 (18.09; 33.42) | 20.76 (18.78; 21.91) | 0.130 |
| % EM CCR6+ Tfh: | ||||
| Tfh1 | CCR6–CXCR3+ | 25.26 (12.62; 40.94) | 22.89 (15.90; 28.48) | 0.459 |
| Tfh2 | CCR6–CXCR3– | 6.94 (1.57; 11.70) | 14.23 (9.89; 18.58) | 0.010 |
| Tfh17 | CCR6+CXCR3– | 35.23 (19.69; 46.94) | 41.82 (37.10; 48.29) | 0.148 |
| DP Tfh | CCR6+CXCR3+ | 31.53 (22.40; 37.80) | 18.86 (17.00; 23.48) | 0.007 |
| B Cell Subsets | Phenotype | AOSD (n = 7) | Healthy Control (n = 15) | p Value |
|---|---|---|---|---|
| Med (Q25; Q75) | Med (Q25; Q75) | |||
| Bm1 | IgD+CD38– | 9.39 (5.92; 41.21) | 7.90 (3.70; 9.88) | 0.307 |
| Bm2 | IgD+CD38+ | 50.10 (39.08; 56.04) | 60.41 (52.18; 68.75) | 0.053 |
| Bm2′ | IgD+CD38++ | 3.73 (0.43; 7.12) | 8.21 (5.46; 9.70) | 0.045 |
| Bm3 + Bm4 | IgD–CD38+++ | 1.79 (0.26; 4.31) | 1.61 (1.37; 2.39) | 0.972 |
| eBm5 | IgD–CD38+ | 10.71 (8.66; 13.86) | 13.86 (11.27; 17.82) | 0.105 |
| Bm5 | IgD–CD38– | 15.80 (9.89; 19.22) | 6.30 (4.54; 8.19) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myachikova, V.; Kudryavtsev, I.; Rubinstein, A.; Aquino, A.; Isakov, D.; Golovkin, A.; Maslyanskiy, A. Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still’s Disease. Curr. Issues Mol. Biol. 2024, 46, 1177-1191. https://doi.org/10.3390/cimb46020075
Myachikova V, Kudryavtsev I, Rubinstein A, Aquino A, Isakov D, Golovkin A, Maslyanskiy A. Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still’s Disease. Current Issues in Molecular Biology. 2024; 46(2):1177-1191. https://doi.org/10.3390/cimb46020075
Chicago/Turabian StyleMyachikova, Valentina, Igor Kudryavtsev, Artem Rubinstein, Arthur Aquino, Dmitry Isakov, Alexey Golovkin, and Alexey Maslyanskiy. 2024. "Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still’s Disease" Current Issues in Molecular Biology 46, no. 2: 1177-1191. https://doi.org/10.3390/cimb46020075
APA StyleMyachikova, V., Kudryavtsev, I., Rubinstein, A., Aquino, A., Isakov, D., Golovkin, A., & Maslyanskiy, A. (2024). Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still’s Disease. Current Issues in Molecular Biology, 46(2), 1177-1191. https://doi.org/10.3390/cimb46020075







