Plastoquinone-Derivative SkQ1 Improved the Biliary Intraepithelial Neoplasia during Liver Fluke Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Compounds and Doses
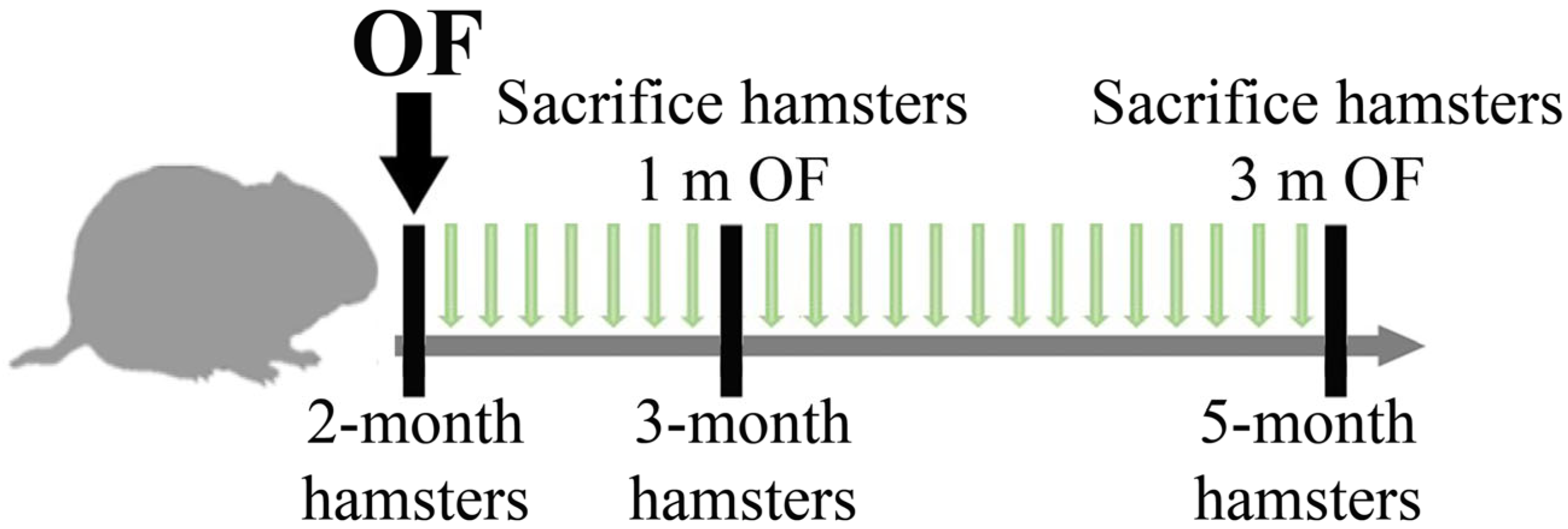
2.3. Animals and Experimental Design
2.4. In Vivo Chemotherapy
2.5. Material Collection, Histopathology and Immunohistochemistry
2.6. Serum Biochemistry
2.7. Total RNA Extraction, cDNA Synthesis and Real-Time PCR
2.8. Western Blotting
2.9. Statistics
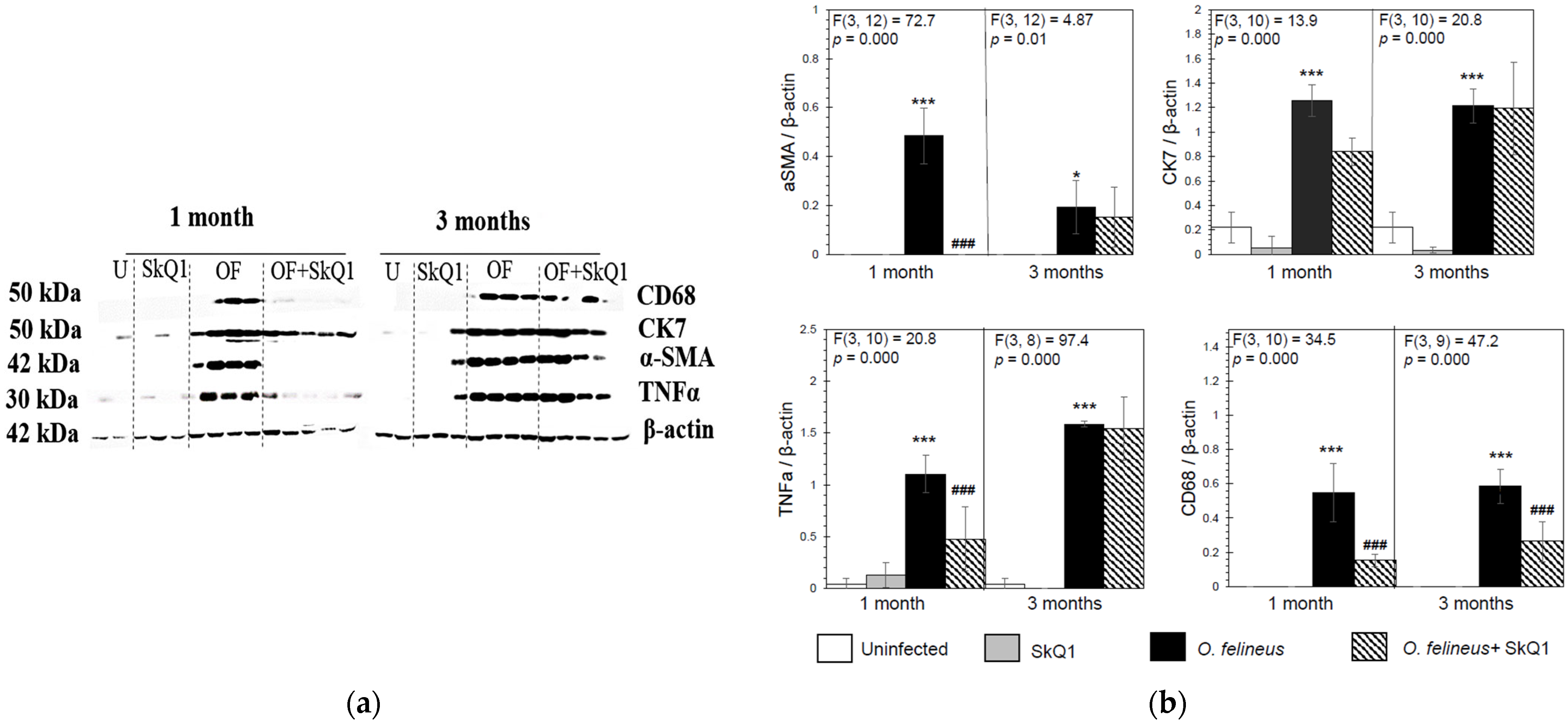
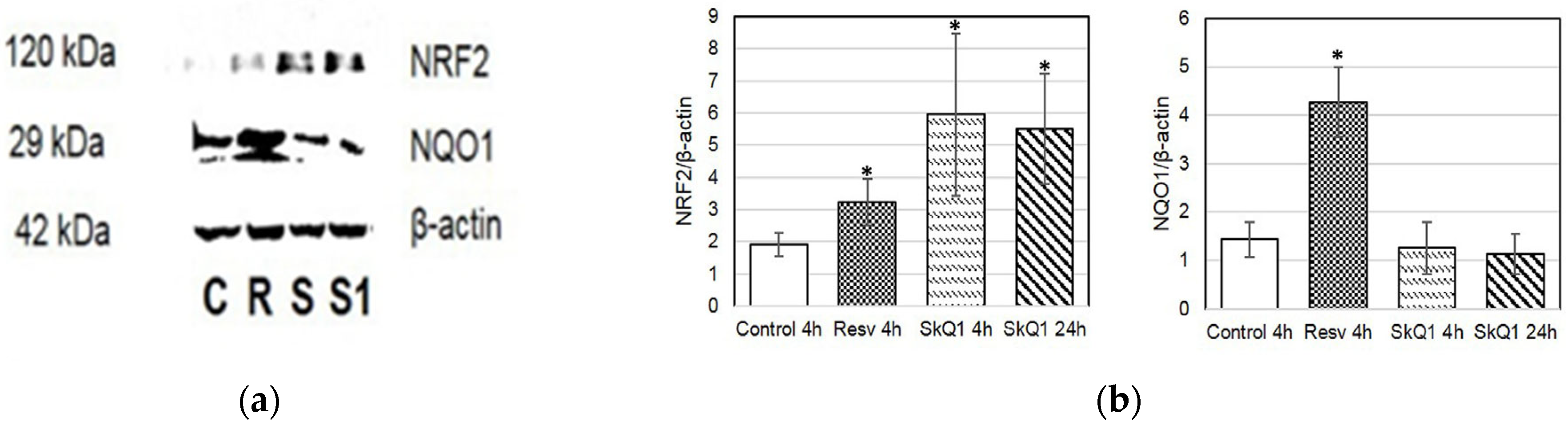
3. Results
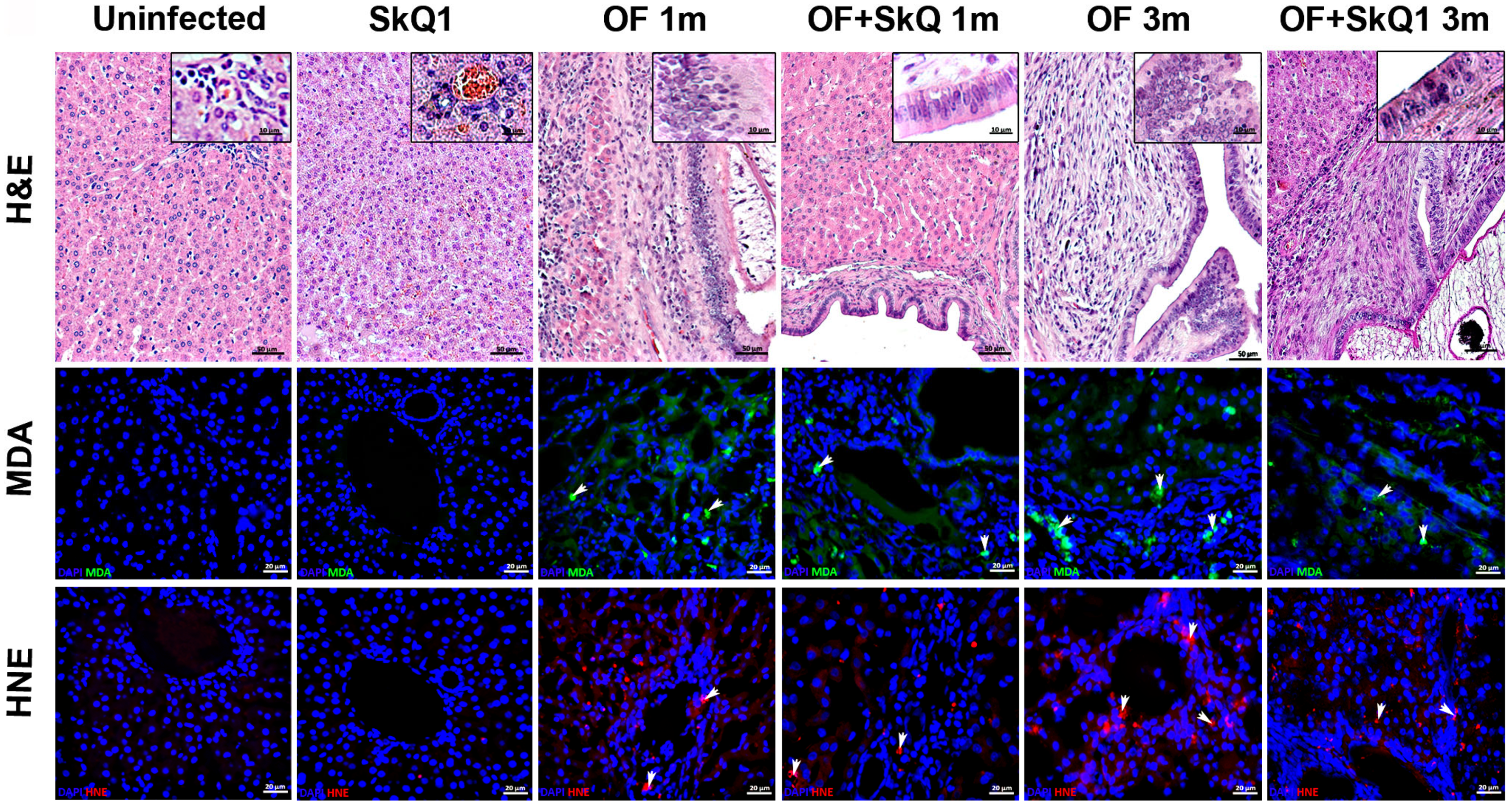
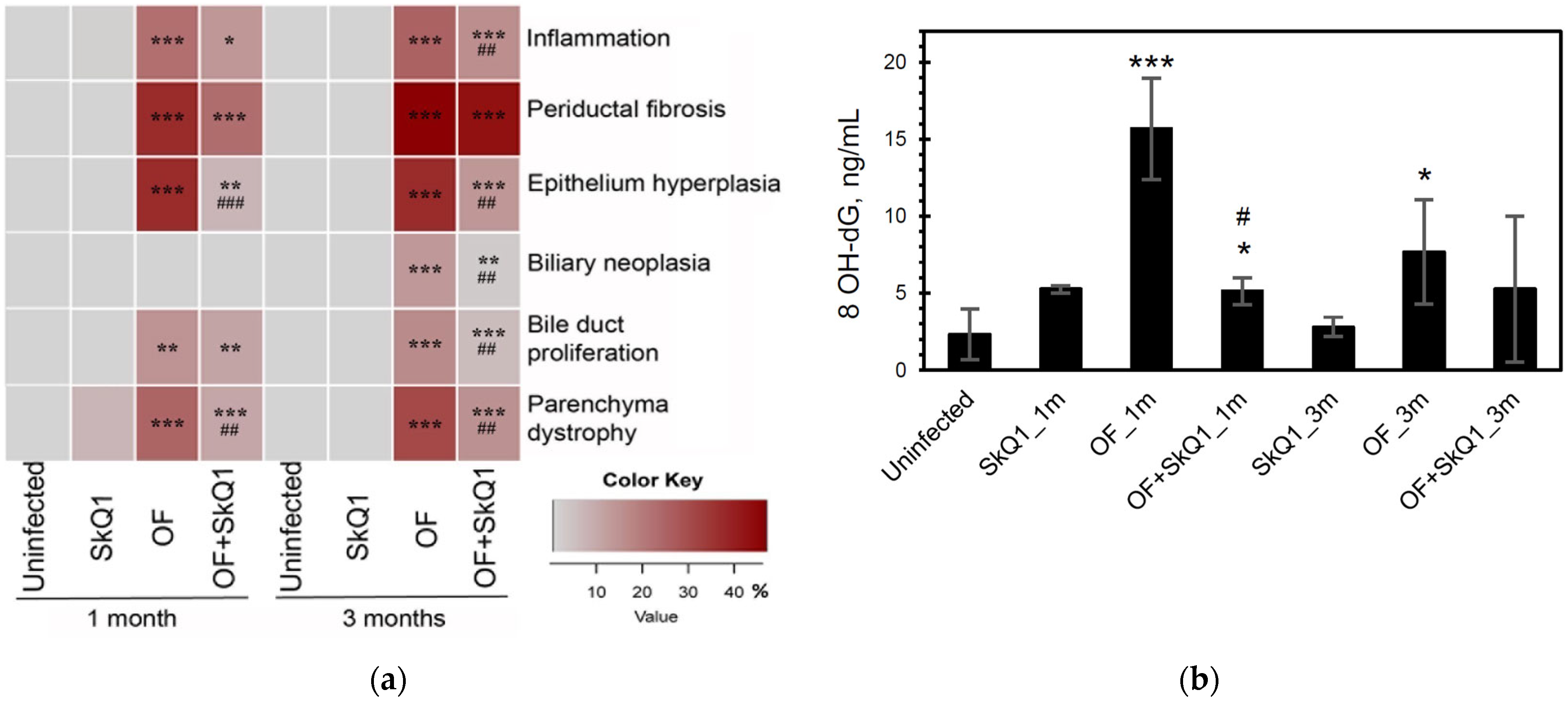
3.1. Liver Histopathology
3.2. Oxidative Lesions
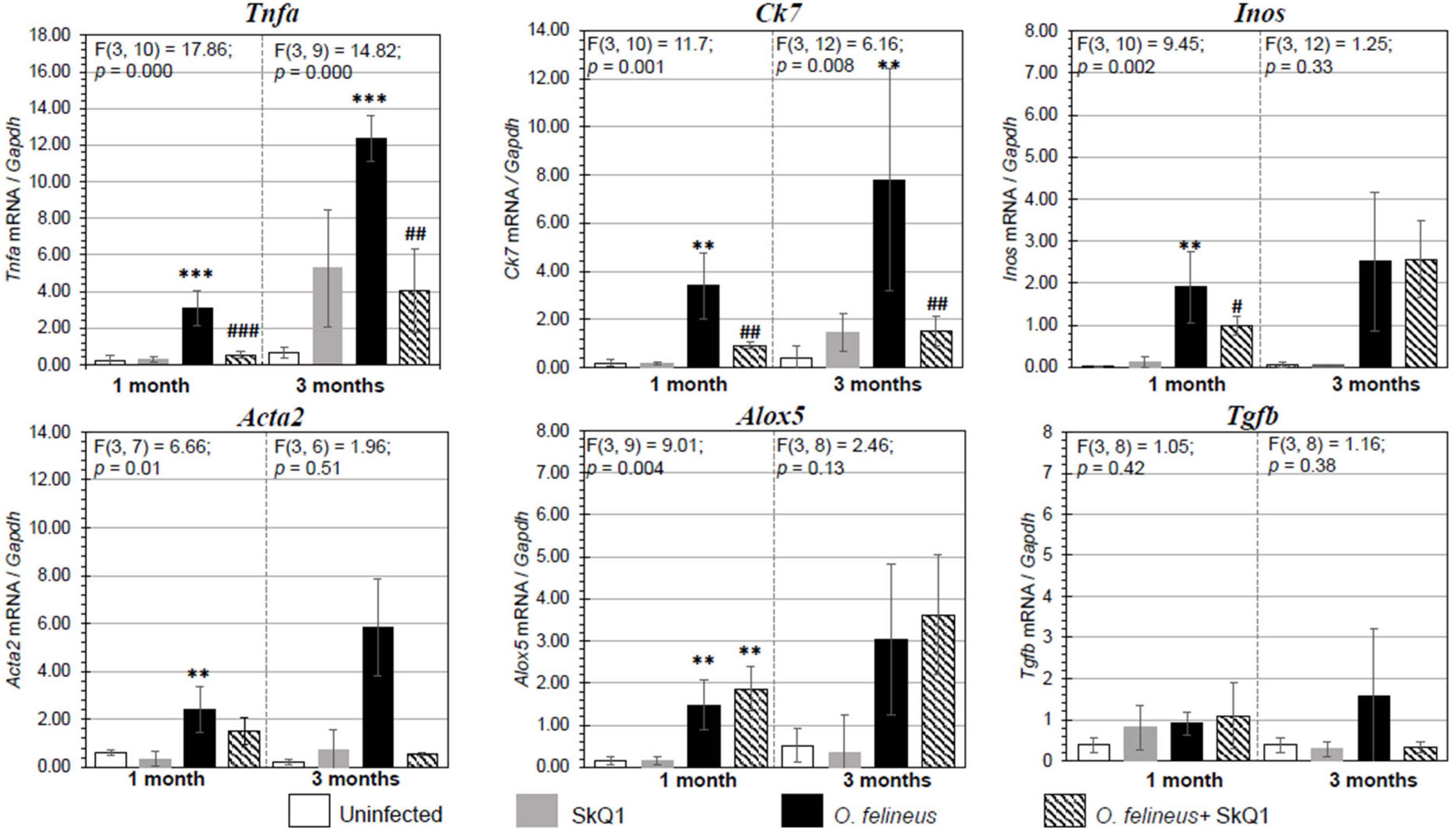
3.3. Fibrosis- and Inflammation-Related Gene Expression
3.4. NRF2 and NQO1
3.5. Evaluation of Anthelmintic Properties of SkQ1 In Vivo
3.6. Serum Biochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beer, S.A. Biology of the Agent of Opisthorchiasis; KMK: Moscow, Russia, 2005; pp. 204–208. ISBN 5-87317-204-8. [Google Scholar]
- Pozio, E.; Armignacco, O.; Ferri, F.; Gomez Morales, M.A. Opisthorchis felineus, an emerging infection in Italy and its implication for the European Union. Acta Trop. 2013, 126, 54–62. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Mordvinov, V.A. Similarities and differences among the Opisthorchiidae liver flukes: Insights from Opisthorchis felineus. Parasitology 2022, 149, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Zaparina, O.; Baginskaya, N.V.; Mordvinov, V.A. Global changes in gene expression related to Opisthorchis felineus liver fluke infection reveal temporal heterogeneity of a mammalian host response. Food Waterborne Parasitol. 2022, 27, e00159. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 341–365. [Google Scholar]
- Bottari, N.B.; Mendes, R.E.; Lucca, N.J.; Schwertz, C.I.; Henker, L.C.; Olsson, D.C.; Piva, M.M.; Sangoi, M.; Campos, L.P.; Moresco, R.N.; et al. Oxidative stress associated with pathological lesions in the liver of rats experimentally infected by Fasciola hepatica. Exp. Parasitol. 2015, 159, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Zaparina, O.G.; Rakhmetova, A.S.; Kolosova, N.G.; Cheng, G.; Mordvinov, V.A.; Pakharukova, M.Y. Antioxidants resveratrol and SkQ1 attenuate praziquantel adverse effects on the liver in Opisthorchis felineus infected hamsters. Acta Trop. 2021, 220, 105954. [Google Scholar] [CrossRef]
- Rehman, A.; Rehman, L.; Ullah, R.; Beg, M.A.; Khan, M.A.H.; Abidi, S.M.A. Oxidative status and changes in the adenosine deaminase activity in experimental host infected with tropical liver fluke, Fasciola gigantica. Acta Trop. 2021, 213, 105753. [Google Scholar] [CrossRef]
- Saichua, P.; Yakovleva, A.; Kamamia, C.; Jariwala, A.R.; Sithithaworn, J.; Sripa, B.; Brindley, P.J.; Laha, T.; Mairiang, E.; Pairojkul, C.; et al. Levels of 8-OxodG Predict Hepatobiliary Pathology in Opisthorchis viverrini Endemic Settings in Thailand. PLoS Negl. Trop. Dis. 2015, 31, e0003949. [Google Scholar] [CrossRef]
- Charoensuk, L.; Pinlaor, P.; Wanichwecharungruang, S.; Intuyod, K.; Vaeteewoottacharn, K.; Chaidee, A.; Yongvanit, P.; Pairojkul, C.; Suwannateep, N.; Pinlaor, S. Nanoencapsulated curcumin and praziquantel treatment reduces periductal fibrosis and attenuates bile canalicular abnormalities in Opisthorchis viverrini-infected hamsters. Nanomedicine 2016, 12, 21–32. [Google Scholar] [CrossRef]
- Jamnongkan, W.; Thanee, M.; Yongvanit, P.; Loilome, W.; Thanan, R.; Kimawaha, P.; Boonmars, T.; Silakit, R.; Namwat, N.; Techasen, A. Antifibrotic effect of xanthohumol in combination with praziquantel is associated with altered redox status and reduced iron accumulation during liver fluke-associated cholangiocarcinogenesis. PeerJ 2018, 22, e4281. [Google Scholar] [CrossRef]
- Turkseven, S.; Bolognesi, M.; Brocca, A.; Pesce, P.; Angeli, P.; Di Pascoli, M. Mitochondria-targeted antioxidant mitoquinone attenuates liver inflammation and fibrosis in cirrhotic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, S.; Tang, H.; Fang, W.; Chen, K.; Chen, X. CoQ10 protects against acetaminophen-induced liver injury by enhancing mitophagy. Toxicol. Appl. Pharmacol. 2021, 410, 115355. [Google Scholar] [CrossRef] [PubMed]
- Demiroren, K.; Basunlu, M.T.; Erten, R.; Cokluk, E. A comparison of the effects of thymoquinone, silymarin and N-acetylcysteine in an experimental hepatotoxicity. Biomed. Pharmacother. 2018, 106, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G.; et al. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta 2009, 1787, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, M.V.; Antonenko, Y.N.; Anisimov, V.N.; Chernyak, B.V.; Cherepanov, D.A.; Chistyakov, V.A.; Egorov, M.V.; Kolosova, N.G.; Korshunova, G.A.; Lyamzaev, K.G.; et al. Mitochondrial-targeted plastoquinone derivatives. Effect on senescence and acute age-related pathologies. Curr. Drug Targets 2011, 12, 800–826. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Romaschenko, V.P.; Galkin, I.I.; Zakharova, V.V.; Pletjushkina, O.Y.; Chernyak, B.V.; Popova, E.N. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging 2014, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Pakharukov, Y.V.; Mordvinov, V.A. Effects of miconazole/clotrimazole and praziquantel combinations against the liver fluke Opisthorchis felineus in vivo and in vitro. Parasitol. Res. 2018, 117, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Van Hoosier, G.L.; McPherson, C.W. Laboratory Hamsters; Academic Press: Orlando, FL, USA, 1987. [Google Scholar]
- Vnukov, V.V.; Gutsenko, O.I.; Milutina, N.P.; Kornienko, I.V.; Ananyan, A.A.; Danilenko, A.O.; Panina, S.B.; Plotnikov, A.A.; Makarenko, M.S. Influence of SkQ1 on Expression of Nrf2 Gene, ARE-controlled genes of antioxidant enzymes and their activity in rat blood leukocytes under oxidative stress. Biochemistry 2015, 80, 1598–1605. [Google Scholar] [CrossRef]
- Alavi, M.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Resveratrol mediates its anti-cancer effects by Nrf2 signaling pathway activation. Cancer Cell Int. 2021, 21, 579. [Google Scholar] [CrossRef]
- Mordvinov, V.A.; Ponomarev, D.V.; Pakharukov, Y.V.; Pakharukova, M.Y. Anthelmintic activity of antioxidants: In vitro effects on the liver fluke Opisthorchis felineus. Pathogens 2021, 10, 284. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondriatargeted plastoquinone derivatives as tools to interrupt execution of the aging program. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry 2008, 73, 1273–1287. [Google Scholar]
- Kawanishi, S.; Hiraku, Y. Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid. Redox. Signal 2006, 8, 1047–1058. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Prueksapanich, P.; Piyachaturawat, P.; Aumpansub, P.; Ridtitid, W.; Chaiteerakij, R.; Rerknimitr, R. Liver fluke-associated biliary tract cancer. Gut Liver 2017, 12, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Gallo, C.; Invernizzi, P.; Massironi, S. Any role for microbiota in cholangiocarcinoma? A Comprehensive Review. Cells 2023, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Lishai, E.A.; Zaparina, O.; Baginskaya, N.V.; Hong, S.-J.; Sripa, B.; Mordvinov, V.A. Opisthorchis viverrini, Clonorchis sinensis and Opisthorchis felineus liver flukes affect mammalian host microbiome in a species-specific manner. PLoS Negl. Trop. Dis. 2023, 17, e0011111. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Zaparina, O.; Hong, S.J.; Sripa, B.; Mordvinov, V.A. A comparative study of Helicobacter pylori infection in hamsters experimentally infected with liver flukes Opisthorchis felineus, Opisthorchis viverrini, or Clonorchis sinensis. Sci. Rep. 2021, 11, 7789. [Google Scholar] [CrossRef]
- Chienwichai, P.; Thiangtrongjit, T.; Tipthara, P.; Tarning, J.; Adisakwattana, P.; Reamtong, O. Untargeted serum metabolomics analysis of Trichinella spiralis-infected mouse. PLoS Negl. Trop. Dis. 2023, 17, e0011119. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Fedorov, A.V.; Ilyinskaya, O.P.; Zinovkin, R.A.; Chernyak, B.V. The role of reactive oxygen in mast cell degranulation. Biochemistry 2016, 81, 1564–1577. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Averina, O.A.; Vasilyeva, T.V.; Pletiushkina, O.Y.; Popova, E.N.; Fedorov, A.V.; Chernyak, B.V.; Shishkina, V.S.; Ilinskaya, O.P. Mitochondria Targeted Antioxidant SkQ1 (10-(6′-Plastoquinonyl)decyltriphenylphosphonium bromide) inhibits mast cell degranulation in vivo and in vitro. Biochemistry 2017, 82, 1493–1503. [Google Scholar] [CrossRef]
- Song, J.; Sheng, J.; Lei, J.; Gan, W.; Yang, Y. Mitochondrial Targeted Antioxidant SKQ1 ameliorates acute kidney injury by inhibiting ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 2223957. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.G.; Kozhevnikova, O.S.; Muraleva, N.A.; Rudnitskaya, E.A.; Rumyantseva, Y.V.; Stefanova, N.A.; Telegina, D.V.; Tyumentsev, M.A.; Fursova, A.Z. SkQ1 as a tool for controlling accelerated senescence program: Experiments with OXYS rats. Biochemistry 2022, 87, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, P. The Nrf2 transcription factor: A multifaceted regulator of the extracellular matrix. Matrix Biol. Plus. 2021, 10, 100057. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Liu, J.; Klaassen, C.D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol. Appl. Pharmacol. 2012, 262, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Aleksunes, L.M.; Yeager, R.L.; Gyamfi, M.A.; Esterly, N.; Guo, G.L.; Klaassen, C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–664. [Google Scholar] [CrossRef] [PubMed]
- More, V.R.; Cheng, Q.; Donepudi, A.C.; Buckley, D.B.; Lu, Z.J.; Cherrington, N.J.; Slitt, A.L. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab. Dispos. 2013, 41, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Umemura, T.; Kanki, K.; Kodama, Y.; Kitamoto, S.; Saito, K.; Itoh, K.; Yamamoto, M.; Masegi, T.; Nishikawa, A.; et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo [4,5-f] quinoline. Cancer Sci. 2007, 98, 19–24. [Google Scholar] [CrossRef] [PubMed]

| Untreated | SkQ1 | Praziquantel | |
|---|---|---|---|
| Number of animals | 5 | 3 | 5 |
| Mean no. of worms ± SD | 56 ± 9 | 55 ± 12 | 8 ± 6 ** |
| WBR (%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaparina, O.; Kovner, A.; Petrova, V.; Kolosova, N.; Mordvinov, V.; Pakharukova, M. Plastoquinone-Derivative SkQ1 Improved the Biliary Intraepithelial Neoplasia during Liver Fluke Infection. Curr. Issues Mol. Biol. 2024, 46, 1593-1606. https://doi.org/10.3390/cimb46020103
Zaparina O, Kovner A, Petrova V, Kolosova N, Mordvinov V, Pakharukova M. Plastoquinone-Derivative SkQ1 Improved the Biliary Intraepithelial Neoplasia during Liver Fluke Infection. Current Issues in Molecular Biology. 2024; 46(2):1593-1606. https://doi.org/10.3390/cimb46020103
Chicago/Turabian StyleZaparina, Oxana, Anna Kovner, Viktoria Petrova, Nataliya Kolosova, Viatcheslav Mordvinov, and Maria Pakharukova. 2024. "Plastoquinone-Derivative SkQ1 Improved the Biliary Intraepithelial Neoplasia during Liver Fluke Infection" Current Issues in Molecular Biology 46, no. 2: 1593-1606. https://doi.org/10.3390/cimb46020103
APA StyleZaparina, O., Kovner, A., Petrova, V., Kolosova, N., Mordvinov, V., & Pakharukova, M. (2024). Plastoquinone-Derivative SkQ1 Improved the Biliary Intraepithelial Neoplasia during Liver Fluke Infection. Current Issues in Molecular Biology, 46(2), 1593-1606. https://doi.org/10.3390/cimb46020103






