Diversity of Improved Diploids and Commercial Triploids from Musa spp. via Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction
2.3. IRAP Analysis
2.4. SSR Analysis
2.5. ISSR Analysis
2.6. Electrophoresis and Data Analysis
3. Results
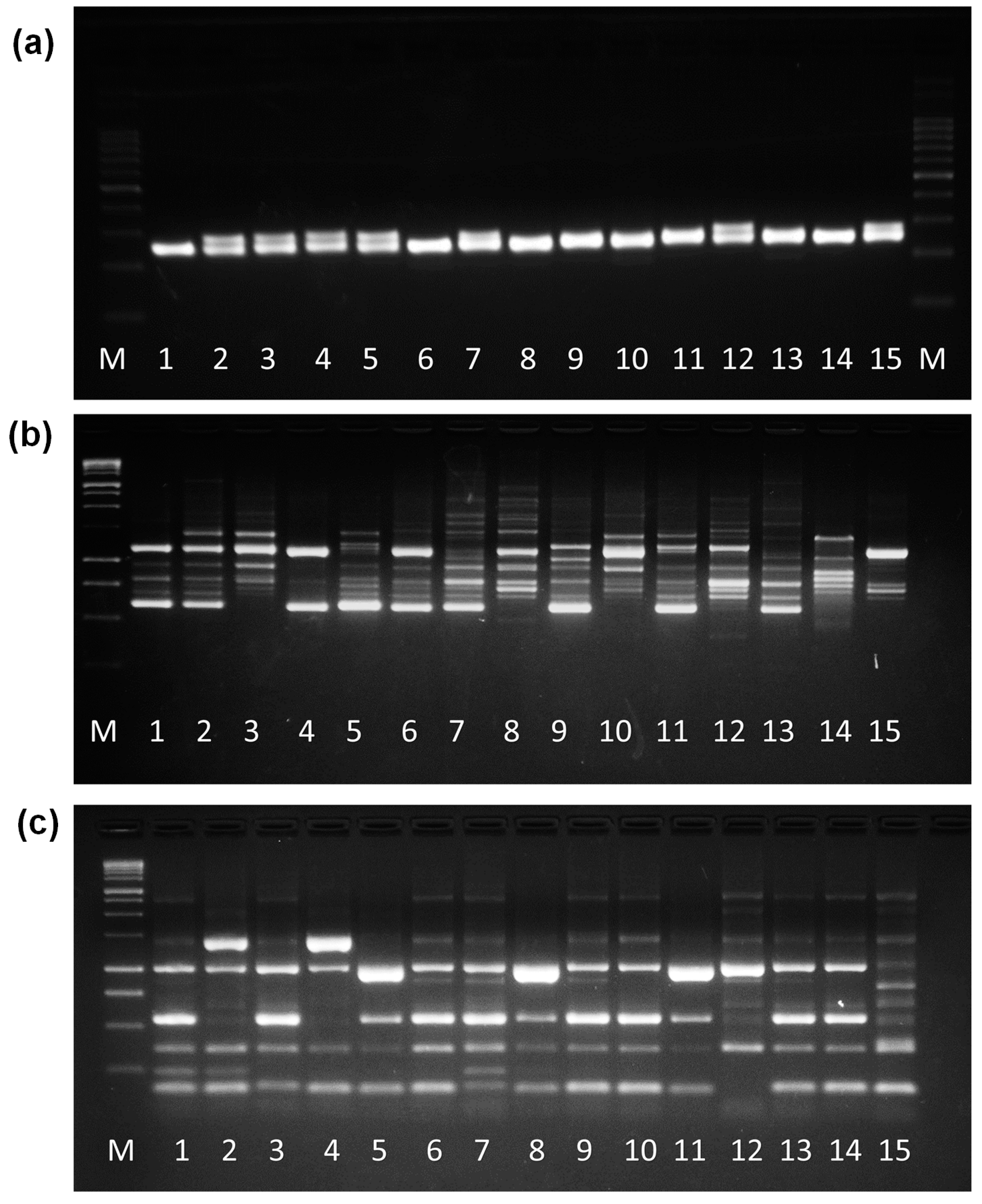
3.1. Molecular Analysis of Gene Polymorphism by DNA Molecular Markers—SSR, ISSR, and IRAP
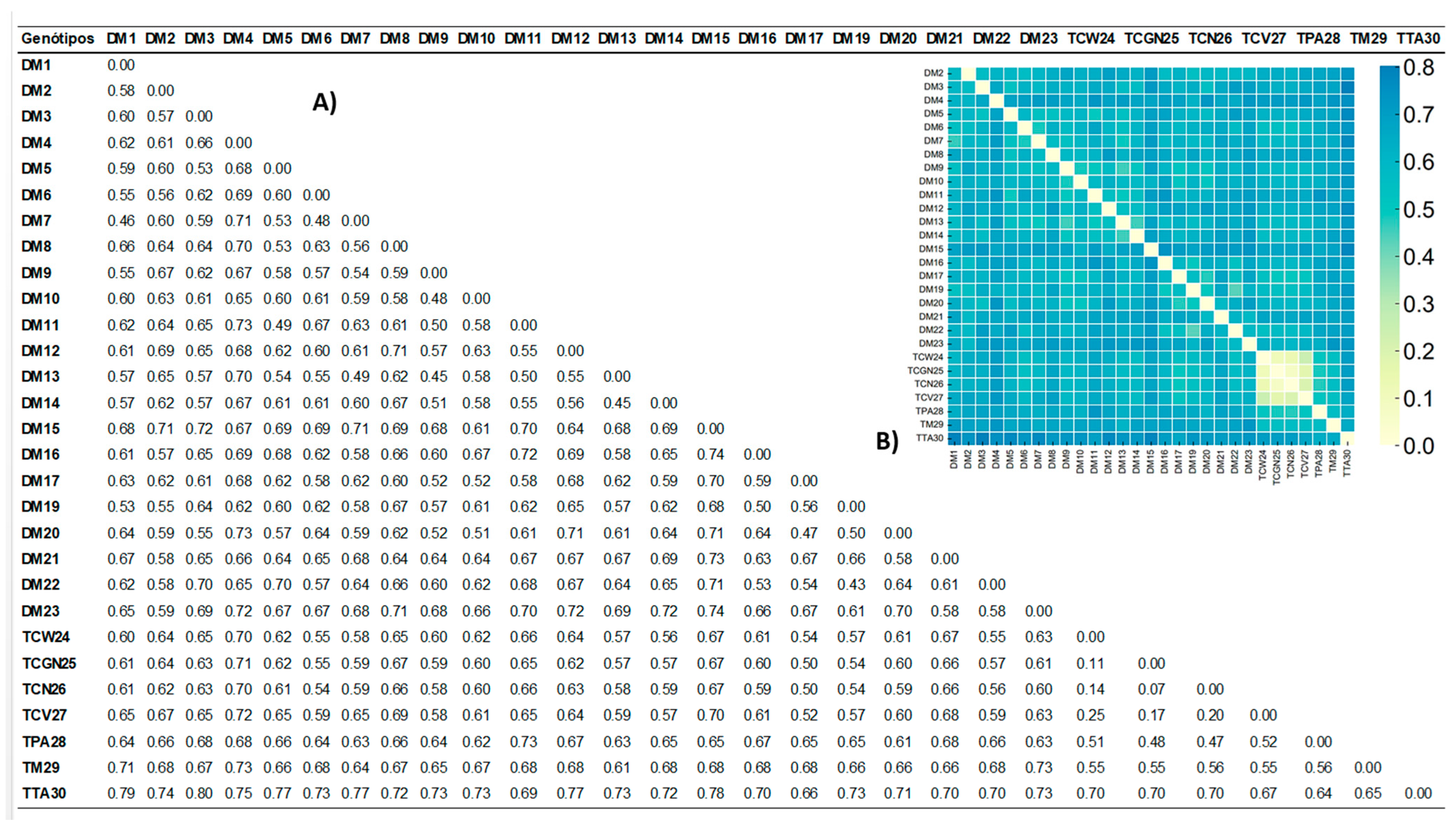
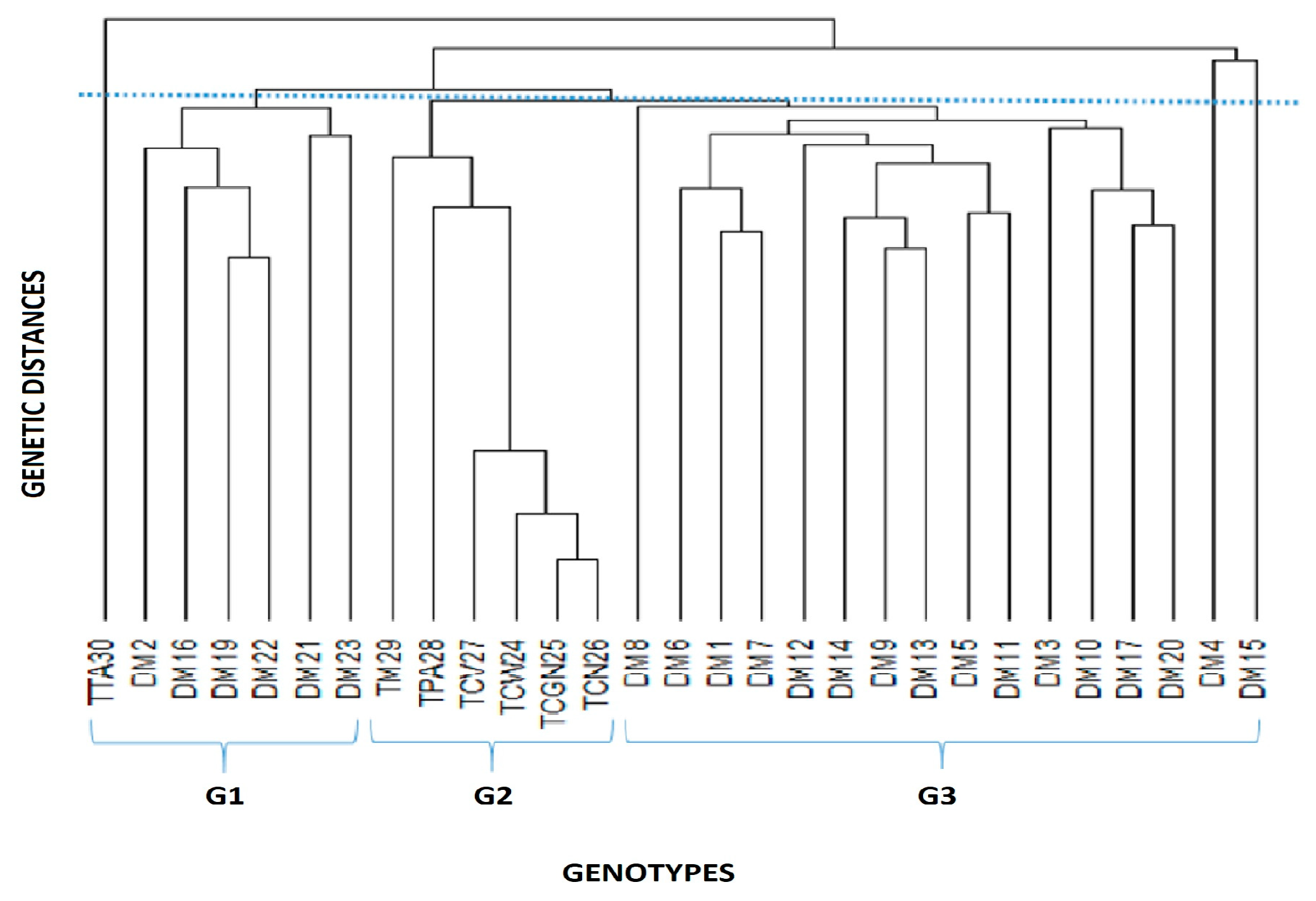
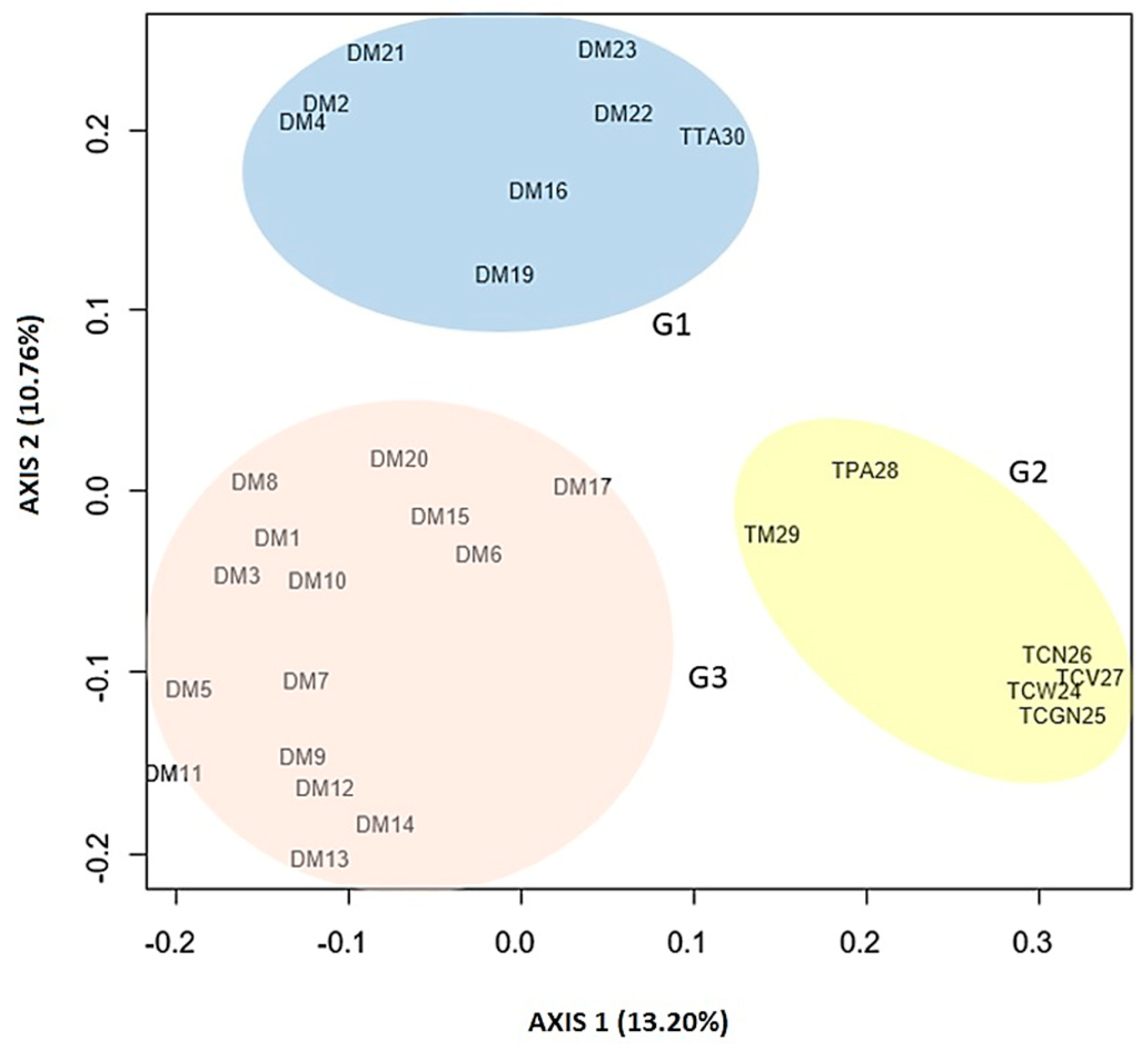
3.2. Genetic Diversity
4. Discussion
Molecular Analysis of Gene Polymorphism and Genetic Diversity
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, K.; Alves, E.J. The banana breeding programme at the Centro Nacional de Pesquisa de Mandioca e Fruticultura (CNPMF), Bahia State, Brazil. Fruits 1981, 39, 154–157. [Google Scholar]
- Shepherd, K. Banana Breeding—Past and Present. Acta Hortic. 1987, 196, 37–44. [Google Scholar] [CrossRef]
- Silva, S.O.; Souza Junior, M.T.; Alves, É.J.; Silveira, J.R.S.; Lima, M.B. Banana Breeding Program at Embrapa. Crop Breed. Appl. Biotechnol. 2001, 1, 399–436. [Google Scholar] [CrossRef]
- Amorim, E.P.; Amorim, V.B.O.; Silva, S.O.; Pillay, M. Quality improvement of cultivated Musa. In Banana Breeding: Progress and Challenges; Pillay, M., Tenkouano, A., Eds.; CRC Press: New York, NY, USA, 2011; pp. 252–280. [Google Scholar]
- Creste, S.; Neto, A.T.; Silva, S.D.; Figueira, A. Genetic characterization of banana cultivars (Musa spp.) from Brazil using microsatellite markers. Euphytica 2003, 132, 259–268. [Google Scholar] [CrossRef]
- Santos, T.T.C.; Amorim, V.B.O.; Santos-Serejo, J.A.; Silva Ledo, C.A.; Haddad, F.; Ferreira, C.F.; Amorim, E.P. Genetic variability among autotetraploid populations of banana plants derived from wild diploids through chromosome doubling using SSR and molecular markers based on retrotransposons. Mol. Breed. 2019, 39, 95. [Google Scholar] [CrossRef]
- Teo, C.H.; Tan, S.H.; Ho, C.L.; Faridah, Q.Z.; Othman, Y.R.; Heslop-Harrison, J.S.; Kalendar, R.; Schulman, A.H. Genome constitution and classification using retrotransposon-based markers in the orphan crop banana. J. Plant Biol. 2005, 48, 96–105. [Google Scholar] [CrossRef]
- Saraswathi, M.S.; Uma, S.; Prasanya Selvam, K.; Ramaraj, S.; Durai, P.; Mustaffa, M.M. Assessing the robustness of IRAP and RAPD marker systems to study intra-group diversity among Cavendish (AAA) clones of banana. J. Hortic. Sci. Biotechnol. 2011, 86, 7–12. [Google Scholar] [CrossRef]
- Simmonds, N.W.; Shepherd, K. The taxonomy and origins of the cultivated bananas. Bot. J. Linn. Soc. 1955, 55, 302–312. [Google Scholar] [CrossRef]
- Häkkinen, M. Reappraisal of sectional taxonomy in Musa (Musaceae). Taxon 2013, 62, 809–813. [Google Scholar] [CrossRef]
- Baurens, F.-C.; Martin, G.; Hervouet, C.; Salmon, F.; Yohomé, D.; Ricci, S.; Rouard, M.; Habas, R.; Lemainque, A.; Yahiaoui, N.; et al. Recombination and Large Structural Variations Shape Interspecific Edible Bananas Genomes. Mol. Bio. Evol. 2019, 36, 97–111. [Google Scholar] [CrossRef]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Fusarium wilt of banana: Biology, epidemiology and management. Int. J. Pest Manag. 2015, 61, 250–263. [Google Scholar] [CrossRef]
- Silva, S.O.; Amorim, E.P.; Santos-Serejo, J.A.; Ferreira, C.F.; Rodriguez, M.A.D. Melhoramento genético da bananeira: Estratégias e tecnologias disponíveis. Rev. Bras. Frutic. 2013, 35, 919–931. [Google Scholar] [CrossRef]
- Centro de Estudos Avançados em Economia Aplicada—CEPEA. Banana. In Anuário Hortifruti Brasil: Retrospectiva 2023 & Perspectiva 2024; Editora Gazeta: Santa Cruz do Sul, Brazil, 2023; pp. 26–27. Available online: https://www.hfbrasil.org.br/br/revista/anuario-hf-brasil-retrospectiva-2023-perspectiva-2024.aspx (accessed on 10 July 2024).
- Liu, X.; Arshad, R.; Wang, X.; Li, W.-M.; Zhou, Y.; Ge, X.-J.; Huang, H.-R. The phased telomere-to-telomere reference genome of Musa acuminata, a main contributor to banana cultivars. Sci. Data 2023, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.O.; Matos, A.P.; Alves, E.J. Melhoramento genético da bananeira. Pesq. Agropec. Bras. 1998, 33, 693–703. [Google Scholar]
- Wang, Z.; Miao, H.; Liu, J.; Xu, B.; Yao, X.; Xu, C.; Zhao, S.; Fang, X.; Jia, C.; Wang, J.; et al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 2019, 5, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Arvas, Y.E.; Marakli, S.; Kaya, Y.; Kalendar, R. The power of retrotransposons in high-throughput genotyping and sequencing. Front. Plant Sci. 2023, 14, 1174339. [Google Scholar] [CrossRef]
- Kalendar, R.; Flavell, A.J.; Ellis, T.H.N.; Sjakste, T.; Moisy, C.; Schulman, A.H. Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 2011, 106, 520–530. [Google Scholar] [CrossRef]
- Chabannes, M.; Gabriel, M.; Aksa, A.; Galzi, S.; Dufayard, J.F.; Iskra-Caruana, M.L.; Muller, E. Badnaviruses and banana genomes: A long association sheds light on Musa phylogeny and origin. Mol. Plant Pathol. 2021, 22, 216–230. [Google Scholar] [CrossRef]
- Biswas, M.K.; Bagchi, M.; Biswas, D.; Harikrishna, J.A.; Liu, Y.; Li, C.; Sheng, O.; Mayer, C.; Yi, G.; Deng, G. Genome-Wide Novel Genic Microsatellite Marker Resource Development and Validation for Genetic Diversity and Population Structure Analysis of Banana. Genes 2020, 11, 1479. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, H.; Zhang, M.; Qiao, G.; Shen, X. IRAPs in Combination with Highly Informative ISSRs Confer Effective Potentials for Genetic Diversity and Fidelity Assessment in Rhododendron. Int. J. Mol. Sci. 2023, 24, 6902. [Google Scholar] [CrossRef] [PubMed]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.M.; Shirzadian-Khorramabad, R.; Sabouri, H.; Rabiei, B.; Moghadam, H.H. Identification of Quantitative Trait Loci Related to Salt Tolerance of Indica Rice RIL Population in Different Growth Stages. Russ. J. Genet. 2022, 58, 1091–1103. [Google Scholar] [CrossRef]
- Makhtoum, S.; Sabouri, H.; Gholizadeh, A.; Ahangar, L.; Katouzi, M. QTLs Controlling Physiological and Morphological Traits of Barley (Hordeum vulgare L.) Seedlings under Salinity, Drought, and Normal Conditions. BioTech 2022, 11, 26. [Google Scholar] [CrossRef]
- Pakhrou, O.; Medraoui, L.; Yatrib, C.; Alami, M.; Filali-Maltouf, A.; Belkadi, B. Assessment of genetic diversity and population structure of an endemic Moroccan tree (Argania spinosa L.) based in IRAP and ISSR markers and implications for conservation. Physiol. Mol. Biol. Plants 2017, 23, 651–661. [Google Scholar] [CrossRef]
- AGRITEMPO. Agritempo: Sistema de Monitoramento Agrometeorológico. Available online: https://www.agritempo.gov.br/en/ (accessed on 22 July 2024).
- Amorim, E.P.; Santos-Serejo, J.A.; Amorim, V.B.O.; Ferreira, C.F.; Silva, S. Banana breeding at Embrapa cassava and fruits. Acta Hortic. 2013, 986, 171–176. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; Haddad, F.; de Oliveira Amorim, V.B.; Ferreira, C.F.; de Oliveira, S.A.S.; Amorim, E.P. Agronomic characterization and identification of banana genotypes resistant to Fusarium wilt race 1. Eur. J. Plant Pathol. 2019, 155, 1093–1103. [Google Scholar] [CrossRef]
- Nascimento, F.d.S.; Sousa, Y.M.; Rocha, A.d.J.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Sources of black Sigatoka resistance in wild banana diploids. Rev. Bras. Frutic. 2020, 42, e-038. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; de Jesus Rocha, A.; Haddad, F.; de Oliveira Amorim, V.B.; Ferreira, C.F.; Amorim, E.P. Selection of Diploid and Tetraploid Banana Hybrids Resistant to Pseudocercospora fijiensis. Agronomy 2021, 11, 2483. [Google Scholar] [CrossRef]
- Santana, W.S.; Rocha, A.D.J.; Da Silva, W.B.; Amorim, V.B.O.D.; Ramos, A.P.D.S.; Haddad, F.; Amorim, E.P. Selection of Improved Banana Diploid Resistant to Fusarium oxysporum f. sp. cubense Races 1 and Subtropical 4. Agronomy 2024, 14, 1277. [Google Scholar] [CrossRef]
- Ferreira, C.F.; Gutierrez, D.L.; Kreuze, J.F.; Iskra-Caruana, M.L.; Chabannes, M.; Barbosa, A.C.O.; Santos, T.A.; Silva, A.G.S.; Santos, R.M.F.; Amorim, E.P.; et al. Rapid plant DNA and RNA extraction protocol using a bench drill. Genet. Mol. Res. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Baumel, A.; Ainouche, M.; Kalendar, R.; Schulman, A.H. Retrotransposons and genomic stability in populations of the young allopolyploide species Spartina anglica C.E. Hubbard (Poaceae). Mol. Biol. Evol. 2002, 19, 1218–1227. [Google Scholar] [CrossRef]
- Christelová, P.; Valárik, M.; Hřibová, E.; Houwe, I.V.D.; Channelière, S.; Roux, N.; Doležel, J. A platform for efficient genotyping in Musa using microsatellite markers. AoB Plants 2011, 2011, plr024. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: https://www.R-project.org/ (accessed on 10 July 2024).
- Mingoti, S.A. Análise de Dados Através de Métodos de Estatística Multivariada: Uma Abordagem Aplicada; Editora UFMG: Belo Horizonte, Brazil, 2005. [Google Scholar]
- Bered, F.; Barbosa Neto, J.F.; Rocha, B.M.; Pegoraro, D.G.; Vacaro, E.; Carvalho, F.I.F. Characterization of wheat germplasm through the cycle and height adaptive characters. Pesq. Agropec. Bras. 2002, 37, 145–150. [Google Scholar] [CrossRef]
- Biswas, M.K.; Biswas, D.; Yi, G.; Deng, G. The Musa Marker Database: A Comprehensive Genomic Resource for the Improvement of the Musaceae Family. Agronomy 2024, 14, 838. [Google Scholar] [CrossRef]
- Dantas, J.L.L.; Shepherd, K.; Soares Filho, W.S.; Cordeiro, Z.J.M.; Silva, S.O.; Alves, E.J.; Souza, A.S.; Oliveira, M.A. Programa de Melhoramento Genético da Bananeira em Execução no CNPMF/Embrapa; Documentos, 47; Embrapa-CNPMF: Cruz das Almas, Brzsil, 1993; p. 43. [Google Scholar]
- Shepherd, K.; Dantas, J.L.L.; Goutant-Bakry, M.-E.; Dzoyem, C.U.D.; Bakry, F. Development and functioning of the embryo sac in four triploid banana cultivars. Pesq. Agropec. Bras. 2023, 58, e03204. [Google Scholar] [CrossRef]
- Silva, M.S.; Goes, N.H.; Santos-Serejo, J.A.; Ferreira, C.F.; Amorim, E.P. Phenolic compounds and oxidative enzymes involved in female fertility in banana plants of the Cavendish subgroup. Plants 2021, 10, 2790. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Santana, A.N.; Santos-Serejo, J.A.; Ferreira, C.F.; Amorim, E.P. Morphoanatomy and histochemistry of septal nectaries related to female fertility in banana plants of the ‘Cavendish’ subgroup. Plants 2022, 11, 1177. [Google Scholar] [CrossRef]
- Donato, S.L.R.; Arantes, A.M.; Silva, S.O.E.; Cordeiro, Z.J.M. Phytotechnical behavior of ‘Prata-Anã’ banana and progeny hybrids. Pesq. Agropec. Bras. 2009, 44, 1608–1615. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Satovic, Z.; Pêgo, S.; Fevereiro, P. Assessing the genetic diversity of Portuguese maize germoplasm using microsatellite markers. Euphytica 2004, 137, 6372. [Google Scholar] [CrossRef]
- Amorim, E.P.; Lessa, L.S.; Ledo, C.A.S.; Amorim, V.B.O.; Reis, R.V.; Santos-Serejo, J.A.; Silva, S.O. Agronomic and molecular characterization of diploid improved banana genotypes. Rev. Bras. Frutic. 2009, 31, 154–161. [Google Scholar] [CrossRef]
- Amorim, E.P.; Reis, R.V.; Santos-Serejo, J.A.; Amorim, V.B.O.; Silva, S.O. Genetic variability estimated in banana diploids through microsatellite markers. Pesq. Agropec. Bras. 2008, 43, 1045–1052. [Google Scholar] [CrossRef]
- Mattos-Moreira, L.; Ferreira, C.F.; Amorim, E.P.; Pirovani, C.P.; Filho, M.A.C.; Ledo, C.A.S. Diferentially expressed proteins associated with drought tolerance in bananas (Musa spp.). Acta Physiol. Plant. 2018, 40, 60. [Google Scholar] [CrossRef]
- Rocha, A.J.; Soares, J.M.S.; Nascimento, F.S.; Rocha, A.S.; Amorim, V.B.O.; Ramos, A.P.S.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Molecular, Histological and Histochemical Responses of Banana Cultivars Challenged with Fusarium oxysporum f. sp. cubense with Different Levels of Virulence. Plants 2022, 11, 2339. [Google Scholar] [CrossRef]
- Nascimento, F.S.; Mascarenhas, M.S.; Boaventura, S.C.; Souza, C.C.H.; Ramos, A.P.S.; Rocha, A.J.; Soaress, J.M.S.; Diniz, E.E.C.; Mendes, T.A.O.; Ferreira, C.F.; et al. Phytoene Desaturase (PDS) Gene-Derived Markers Identify “A” and “B” Genomes in Banana (Musa spp.). Horticulturae 2024, 10, 294. [Google Scholar] [CrossRef]
| Ploidy Level | Code | Genotype | Genealogy |
|---|---|---|---|
| AA | DM1 | CNPMF0612zw | [(M53 × Madu)] × SH3263 |
| AA | DM2 | CNPMF 0998zw | [(Borneo × Guyod)] × [(Borneo × Guyod) × SH3263] |
| AA | DM3 | CNPMF 0534zw | [(Calcutta4 × Madang)] × [(Maccensis-FHIA) × (Tjau Lagada)] |
| AA | DM4 | CNPMF 1323zw | [(Malaccensis-FHIA × Sinwobogi)] × [(Calcutta 4 × Madang) × (Borneo × Madang)] |
| AA | DM5 | 001016-01w | Borneo × Guyod |
| AA | DM6 | M53 | [(Malaccensis − Kedah × Banksii − Samoa)] × [(Paka × Banksii − Samoa)] |
| AA | DM7 | 086094-20w | [(Calcutta 4 × Heva)] × SH3263 |
| AA | DM8 | 058054-03w | [(Calcutta 4 × Pahang)] × [(Borneo × Madang)] |
| AA | DM9 | 013018-01w | Malaccensis-FHIA × Sinwobogi |
| AA | DM10 | 013019-01w | Malaccensis-FHIA × Tjau Lagada |
| AA | DM11 | 013004-04w | Malaccensis-FHIA × Madang |
| AA | DM12 | 050012-02w | Pahang × [(Lidi × SH3263)] |
| AA | DM13 | CNPMF 0542zw | [(Malaccensis-FHIA × Sinwobogi)] × SH3263 |
| AA | DM14 | CNPMF 0496zw | [(M61 × Lidi)] × [(Terrinha × Calcutta 4)] |
| AA | DM15 | CNPMF 0519zw | Self (diployd wild Tambi) |
| AA | DM16 | 042085-02w | [(Madu × Calcutta 4)] × M53 |
| AA | DM17 | CNPMF 0536zw | [(Calcutta 4 × Madang)] × [(Malaccensis-FHIA × Tjau Lagada)] |
| AA | DM18 * | CNPMF 0357 | [(Prata-Anã)] × [(M53 (BGB 06)) × M48] |
| AA | DM19 | CNPMF 0731zw | [(Malaccensis-FHIA × Madang)] × [(Tuu Gia × Madang)] |
| AA | DM20 | CNPMF 0557zw | [(M61 × Lidi)] × [(Malaccensis-FHIA × Tjau Lagada)] |
| AA | DM21 | CNPMF 0513zw | [(M61 × Lidi)] × [(M53 (BGB 06)) × (Kumburgh)] |
| AA | DM22 | CNPMF 0993zw | [(Borneo × Guyod) × (Tuu Gia × Madang)] × [Khai × [(Calcutta 4 × Madang)] |
| AA | DM23 | 028003-01f | Tuu Gia × Madang |
| AAA | TCW24 | Williams | Commercial banana varieties |
| AAA | TCGN25 | Grande Naine | Commercial banana varieties |
| AAA | TCN26 | Nanica | Commercial banana varieties |
| AAA | TCV27 | Valery | Commercial banana varieties |
| AAB | TPA28 | Prata-Anã | Commercial banana varieties |
| AAB | TM29 | Maçã | Commercial banana varieties |
| AAB | TTA30 | Terra-Anã | Plantain |
| Primers | Family of Origin | Sequence (5′-3′) | Reference |
|---|---|---|---|
| 5′LTR2 ← | BARE 1 | 5′-ATC ATT GCC TCT AGG GCA TAA TTC-3′ | [7] |
| 3′LTR → | BARE 1 | 5′-TGT TTC CCA TGC GAC GTT CCC CAA CA-3′ | [7] |
| Sukkula → | Sukkula | 5′-GAT AGG GTC GCA TCT TGG GCG TGA C-3′ | [7] |
| Nikita → | Nikita | 5′-CGC ATT TGT TCA AGC CTA AAC C-3′ | [7] |
| LTR6149 → | BARE 1 | 5′-CTC GCT CGC CCA CTA CAT CAA CCG CGT TTA TT-3′ | [7] |
| LTR6150 ← | BARE 1 | 5′-CTG GTT CGG CCC ATG TCT ATG TAT CCA CAC ATG TA-3′ | [7] |
| CO795 | BARE 1 | 5′-TCC CAT GCG ACG TTC CCC-3′ | [23] |
| C0945 | Sabrina | 5′-GCA AGC TTC CGT TTC CGC-3′ | [23] |
| Marker | T (°C) * | Motif | Reference |
|---|---|---|---|
| mMaCIR 01 | 55.0 | (GA)20 | [36] |
| mMaCIR 07 | 53.0 | (GA)13 | [36] |
| mMaCIR 08 | 55.0 | (TC)6N24(TC)7 | [36] |
| mMaCIR 24 | 52.0 | (TC)7 | [36] |
| mMaCIR 40 | 54.0 | (GA)13 | [36] |
| mMaCIR 150 | 54.0 | (CA)10 | [36] |
| mMaCIR 45 | 58.0 | (TA)4CA(CTCGA)4 | [36] |
| mMaCIR 196 | 55.0 | (TA)4, (TC)17, (TC)3 | [36] |
| mMaCIR 231 | 55.0 | (TC)10 | [36] |
| mMaCIR 13 | 57.0 | (GA)16N76(GA)8 | [36] |
| mMaCIR 39 | 52.0 | (CA)5GATA(GA)5 | [36] |
| mMaCIR 152 | 54.0 | (CTT)18, (CT)17, (CA)6 | [36] |
| Markers | Name | T (°C) * | Sequence | Reference |
|---|---|---|---|---|
| ISSR 92 | TriGAC 3′RC | 45.0 | GACGACGACGACGACRC | Embrapa |
| ISSR 72 | TriTCC 3′RC | 50.0 | TCCTCCTCCTCCTCCRC | Embrapa |
| ISSR 95 | TriGTT 3′RC | 50.0 | GTTGTTGTTGTTGTTRC | Embrapa |
| ISSR 47 | TriTGT5′CY | 50.0 | CYTGTTGTTGTTGTTGT | Embrapa |
| ISSR 29 | TriCAC3′RC | 50.0 | CACCACCACCACCACRC | Embrapa |
| ISSR 07 | DiCA5′CY | 48.0 | CYCACACACACACACACA | Embrapa |
| ISSR 57 | TriACC 3′RC | 50.0 | ACCACCACCACCACCRC | Embrapa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, J.R.; Oliveira, W.D.d.S.; Junior, L.C.d.S.; Nascimento, F.d.S.; Moreira, R.F.C.; Ramos, A.P.d.S.; Santos-Serejo, J.A.d.; Amorim, E.P.; Jesus, R.D.M.d.; Ferreira, C.F. Diversity of Improved Diploids and Commercial Triploids from Musa spp. via Molecular Markers. Curr. Issues Mol. Biol. 2024, 46, 11783-11796. https://doi.org/10.3390/cimb46110700
Sampaio JR, Oliveira WDdS, Junior LCdS, Nascimento FdS, Moreira RFC, Ramos APdS, Santos-Serejo JAd, Amorim EP, Jesus RDMd, Ferreira CF. Diversity of Improved Diploids and Commercial Triploids from Musa spp. via Molecular Markers. Current Issues in Molecular Biology. 2024; 46(11):11783-11796. https://doi.org/10.3390/cimb46110700
Chicago/Turabian StyleSampaio, Juliana Rodrigues, Wanderley Diaciso dos Santos Oliveira, Luiz Carlos de Souza Junior, Fernanda dos Santos Nascimento, Ricardo Franco Cunha Moreira, Andresa Priscila de Souza Ramos, Janay Almeida dos Santos-Serejo, Edson Perito Amorim, Renata Darilia Moraes de Jesus, and Claudia Fortes Ferreira. 2024. "Diversity of Improved Diploids and Commercial Triploids from Musa spp. via Molecular Markers" Current Issues in Molecular Biology 46, no. 11: 11783-11796. https://doi.org/10.3390/cimb46110700
APA StyleSampaio, J. R., Oliveira, W. D. d. S., Junior, L. C. d. S., Nascimento, F. d. S., Moreira, R. F. C., Ramos, A. P. d. S., Santos-Serejo, J. A. d., Amorim, E. P., Jesus, R. D. M. d., & Ferreira, C. F. (2024). Diversity of Improved Diploids and Commercial Triploids from Musa spp. via Molecular Markers. Current Issues in Molecular Biology, 46(11), 11783-11796. https://doi.org/10.3390/cimb46110700






