A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Variables
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
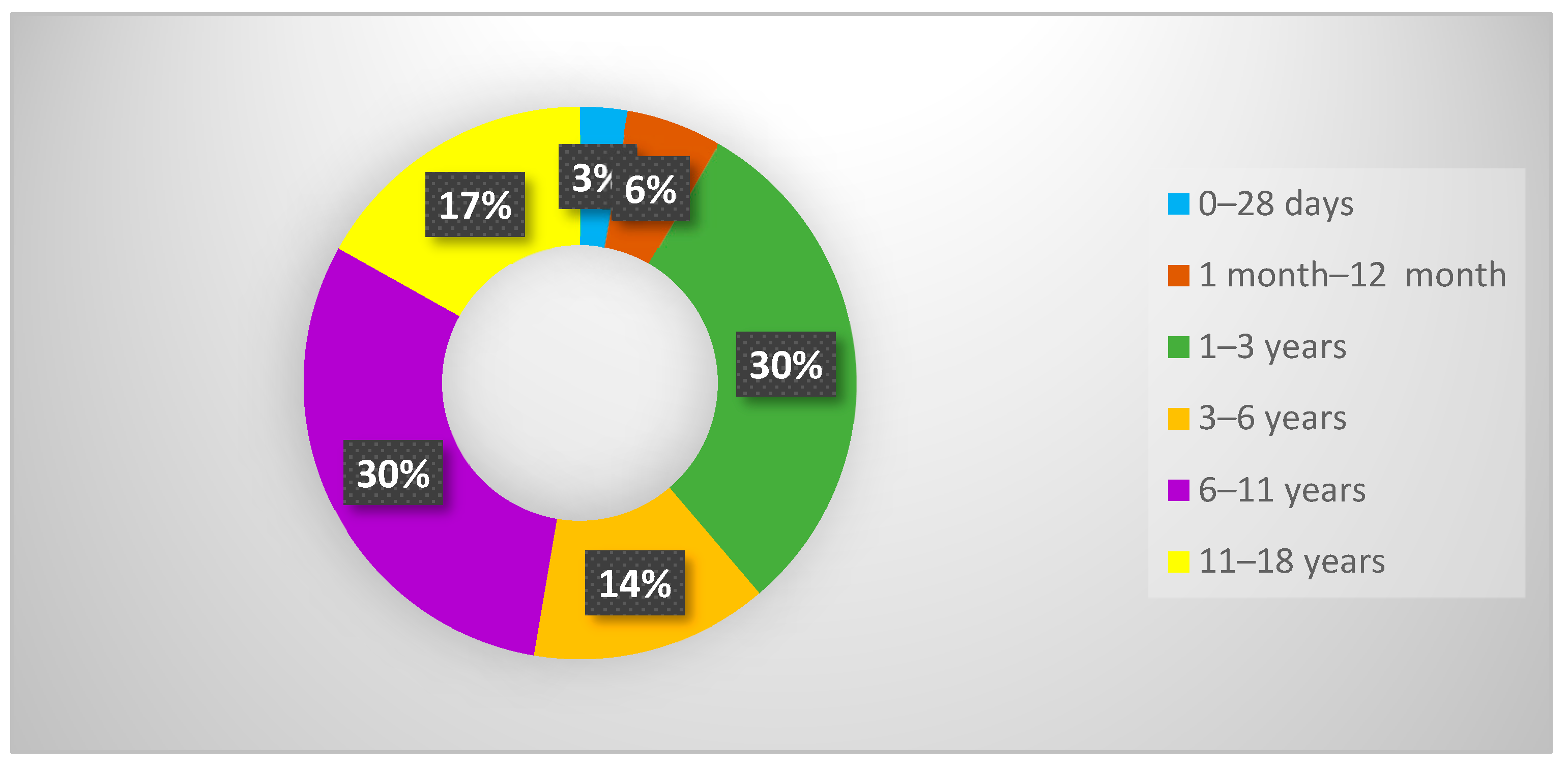
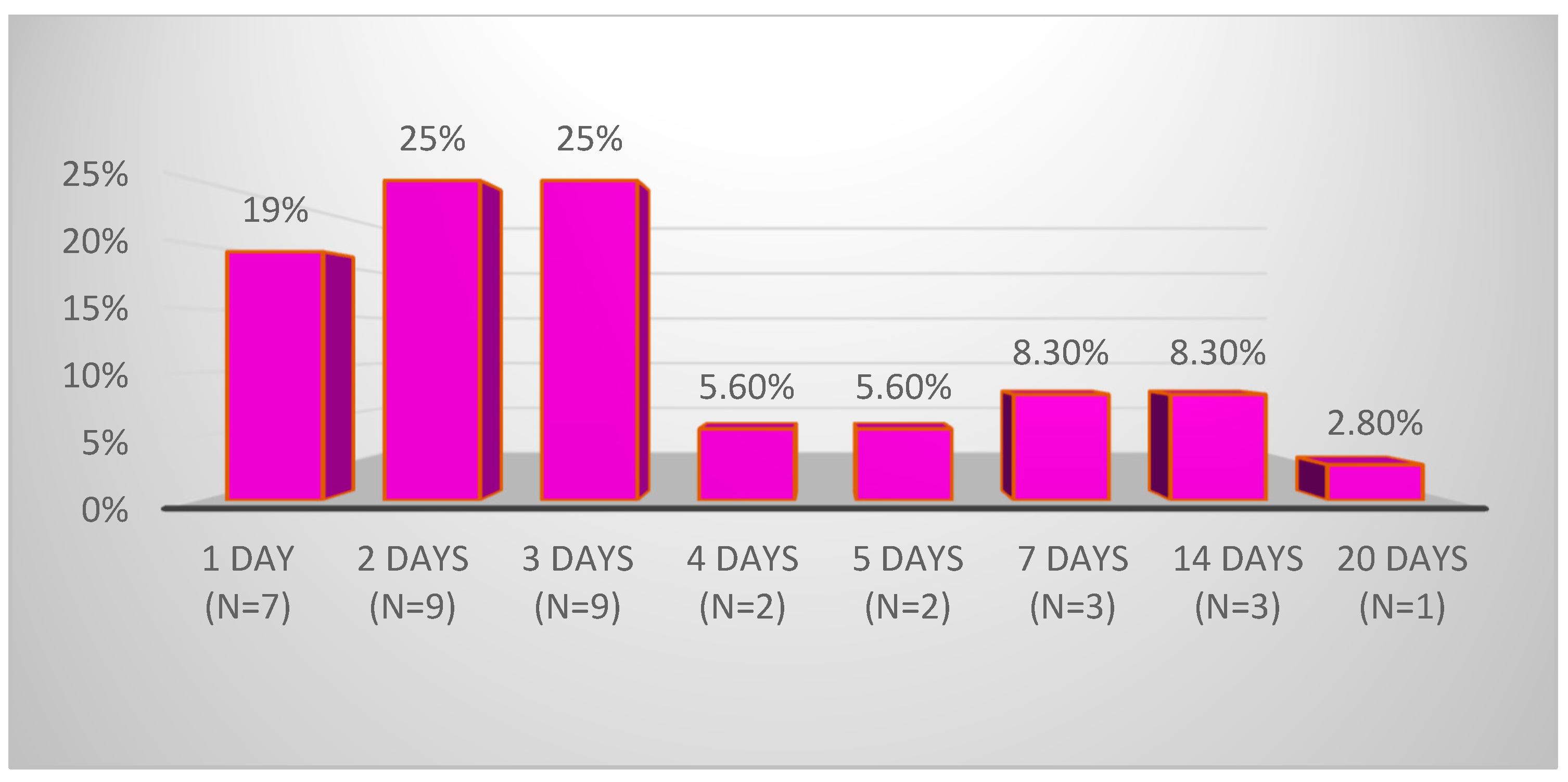
3.1. Demographic Data
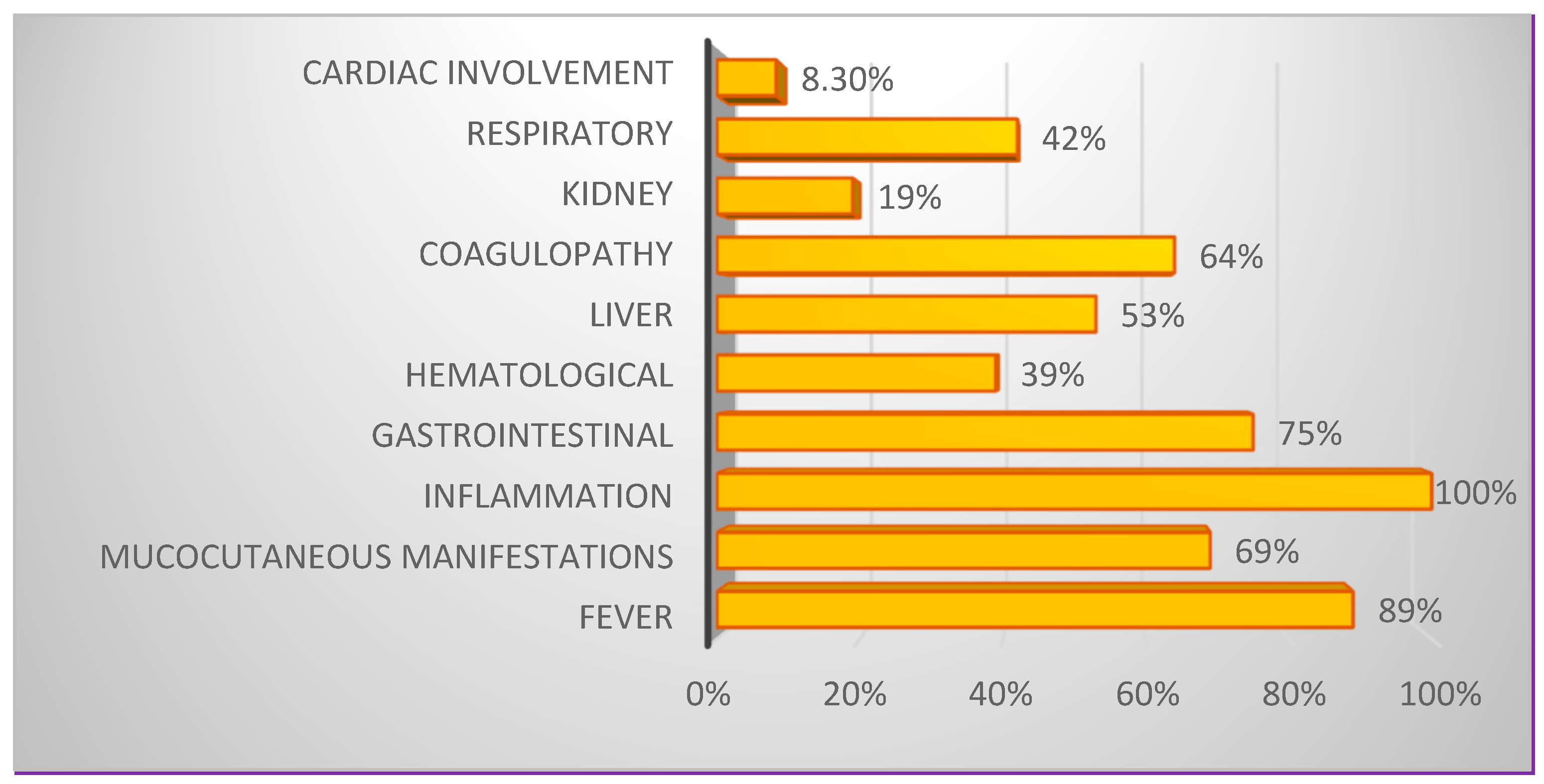
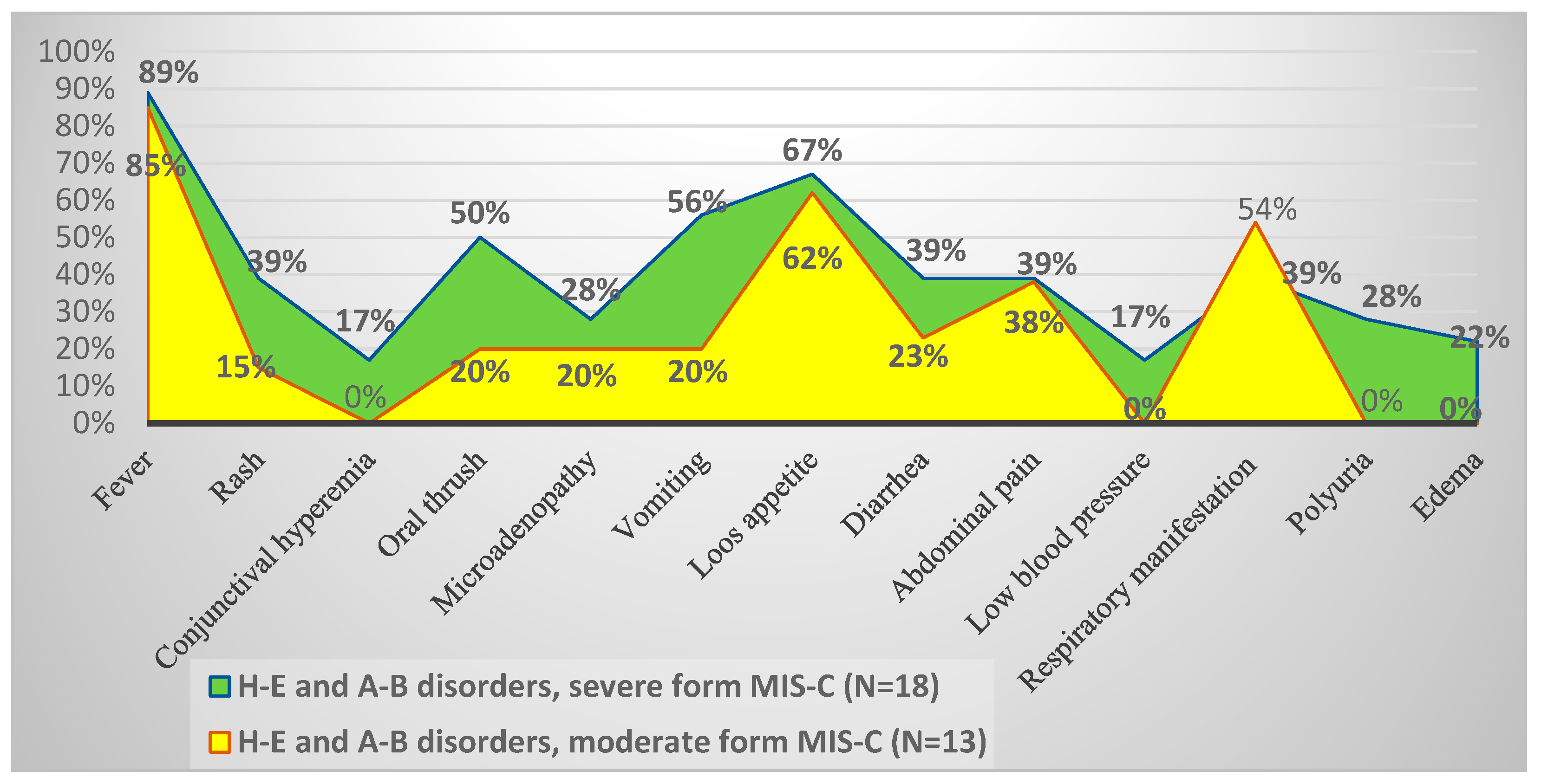
3.2. Constellations of Symptoms and Variations in Biological Parameters in MIS-C
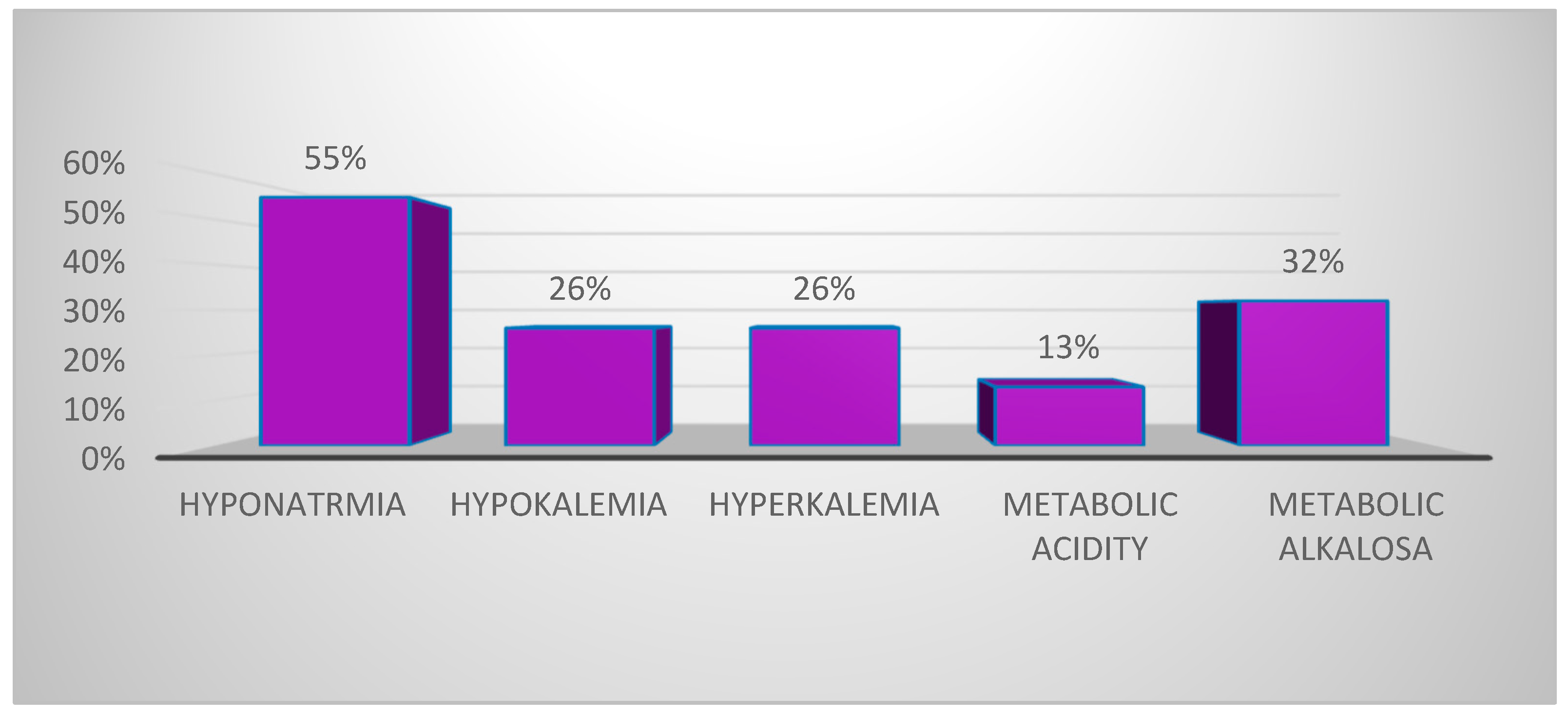
3.3. Hydroelectrolytic and Acid–Base Disorders in the Context of MIS-C
3.3.1. Hyponatremia in MIS-C Settings
3.3.2. Potassium Ion Concentration Disorders
3.3.3. Bicarbonate Concentration Disorders (ECO2)
4. Discussion
4.1. Hydroelectrolytic Disturbances in Severe Forms of MIS-C
4.1.1. Hyponatremia: Characteristics and Complications in Patients with MIS-C
4.1.2. Hypokalemia/Hyperkalemia Characteristics and Complications in MIS-C Patients
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-B | Acid–base |
| ADH | Antidiuretic hormone |
| CDC | Centers for Disease Control and Prevention |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-reactive protein |
| CTSE | Council of State and Territorial Epidemiologists |
| ESR | Erythrocyte sedimentation rate |
| H-E | Hydroelectrolyte |
| MIS-C | Multisystem inflammatory syndrome in children, associated with COVID-19 |
| PICU | Pediatric intensive care unit |
| R.A. | Alkaline reserve (synonym ECO2-bicarbonate) |
| RT-PCR | Real-time polymerase chain reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-Related Coronavirus 2 |
| WHO | World Health Organization |
References
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Parri, N.; Lenge, M.; Buonsenso, D.; Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with COVID-19 in Pediatric Emergency Departments in Italy. N. Engl. J. Med. 2020, 383, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.G.; Mills, M.; Suarez, D.; Hogan, C.A.; Yeh, D.; Segal, J.B.; Nguyen, E.L.; Barsh, G.R.; Maskatia, S.; Mathew, R. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp. Pediatr. 2020, 10, 537–540. [Google Scholar] [CrossRef]
- Levin, M. Childhood Multisystem Inflammatory Syndrome—A New Challenge in the Pandemic. N. Engl. J. Med. 2020, 383, 393–395. [Google Scholar] [CrossRef]
- Kundu, A.; Maji, S.; Kumar, S.; Bhattacharya, S.; Chakraborty, P.; Sarkar, J. Clinical aspects and presumed etiology of multisystem inflammatory syndrome in children (MIS-C): A review. Clin. Epidemiol. Glob. Health 2022, 14, 100966. [Google Scholar] [CrossRef]
- Rapid Risk Assessment: Paediatric Inflammatory Multisystem Syndrome and SARS-CoV-2 Infection in Children. Available online: https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment (accessed on 12 July 2024).
- HAN Archive|Health Alert Network (HAN). Available online: https://emergency.cdc.gov/han/dir.asp (accessed on 12 July 2024).
- COVID: WHO Says Pandemic Remains a Global Health Emergency|CTV News. Available online: https://www.ctvnews.ca/health/coronavirus/who-says-covid-19-remains-a-global-health-emergency-1.5984249 (accessed on 12 July 2024).
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar] [CrossRef]
- Godfred-Cato, S. COVID-19–Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. Mmwr. Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef]
- Patel, J.M. Multisystem Inflammatory Syndrome in Children (MIS-C). Curr. Allergy Asthma Rep. 2022, 22, 53–60. [Google Scholar] [CrossRef]
- Post, A.; Dullaart, R.P.; Bakker, S.J. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur. J. Intern. Med. 2020, 75, 109. [Google Scholar] [CrossRef]
- Berni, A.; Malandrino, D.; Parenti, G.; Maggi, M.; Poggesi, L.; Peri, A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: May all fit together? J. Endocrinol. Investig. 2020, 43, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, H.; Richard, M.C.; Chouihed, T.; Goffinet, N.; Le Bastard, Q.; Freund, Y.; Kratz, A.; Dubroux, M.; Masson, D.; Figueres, L.; et al. Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: A case–control study. Intern. Emerg. Med. 2021, 16, 1945–1950. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Geng, X.; Chen, R.; Zhang, P.; Lin, J.; Teng, J.; Zhang, X.; Ding, X. Dysnatremia is an Independent Indicator of Mortality in Hospitalized Patients. Med. Sci. Monit. 2017, 23, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- Atila, C.; Sailer, C.O.; Bassetti, S.; Tschudin-Sutter, S.; Bingisser, R.; Siegemund, M.; Osswald, S.; Rentsch, K.; Rueegg, M.; Schaerli, S.; et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur. J. Endocrinol. 2021, 184, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Tzoulis, P.; Waung, J.A.; Bagkeris, E.; Hussein, Z.; Biddanda, A.; Cousins, J.; Dewsnip, A.; Falayi, K.; McCaughran, W.; Mullins, C.; et al. Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, 1637–1648. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Uppal, N.N.; Sharma, P.; Khanin, Y.; Shah, H.H.; Malieckal, D.A.; Bellucci, A.; Mala Sachdeva, M.; Rondon-Berrios, H.; Jhaveri, K.D.; et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol. Dial. Transplant. 2021, 36, 1135–1138. [Google Scholar] [CrossRef]
- Tzoulis, P. Prevalence, prognostic value, pathophysiology, and management of hyponatraemia in children and adolescents with COVID-19. Acta Biomed. 2021, 92, e2021474. [Google Scholar] [CrossRef]
- Basalely, A.; Gurusinghe, S.; Schneider, J.; Shah, S.S.; Siegel, L.B.; Pollack, G.; Singer, P.; Castellanos-Reyes, L.J.; Fishbane, S.; Jhaveri, K.D.; et al. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. 2021, 100, 138–145. [Google Scholar] [CrossRef]
- Melgar, M.; Lee, E.H.; Miller, A.D.; Lim, S.; Brown, C.M.; Yousaf, A.R.; Zambrano, L.D.; Belay, E.D.; Godfred-Cato, S.; Abrams, J.Y.; et al. Council of State and Territorial Epidemiologists/CDC Surveillance Case Definition for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection—United States. MMWR. Recomm. Rep. 2022, 71, 1–14. [Google Scholar] [CrossRef]
- Son, M.B.F.; Burns, J.C.; Newburger, J.W. A New Definition for Multisystem Inflammatory Syndrome in Children. Pediatrics 2023, 151, e2022060302. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19)|COVID-19|CDC. Available online: https://www.cdc.gov/covid/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq-children.html (accessed on 5 October 2024).
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. BioMed Res. Int. 2021, 2021, 6667047. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Lv, W.; Wu, M.; Ren, Y.; Zeng, N.; Deng, P.; Zeng, H.; Zhang, Q.; Wu, Y. Coronavirus Disease 2019: Coronaviruses and Kidney Injury. J. Urol. 2020, 204, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.-H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Giuliani, C.; Parenti, G.; Norello, D.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Moderate Hyponatremia Is Associated with Increased Risk of Mortality: Evidence from a Meta-Analysis. PLoS ONE 2013, 8, e80451. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2020, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.A.; Zink, A.K.; Weißer, C.W.; Vogt, U.; Michelsen, A.; Priebe, H.-J.; Mols, G. Hypernatremia—A Manifestation of COVID-19: A Case Series. A&A Pract. 2020, 14, e01295. [Google Scholar] [CrossRef]
- Dalal, N.; Pfaff, M.; Silver, L.; Glater-Welt, L.; Sethna, C.; Singer, P.; Castellanos-Reyes, L.; Basalely, A. The prevalence and outcomes of hyponatremia in children with COVID-19 and multisystem inflammatory syndrome in children (MIS-C). Front. Pediatr. 2023, 11, 1209587. [Google Scholar] [CrossRef]
- Mardi, P.; Esmaeili, M.; Iravani, P.; Abdar, M.E.; Pourrostami, K.; Qorbani, M. Characteristics of Children With Kawasaki Disease-Like Signs in COVID-19 Pandemic: A Systematic Review. Front. Pediatr. 2021, 9, 625377. [Google Scholar] [CrossRef] [PubMed]
- Reiff, D.D.; Mannion, M.L.; Samuy, N.; Scalici, P.; Cron, R.Q. Distinguishing active pediatric COVID-19 pneumonia from MIS-C. Pediatr. Rheumatol. 2021, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S. Significance of hyponatremia in Kawasaki disease. Pediatr. Int. 2020, 62, 259. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Lavagno, C.; Milani, G.P.; Uestuener, P.; Simonetti, G.D.; Casaulta, C.; Bianchetti, M.G.; Fare, P.B.; Lava, S.A.G. Hyponatremia in children with acute respiratory infections: A reappraisal. Pediatr. Pulmonol. 2017, 52, 962–967. [Google Scholar] [CrossRef]
- Ciortea, D.-A.; (Cliveți), C.L.P.; Bezman, L.B.; Vivisenco, I.C.; Berbece, S.I.; Gurău, G.; Matei, M.N.; Nechita, A. Diagnostic Utility of Copeptin in Pediatric Patients with Polyuria-Polydipsia Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 10743. [Google Scholar] [CrossRef]
- Yousaf, Z.; Al-Shokri, S.D.; Al-Soub, H.; Mohamed, M.F.H. COVID-19-associated SIADH: A clue in the times of pandemic! Am. J. Physiol. Metab. 2020, 318, E882–E885. [Google Scholar] [CrossRef]
- Zieg, J. Pathophysiology of Hyponatremia in Children. Front. Pediatr. 2017, 5, 213. [Google Scholar] [CrossRef]
- Park, S.J.; Shin, J.I. Inflammation and hyponatremia: An underrecognized condition? Korean J. Pediatr. 2013, 56, 519–522. [Google Scholar] [CrossRef]
- Mills, T.; Trivedi, A.; Tremoulet, A.H.M.; Hershey, D.; Burns, J.C. Hyponatremia in Patients With Multisystem Inflammatory Syndrome in Children. Pediatr. Infect. Dis. J. 2021, 40, e344–e346. [Google Scholar] [CrossRef]
- Verbalis, J.G.; Goldsmith, S.R.; Greenberg, A.; Korzelius, C.; Schrier, R.W.; Sterns, R.H.; Thompson, C.J. Diagnosis, Evaluation, and Treatment of Hyponatremia: Expert Panel Recommendations. Am. J. Med. 2013, 126, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 2014, 170, G1–G47. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Narang, A.; Singhi, S. Hyponatraemia and the inappropriate ADH syndrome in pneumonia. Ann. Trop. Paediatr. 1992, 12, 455–462. [Google Scholar] [CrossRef]
- Feld, L.G.; Neuspiel, D.R.; Foster, B.A.; Leu, M.G.; Garber, M.D.; Austin, K.; Basu, R.K.; Conway, E.E.; Fehr, J.J.; Hawkins, C.; et al. Clinical Practice Guideline: Maintenance Intravenous Fluids in Children. Pediatrics 2018, 142, e20183083. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Cheng, M.H.; Zhang, S.; Porritt, R.A.; Noval Rivas, M.; Paschold, L.; Willscher, E.; Binder, M.; Arditi, M.; Bahar, I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 25254–25262. [Google Scholar] [CrossRef]
- Hunt, R.H.; East, J.E.; Lanas, A.; Malfertheiner, P.; Satsangi, J.; Scarpignato, C.; Webb, G.J. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig. Dis. 2021, 39, 119–139. [Google Scholar] [CrossRef]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Tan, C.; Huang, Y.; Shi, F.; Tan, K.; Ma, Q.; Chen, Y.; Jiang, X.; Li, X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020, 92, 856–862. [Google Scholar] [CrossRef]
- Fleck, A.; Hawker, F.; Wallace, P.; Raines, G.; Trotter, J.; Ledingham, I.; Calman, K. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet 1985, 325, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Belden, Z.; Deiuliis, J.A.; Dobre, M.; Rajagopalan, S. The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature. Am. J. Nephrol. 2017, 46, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]

| Electrolytes | Age Group | Level Range |
|---|---|---|
| Sodium (mmol/L) | <3 years | 135–142 |
| 3–<6 years | 135–142 | |
| 6–<16 years | 136–143 | |
| >16 years (Females) | 137–142 | |
| >16 years (Males) | 137–143 | |
| Kalium (mmol/L) | 0–<6 years (Females/Males) | 3.9–4.6 |
| >6 years (Females/Males) | 3.8–4.9 | |
| ECO2 (mmol/L) | 0–<15 days (Female/Males) | 5–20 |
| 15 days–<1 year (Females/Males) | 10–24 | |
| 1–<5 years (Females/Males) | 14–24 | |
| 5–<15 years (Females/Males) | 17–26 | |
| 5–<19 years (Females) | 17–26 | |
| 5–<19 years (Males) | 18–28 |
| Variables | Total Batch MIS-C N = 36 | Severe Form MIS-C N = 20 | Moderate Form MIS-C N = 16 | p-Value |
|---|---|---|---|---|
| Categorial variables N (%) | ||||
| Female sex Male sex | 21 (58.3) 15 (41.7) | 11 (55) 9 (45) | 10 (62.5) 6 (37.5) | 0.65 |
| Pediatric surgery | 6 (16.7) | 4 (20) | 2 (12.5) | 0.67 |
| Hospitalized in PICU | 19 (52.8) | 19 (95) | 0.0 (0.0) | <0.001 |
| Numerical variables—Median (IQR) | ||||
| Age (years) | 4.0 (1.75–9.25) | 5.0 (2.0–9.0) | 3.5 (1.0–12.0) | 0.85 |
| Initial manifestations (days) | 3 (2.0–4.25) | 3 (1.25–3.0) | 3 (2.0–5.0) | 0.40 |
| Hospitalization duration (days) | 10.0 (7.0–12.0) | 12.0 (10.0–14.5) | 7.0 (6.0–9.0) | <0.001 |
| Symptoms N (%) | ||||
| Fever duration >24/48 h >72 h | 27 (75) 5 (13.9) | 15 (75) 3 (15) | 12 (75) 2 (12.5) | >0.99 |
| Skin rash | 7 (19.4) | 5 (25) | 2 (12.5) | 0.43 |
| Conjunctival hyperemia | 3 (8.3) | 3 (15) | 0 (0) | >0.99 |
| Oral thrush | 20 (55.6) | 11 (55) | 9 (56.3) | |
| Microadenopathy | 7 (19.4) | 5 (25) | 2 (12.5) | >0.99 |
| Vomiting | 15 (41.7) | 9 (45) | 6 (37.5) | 0.65 |
| Loss of appetite | 18 (50) | 8 (40) | 10 (62.5) | 0.18 |
| Diarrhea | 7 (19.4) | 5 (25) | 2 (12.5) | 0.43 |
| Abdominal pain | 14 (38.9) | 9 (45) | 5 (31.5) | 0.40 |
| Low blood pressure | 3 (8.3) | 2 (10) | 1 (6.3) | >0.99 |
| Respiratory manifestations | 15 (41.7) | 7 (35) | 8 (50) | 0.24 |
| Polyuria | 7 (19.4) | 6 (30) | 1 (6.3) | >0.99 |
| Edema | 4 (11.1) | 4 (20) | 0 (0) | >0.99 |
| Biological parameters—Median (IQR) | ||||
| C-reactive protein [mg/dL] | 15.69 (4.92–26.72) | 20.91(4.91–27.99) | 6.92 (4.84–24.67) | 0.28 |
| Erythrocyte sedimentation rate [mm/h] | 60.5 (29.5–94) | 71 (29.5–94) | 50.2 (28.4–95.8) | 0.62 |
| Procalcitonine [ng/mL] | 1.85 (0.78–6.1) | 3.96 (0.81–6.24) | 1.76 (0.65–3.87) | 0.43 |
| Ferritin [ng/mL] | 168 (102–257) | 177 (99–257) | 135 (103–199) | 0.80 |
| Leukocytes [×103/µL] | 15 (13–22) | 16 (12–20) | 15 (13–23) | 0.75 |
| Absolute lymphocytes [×103/µL] | 1.65 (1.04–2.55) | 1.18 (0.53–2.22) | 1.92 (1.58–2.66) | 0.034 |
| Platelet count [×103/µL] | 274 (210–587) | 302 (150–611) | 265 (235–509) | 0.99 |
| D-dimers [mg/L] | 2.13 (0.9–8.07) | 3 (1–8) | 2 (1–3) | 0.51 |
| Fibrinogen [mg/mL] | 443 (273.75–622) | 439 (361–592) | 463 (253–652) | 0.80 |
| Hemoglobin [mg/dL] | 10.67 (10.01–11.57) | 10.61 (9.83–11.67) | 10.68 (10.3–11.7) | 0.53 |
| Erythrocytes [×10⁶/µL] | 3.99 (3.79–4.58) | 3.90 (3.55–4.22) | 4.56 (4.00–5.15) | 0.057 |
| Ionic calcium [mg/dL] | 4.22 (4–4.36) | 4.15 (3.90–4.34) | 4.27 (4.10–4.37) | 0.19 |
| Creatinine [mg/dL] | 0.32 (0.25–0.45) | 0.32 (0.21–0.42) | 0.32 (0.27–0.50) | 0.61 |
| Serum sodium [mmol/L] | 135 (133–137) | 135 (133–136) | 136 (132.5–138) | 0.28 |
| Serum potassium [mmol/L] | 4.01 (3.66–4.47) | 3.93 (3.26–4.32) | 4.27 (3.97–4.95) | 0.070 |
| Bicarbonate [mmol/L] | 22 (18.75–25) | 24.5 (18–31) | 21.0 (19.5–24) | 0.22 |
| Total protein [g/dL] | 6.3 (6.1–6.8) | 6.30 (5.40–6.80) | 6.45 (6.30–7.20) | 0.17 |
| Aspartate aminotransferase [U/L] | 37 (25.5–52) | 38 (30–95.5) | 32.1 (23.8–39.7) | 0.20 |
| Alanine aminotransferase [U/L] | 22 (16–37) | 29 (21.7–53.5) | 18 (14–21.6) | 0.003 |
| Urine density | 1013 (1007–1027) | 1011.5 (1007–1024.75) | 1013 (1008–1020) | >0.99 |
| Variable | MIS-C with H-E and A-B Disorders N = 31 | H-E and A-B Disorders, Severe-Form MIS-C N = 18 | H-E and A-B Disorders, Moderate-Form MIS-C N = 13 |
|---|---|---|---|
| Categorial variables N (%) | |||
| Female:male ratio | 1.38:1 | 1.25:1 | 1.6:1 |
| Pediatric surgery | 5 (16.1) | 3 (16.7) | 2 (15.4) |
| Hospitalized in PICU | 17 (54.8) | 17 (94.4) | 0 (0.0) |
| Numerical variables—Median (IQR) | |||
| Age (years) | 4 (1.5–9.5) | 5.5 (2–9) | 3 (1–10) |
| Onset of manifestation (days) | 3 (2–4.5) | 3 (1.25–3) | 3 (2–5) |
| Hospitalization duration (days) | 10 (7–12.5) | 12 (11–14.75) | 7 (6–8) |
| Biological markers—Median (IQR) | |||
| C-reactive protein [0–0.5 mg/dL] | 15.71 (5.04–26.93) | 17.09 (4.8–27.56) | 14.09 (6.54–25.39) |
| Erythrocyte sedimentation rate [2–12 mm/h] | 66 (30–93.4) | 71 (30.75–91.25) | 55 (30–93) |
| Procalcitonin [0–0.5 ng/mL] | 1.9 (0.78–5.96) | 3.96 (0.84–5.96) | 1.73 (0.63–4.54) |
| Ferritin [13–68 ng/mL] | 168 (109.5–271) | 173 (107.6–337.5) | 151.5 (113.5–220.75) |
| Leukocytes [4–12 × 103/ µL] | 17.32 (12.85–24.44) | 16.66 (12.32–20.69) | 15.29 (13.75–23.47) |
| Absolute lymphocytes [×103/ µL] | 1.58 (1.01–2.6) | 1.18 (0.51–2.30) | 2.54 (1.58–2.69) |
| Platelet count [×103 /µL] | 272.3 (209.5–592.4) | 301.5 (188.7–563.7) | 258.2 (241–609.5) |
| D-dimers [mg/L] | 1.85 (0.8–5.28) | 2.39 (0.9–9.46) | 1.72 (0.71–2.68) |
| Fibrinogen [mg/mL] | 443 (264.75–637) | 478 (294.25–656.5) | 463 (255.5–642) |
| Hemoglobin [g/dL] | 10.78 (9.98–11.9) | 10.78 (9.8–11.8) | 10.8 (10.4–11.1) |
| Ionic calcium [mg/dL] | 4.23 (4–4.36) | 4.18 (3.9–4.34) | 4.3 (4.16–4.37) |
| Creatinine [mg/dL] | 0.31 (0.24–0.46) | 0.32 (0.23- 0.43) | 0.29 (0.26–0.49) |
| Seric sodium [mmol/L] | 135 (132.5–136.5) | 134.5 (133–135) | 135 (132–138) |
| Seric potassium [mmol/L] | 3.96 (3.64–4.8) | 3.92 (3.19–4.33) | 4.4 (3.91–4.96) |
| Bicarbonate (ECO2) [mmol/L] | 22 (18–26.5) | 25 (18–31) | 21 (19–24) |
| Total protein [g/dL] | 6.3 (6–6.9) | 6.3 (5.6–6.8) | 6.4 (6.3–6.9) |
| Aspartate aminotransferase [U/L] | 36 (26.2–52) | 29 (22–51.5) | 31 (24–39) |
| Alanine aminotransferase [U/L] | 22 (16.5–40) | 38 (30–61) | 17 (15–20) |
| Urine density | 1012 (1007–1023) | 1011.5 (1007–1024.75) | 1013 (1008–1020) |
| Variables | HipoNa N = 17 | HipoK N = 10 | HiperK N = 8 | Metabolic Acidosis N = 4 | Metabolic Alkalosis N = 10 |
|---|---|---|---|---|---|
| Categorial variables N (%) | |||||
| Female:male ratio | 1:1.12 | 1.5:1 | 3:1 | 1:3 | 1.5:1 |
| Pediatric surgery (N = 31) | 4 (12.9) | 3 (9.7) | 1 (3.2) | 1 (3.2) | 3 (9.7) |
| Admission into PICU (n = 17) | 10 (58.8) | 7 (41.1) | 2 (11.7) | 3 (17.6) | 8 (47) |
| Severe-form MIS-C with H-E and A-B disorders | 10 (58.8) | 8 (80) | 3 (37.5) | 2 (50) | 9 (90) |
| Moderate-form MIS-C with H-E and A-B disorders | 7 (41.2) | 2 (20) | 5 (62.5) | 2 (50) | 1 (10) |
| Numerical variables—Median (IQR) | |||||
| Age (years) | 9 (4–10) | 7.5 (1.3–9) | 1 (0.0–2.5) | 7.5 (6.3–9.8) | 8.5 (4–13) |
| Duration of hospitalization (days) | 8.5 (5.5–11.5) | 12.5 (11.5–15) | 8 (6.8–11.3) | 9 (6.8–11.8) | 12 (9.3–15) |
| Symptoms N (%) | |||||
| Fever | 17 (100) | 10 (100) | 7 (87.5) | 3 (75) | 3 (30) |
| Cough, dyspnea | 3 (17.6) | 2 (20) | 2 (25) | 3 (75) | 4 (40) |
| Loss of appetite | 8 (47) | 6 (60) | 6 (75) | 3 (75) | 3 (30) |
| Abdominal pain | 6 (35.3) | 3 (30) | 3 (37.5) | 3 (75) | 4 (40) |
| Vomiting | 6 (35.3) | 7 (70) | 7 (87.5) | 3 (75) | 4 (40) |
| Diarrhea | 5 (29.4) | 1 (10) | 1 (12.5) | 2 (50) | 2 (20) |
| Skin rash | 10 (58.9) | 3 (30) | 3 (37.5) | 0 (0.0) | 4 (40) |
| Oral thrush | 4 (23.5) | 3 (30) | 3 (37.5) | 0 (0.0) | 1 (10) |
| Conjunctival hyperemia | 3 (17.6) | 2 (20) | 2 (25) | 0 (0.0) | 1 (10) |
| Microadenopathy | 4 (23.5) | 3 (30) | 3 (37.5) | 3 (75) | 0 (0.0) |
| Edema | 5 (29.4) | 2 (20) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Polyuria | 4 (23.5) | 3 (30) | 3 (37.5) | 0 (0.0) | 3 (30) |
| Biological markers—Median (IQR) | |||||
| C-reactive protein [mg/dL] | 15.71 (7.62–25.67) | 24.51 (9.23–28.71) | 22.99 (4.36–26.72) | 24.74 (17.34–31/64) | 10.41 (4.49–25.1) |
| Erythrocyte sedimentation rate [mm/h] | 75 (30–94) | 66.5 (34–79.2) | 64 (23.5–100) | 80 (57–103) | 30.5 (24.7–66) |
| Procalcitonin [ng/mL] | 3.4 (1.1–6.1) | 3.7 (1.3–6.5) | 3.9 (0.7–7.8) | 1.3 (0.8–2.6) | 1.3 (0.5–6.5) |
| Absolute lymphocytes [×103/µL] | 1.1 (0.4–1.7) | 1.3 (0.3–1.7) | 2.8 (2.6–3.3) | 1.3 (0.9–1.7) | 1.3 (0.3–2.1) |
| Platelet count [×103/µL] | 256 (209.4–308.5) | 227.1 (125.5–443.9) | 531.9 (345.2–720.6) | 277.8 (181.9–528.9) | 454.4 (134.5–636.8) |
| Seric sodium [mmol/L] | 133 (132–134) | 133.5 (132.3–135) | 137 (135–139.5) | 133.5 (132.8–135) | 135 (133.3–137.5) |
| Seric potassium [mmol/L] | 3.91 (3.2–4.1) | 3.16 (2.9–3.58) | 5.2 (5–5.34) | 3.9 (3.8–3.9) | 4.3 (2.9–4.6) |
| Bicarbonate (ECO2) [mmol/L] | 22 (18–31) | 19.5 (17–31) | 22.5 (20.8–25.8) | 15 (13.5–16) | 31.5 (28.8–33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrea, C.L.; Ciortea, D.-A.; Candussi, I.-L.; Gurău, G.; Matei, N.M.; Bergheș, S.-E.; Chirila, S.I.; Berbece, S.I. A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion. Curr. Issues Mol. Biol. 2024, 46, 11438-11459. https://doi.org/10.3390/cimb46100681
Petrea CL, Ciortea D-A, Candussi I-L, Gurău G, Matei NM, Bergheș S-E, Chirila SI, Berbece SI. A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion. Current Issues in Molecular Biology. 2024; 46(10):11438-11459. https://doi.org/10.3390/cimb46100681
Chicago/Turabian StylePetrea (Cliveți), Carmen Loredana, Diana-Andreea Ciortea, Iuliana-Laura Candussi, Gabriela Gurău, Nicoleta Mădălina Matei, Simona-Elena Bergheș, Sergiu Ioachim Chirila, and Sorin Ion Berbece. 2024. "A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion" Current Issues in Molecular Biology 46, no. 10: 11438-11459. https://doi.org/10.3390/cimb46100681
APA StylePetrea, C. L., Ciortea, D.-A., Candussi, I.-L., Gurău, G., Matei, N. M., Bergheș, S.-E., Chirila, S. I., & Berbece, S. I. (2024). A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion. Current Issues in Molecular Biology, 46(10), 11438-11459. https://doi.org/10.3390/cimb46100681






