Pharmacological and Pathological Effects of Mulberry Leaf Extract on the Treatment of Type 1 Diabetes Mellitus Mice
Abstract
1. Introduction
2. Materials and Methods
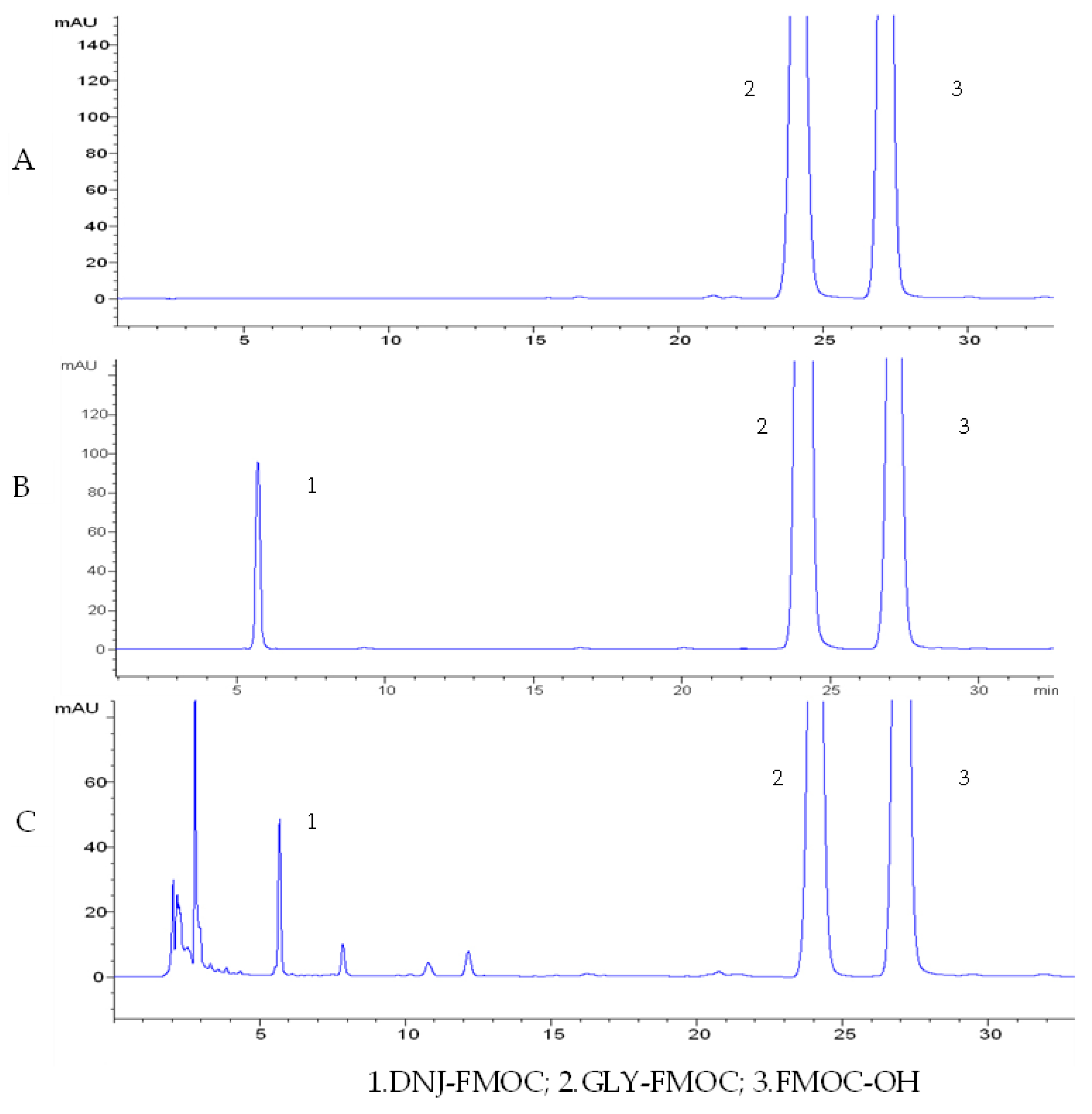
2.1. Preparation of the Experimental Materials
2.2. Reagents and Instruments
2.3. The Diabetic Mouse Model
2.4. Grouping, Doses, and Administration
2.5. Measurement of Plasma Glucose
2.6. Measurement of Antioxidant and Biochemical Indexes
2.7. The Effect of Mulberry Leaf Extract on Glucose Tolerance in Diabetic Mice
2.8. Pathological Examination Methods
2.9. Statistical Analysis
3. Results
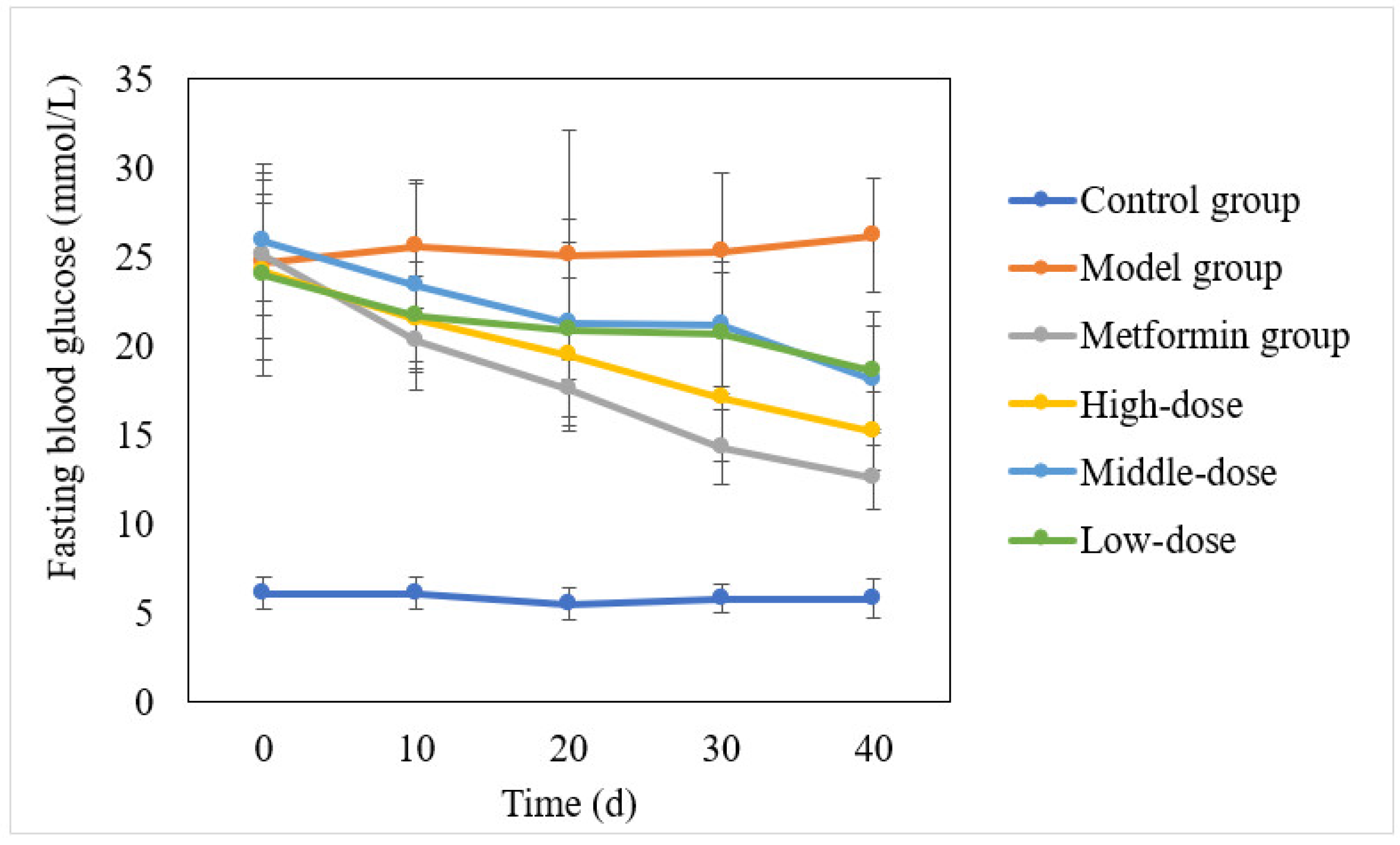
3.1. Effect of the Aqueous Mulberry Leaf Extract on Blood Glucose in T1DM Mice
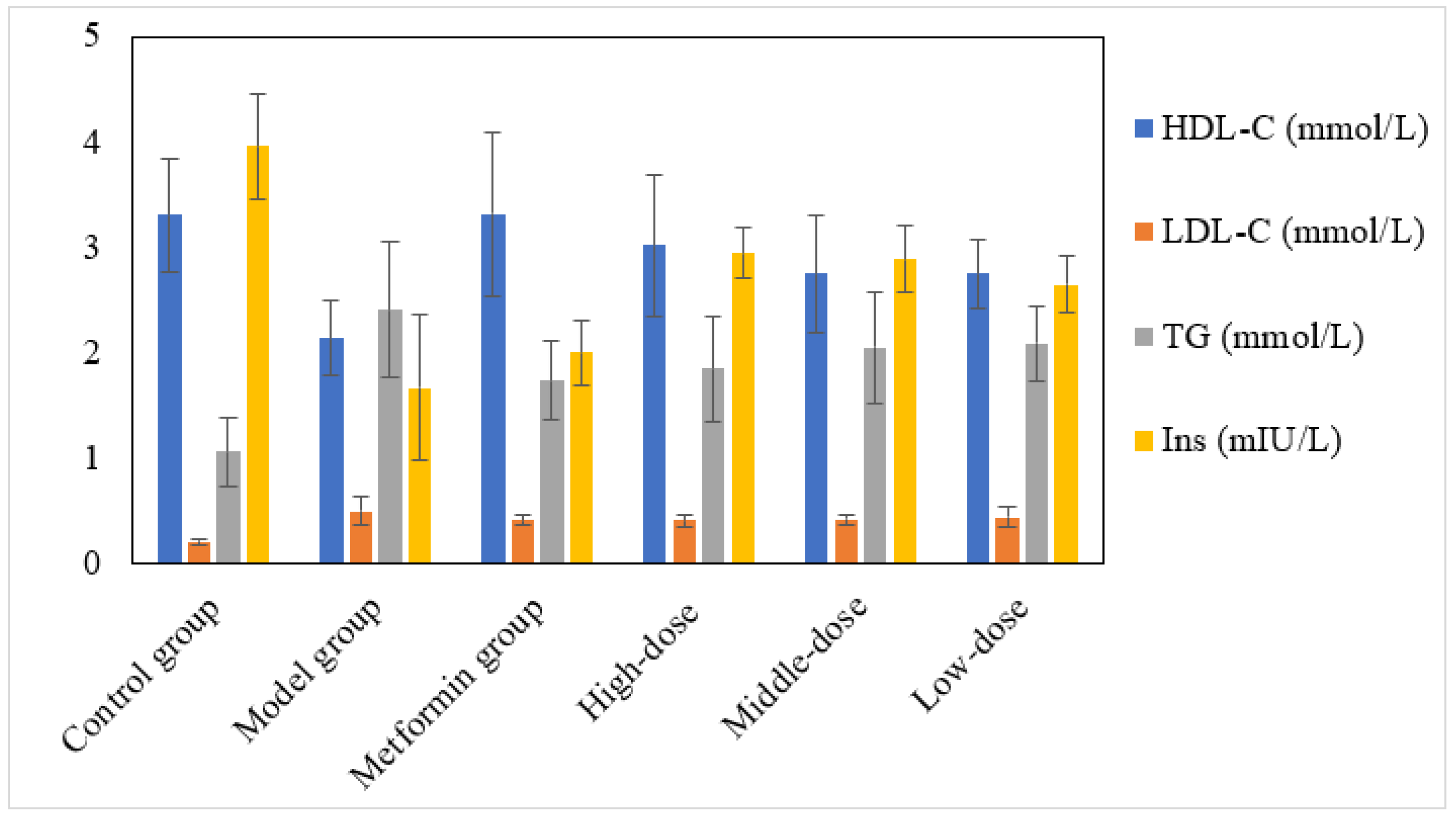
3.2. The Effect of the Aqueous Mulberry Leaf Extract on Serum Lipid (HDL-C, LDL-C, TG) and Insulin Levels in T1DM Mice
3.3. The Effect of the Aqueous Mulberry Leaf Extract on Glucose Tolerance in T1DM Mice
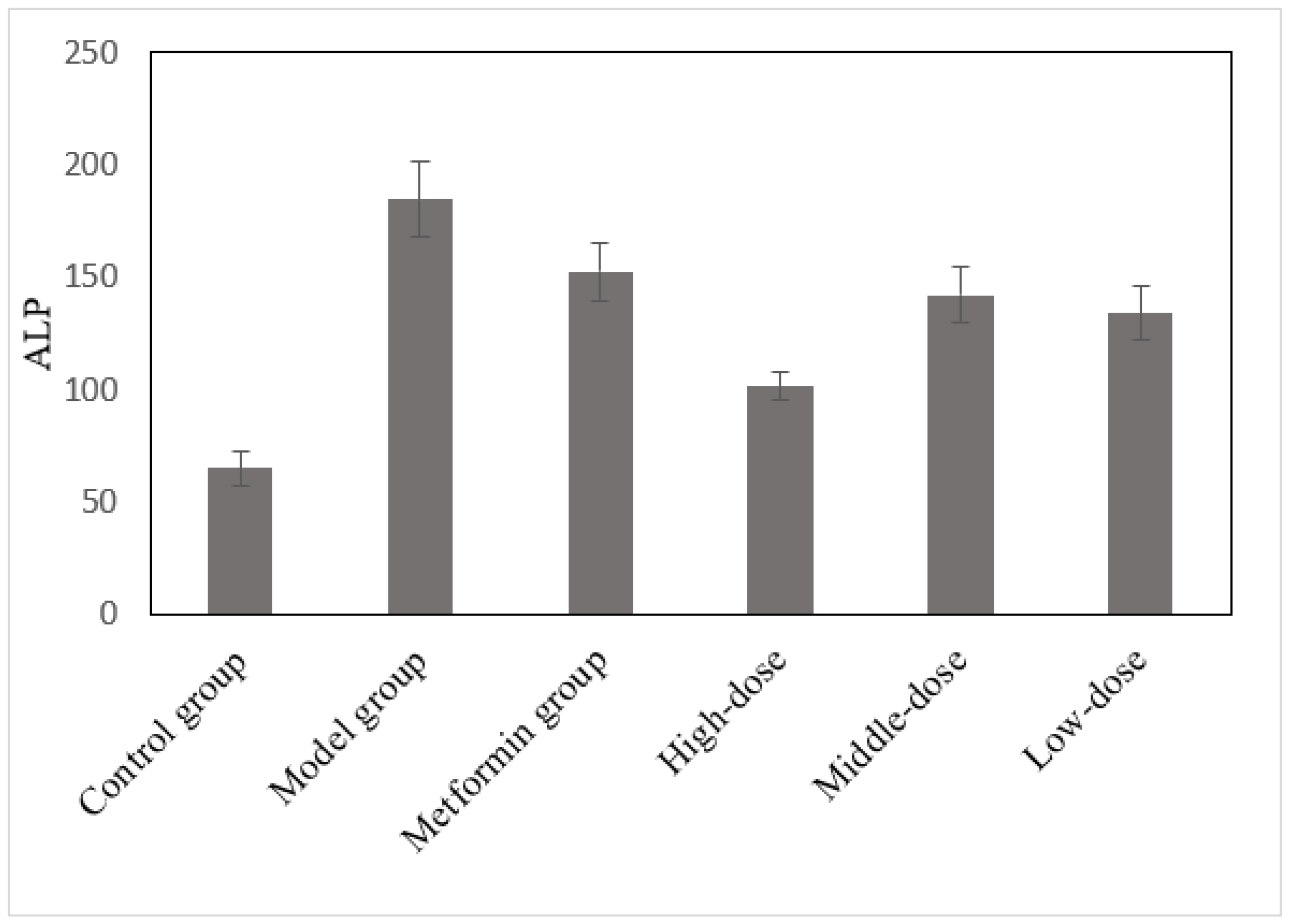
3.4. The Effects of the Aqueous Mulberry Leaf Extract on Alkaline Phosphatase in T1DM Mice
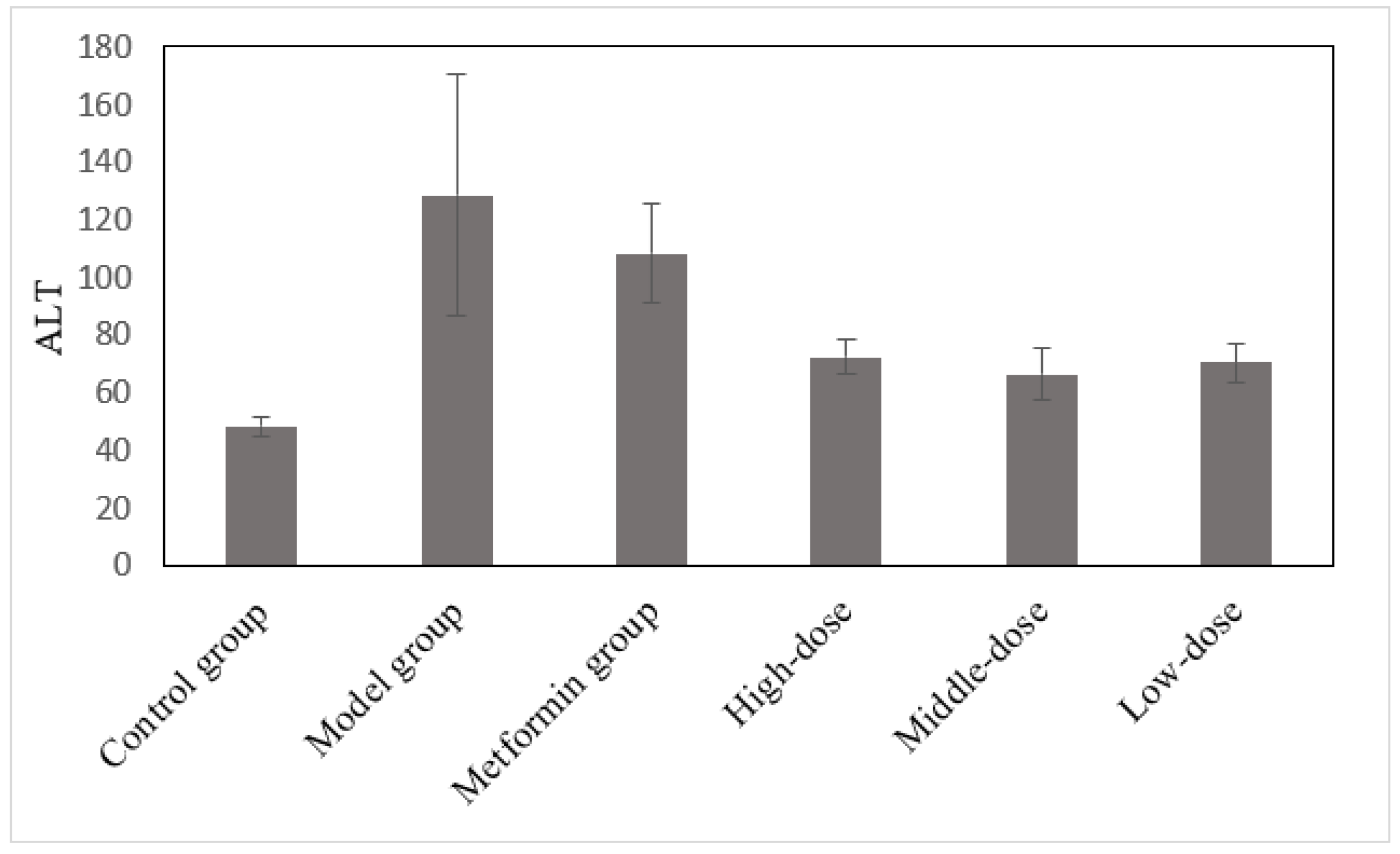
3.5. The Effects of the Aqueous Mulberry Leaf Extract on ALT in T1DM Mice
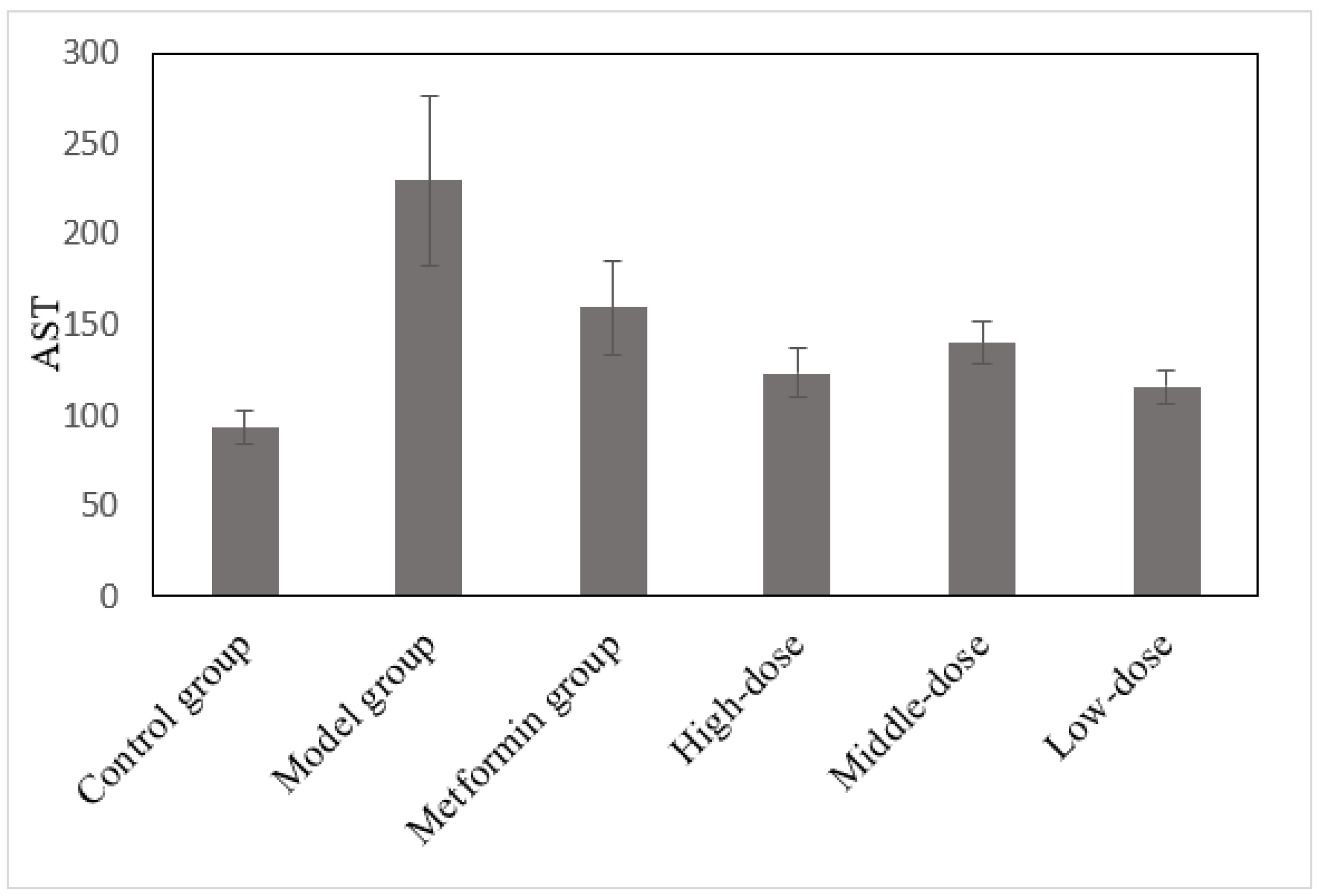
3.6. The Effects of the Aqueous Mulberry Leaf Extract on AST in T1DM Mice
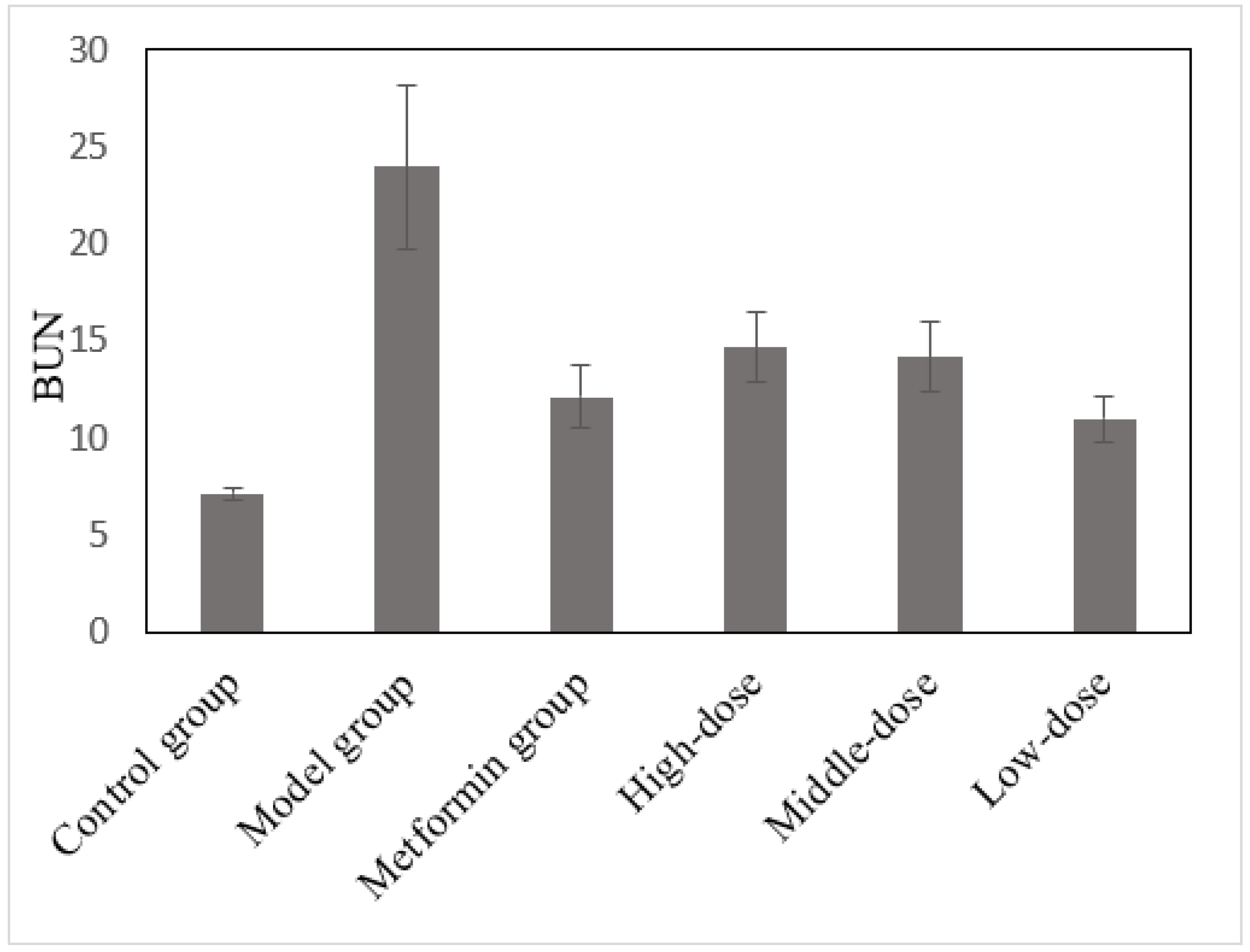
3.7. The Effects of the Aqueous Mulberry Leaf Extract on BUN in T1DM Mice
3.8. The Effects of the Aqueous Mulberry Leaf Extract on CHO in T1DM Mice
3.9. The Effects of the Aqueous Mulberry Leaf Extract on Lactate Dehydrogenase in T1DM Mice
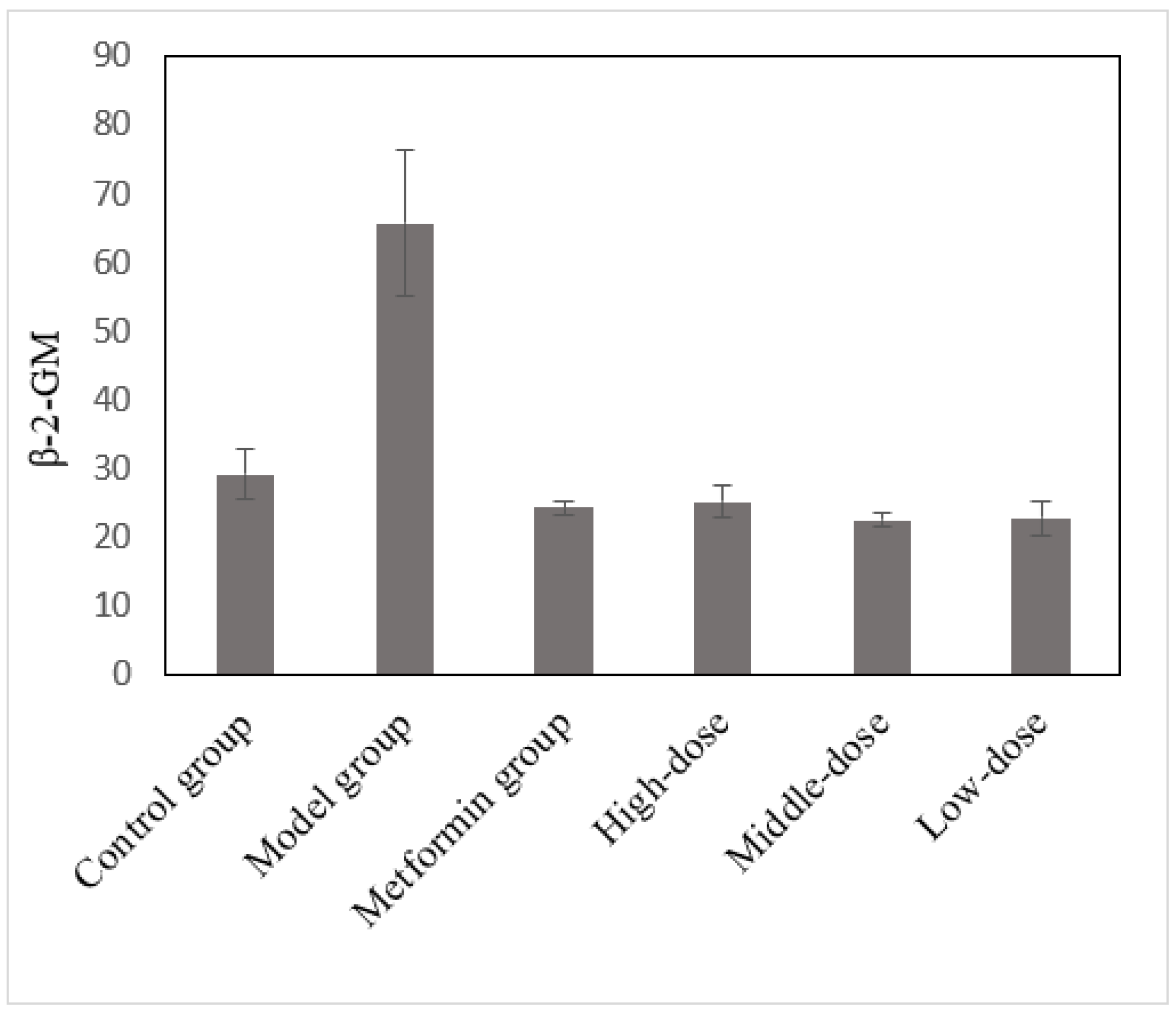
3.10. The Effects of the Aqueous Mulberry Leaf Extract on β-2-Microglobulin in T1DM Mice
3.11. Effect of the Aqueous Mulberry Leaf Extract on Superoxide Dismutase Activity and Malonic Dialdehyde Level in Serum of T1DM Mice
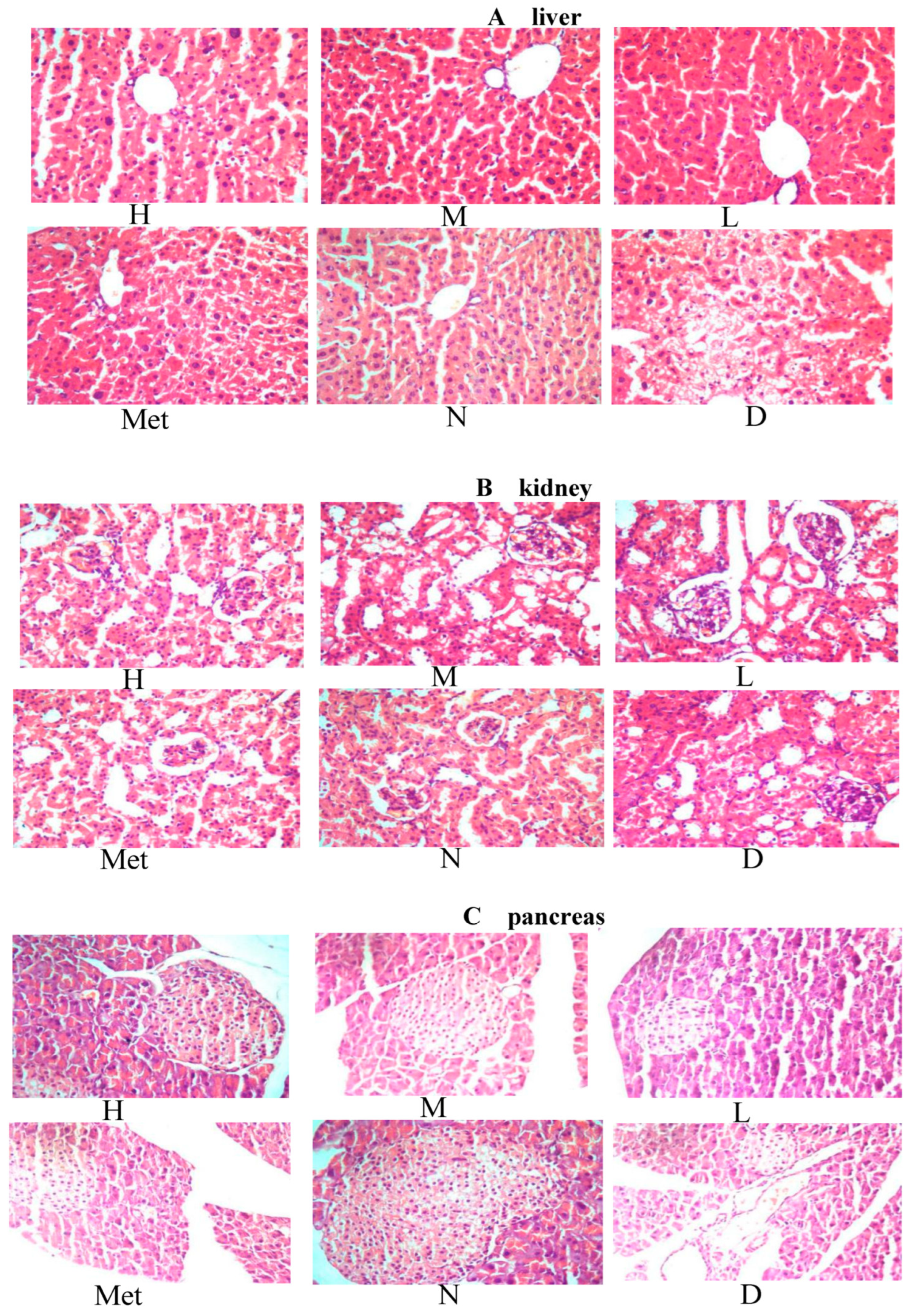
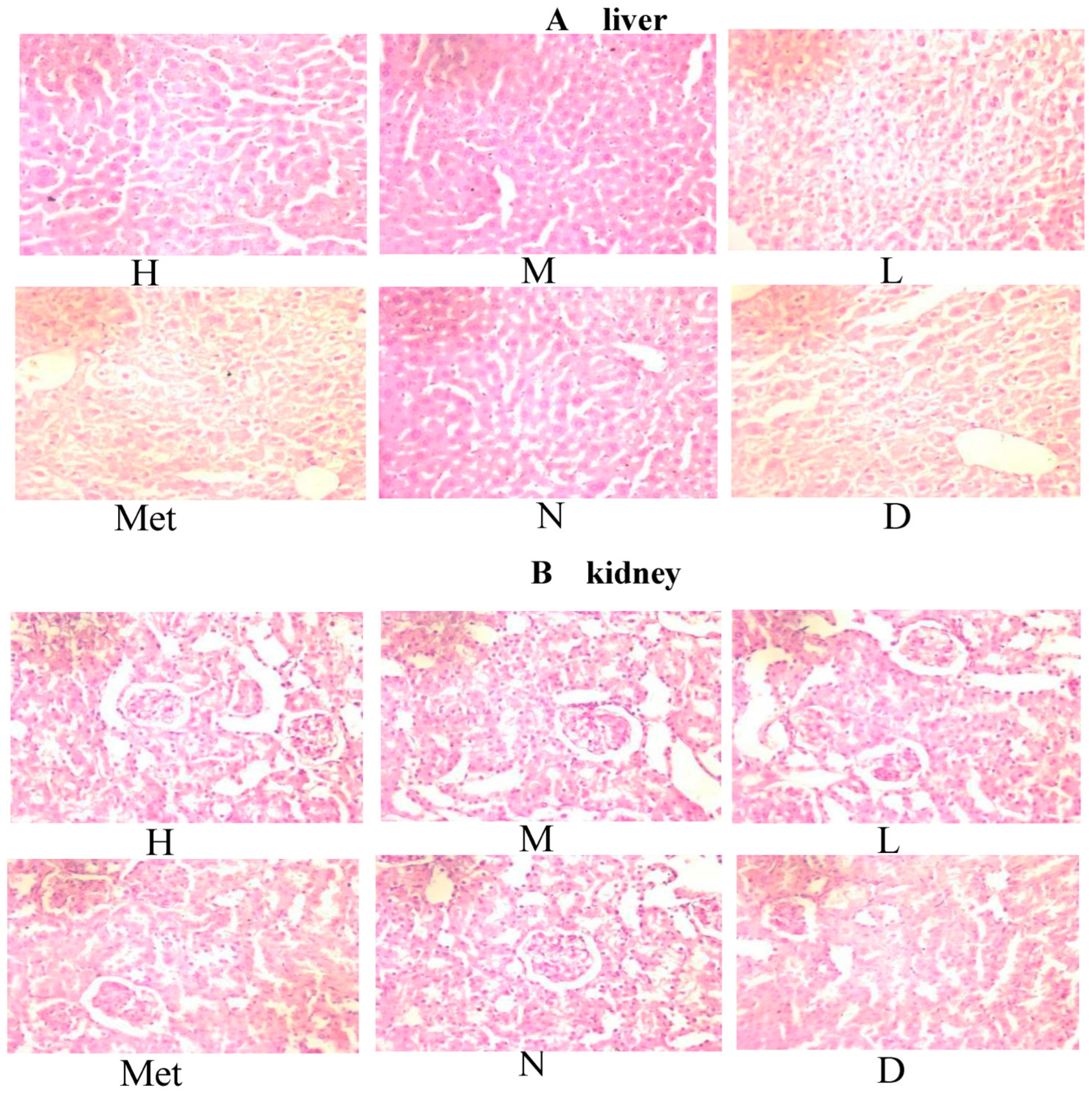
3.12. Effects of the Aqueous Mulberry Leaf Extract on the Liver of Diabetic Mice
3.13. Effect of Aqueous Mulberry Leaf Extract on Kidney in Diabetic Mice
3.14. Effect of the Mulberry Leaf Extract on the Kidney of Diabetic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100S, 153952. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). Diabetes Atlas, 9th ed.; International Diabetes Federation (IDF): Brussels, Belgium, 2019; Available online: https://diabetesatlas.org/en/ (accessed on 24 June 2023).
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Shannon, C.; Merovci, A.; Xiong, J.; Tripathy, D.; Lorenzo, F.; McClain, D.; Abdul-Ghani, M.; Norton, L.; DeFronzo, R.A. Effect of Chronic Hyperglycemia on Glucose Metabolism in Subjects With Normal Glucose Tolerance. Diabetes 2018, 67, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Liu, J.; Zhang, H.; Ji, B.P.; Zhou, F. Addition of mulberry leaf (Morus alba l.) to a diet formula impeded its hypoglycemic effect and exacerbated dyslipidemia in high-fructose- and high-fat-induced cd-1 mice. Emir. J. Food Agric. 2017, 29, 532–538. [Google Scholar] [CrossRef]
- Gurukar, M.S.A.; Chilkunda, N.D. Morus alba leaf bioactives modulate peroxisome proliferator activated receptor gamma in the kidney of diabetic rat and impart beneficial effect. J. Agric. Food Chem. 2018, 66, 7923–7934. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Y.; Mu, W.; Li, Z.; Sun, J.; Wang, B.; Zhong, Z.; Luo, X.; Xie, C.; Huang, Y. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine 2018, 97, e11996. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Mulberry leaf polysaccharides suppress renal fibrosis. Int. J. Biol. Macromol. 2019, 124, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Ouyang, Z.; Duan, J.A. Mulberry leaf active components alleviate type 2 diabetes and its liver and kidney injury in db/db mice through insulin receptor and TGF-β/smads signaling pathway. Biomed. Pharmacother. 2019, 112, 108675. [Google Scholar] [CrossRef]
- Xu, D.Q.; Cheng, S.Y.; Zhang, J.Q.; Lin, H.F.; Chen, Y.Y.; Yue, S.J.; Tian, M.; Tang, Y.P.; Zhao, Y.C. Morus alba L. Leaves–Integration of Their Transcriptome and Metabolomics Dataset: Investigating Potential Genes Involved in Flavonoid Biosynthesis at Different Harvest Times. Front. Plant Sci. 2021, 12, 736332. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Luo, K.; Xiao, Q.; Sun, Z.; Wang, Y.; Cui, C.; Chen, F.; Xu, B.; Shen, W.; Wan, F.; et al. Effect of mulberry leaf or mulberry leaf extract on glycemic traits: A systematic review and meta-analysis. Food Funct. 2023, 14, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Lin, J.; Wu, X.; Pu, H.; Guan, Y.; Xiao, P.; He, C.; Jiang, B. Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front. Pharmacol. 2022, 13, 986931. [Google Scholar] [CrossRef]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of mulberry leaves’ hypoglycemic properties and hypoglycemic mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Xiong, X.S.; Qian, C.L.; Liu, Q.H.; Wen, P.B.; Shi, X.Y.; Dereje, S.B.; Zhang, X.; Wang, L. Network pharmacological analysis of ethanol extract of Morus alba linne in the treatment of type 2 diabetes mellitus. Arab. J. Chem. 2021, 14, 103384. [Google Scholar] [CrossRef]
- Tseng, P.S.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217809. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 6, 5–23. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me3SiN3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef]
- Trapero, A.; Llebaria, A. A prospect for pyrrolidine iminosugars as antidiabetic α-glucosidase inhibitors. J. Med. Chem. 2012, 55, 10345–10346. [Google Scholar] [CrossRef]
- Kojima, Y.; Kimura, T.; Nakagawa, K.; Asai, A.; Hasumi, K.; Oikawa, S.; Miyazawa, T. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J. Clin. Biochem. Nutr. 2010, 47, 155–161. [Google Scholar] [CrossRef]
- Nakamura, T.; Terajima, T.; Ogata, T.; Ueno, K.; Hashimoto, N.; Ono, K.; Yano, S. Establishment and pathophysiological characterization of type 2 diabetic mouse model produced by streptozotocin and nicotinamide. Biol. Pharm. Bull. 2006, 29, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Si, Q.; Yang, S.; Jiao, T.; Zhu, H.; Tian, P.; Wang, L.; Li, X.; Gong, L.; Zhao, J.; et al. Lactic acid bacteria reduce diabetes symptoms in mice by alleviating gut microbiota dysbiosis and inflammation in different manners. Food Funct. 2020, 11, 5898–5914. [Google Scholar] [CrossRef]
- Ma, H.; Bai, L. Effect of Polygonatum odoratum ethanol extract on high glucose-induced tubular epithelial cell apoptosis and oxidative stress. Pak. J. Pharm. Sci. 2021, 34, 1203–1209. [Google Scholar]
- Veissi, M.; Jafarirad, S.; Ahangarpour, A.; Mohaghegh, S.M.; Malehi, A.S. Co-exposure to endocrine disruptors: Effect of bisphenol A and soy extract on glucose homeostasis and related metabolic disorders in male mice. Endocr. Regul. 2018, 52, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, R.; Li, X.; Li, Y.; Zhang, Z.; Huang, S.; Ge, Y.; Chen, X.; Yang, X. Zinc, selenium and chromium co-supplementation improves insulin resistance by preventing hepatic endoplasmic reticulum stress in diet-induced gestational diabetes rats. J. Nutr. Biochem. 2021, 96, 108810. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.S. A High Level of Intestinal Alkaline Phosphatase Is Protective Against Type 2 Diabetes Mellitus Irrespective of Obesity. eBioMedicine 2015, 2, 2016–2023. [Google Scholar] [CrossRef]
- Halling Linder, C.; Englund, U.H.; Narisawa, S.; Millán, J.L.; Magnusson, P. Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum. Bone 2013, 53, 399–408. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Zhu, S.; Liu, B.; Liu, F.; Xu, Y. Mulberry leaf (Morus alba L.): A review of its potential influences in mechanisms of action on metabolic diseases. Pharmacol. Res. 2022, 175, 106029. [Google Scholar] [CrossRef]
- Tanaka, K.; Nanbara, S.; Tanaka, T.; Koide, H.; Hayashi, T. Aminotransferase activity in the liver of diabetic mice. Diabetes Res. Clin. Pract. 1988, 5, 71–75. [Google Scholar] [CrossRef]
- Guan, Y.; Nakano, D.; Zhang, Y.; Li, L.; Tian, Y.; Nishiyama, A. A mouse model of renal fibrosis to overcome the technical variability in ischaemia/reperfusion injury among operators. Sci. Rep. 2019, 9, 10435. [Google Scholar] [CrossRef]
- Cruz, T.B.; Carvalho, F.A.; Matafome, P.N.; Soares, R.A.; Santos, N.C.; Travasso, R.D.; Oliveira, M.J. Mice with Type 2 diabetes present significant alterations in their tissue biomechanical properties and histological features. Biomedicines 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, lactate dehydrogenase. In StatPearls; StatPearls Publishing: London, UK, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557536/ (accessed on 24 June 2023).
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Stahl, F.R.; Jung, R.; Jazbutyte, V.; Ostermann, E.; Tödter, S.; Brixel, R.; Kemmer, A.; Halle, S.; Rose-John, S.; Messerle, M.; et al. Laboratory diagnostics of murine blood for detection of mouse cytomegalovirus (MCMV)-induced hepatitis. Sci. Rep. 2018, 8, 14823. [Google Scholar] [CrossRef] [PubMed]
- Bernier, G.M. Beta 2-Microglobulin: Structure, function and significance. Vox Sang. 1980, 38, 323–327. [Google Scholar] [CrossRef]
- Gómez-Banoy, N.; Guseh, J.S.; Li, G.E.; Rubio-Navarro, A.; Chen, T.; Poirier, B.; Putzel, G.; Rosselot, C.; Pabón, M.A.; Camporez, J.P.; et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat. Med. 2019, 25, 1739–1747. [Google Scholar] [CrossRef]
- Kitada, M.; Zhang, Z.; Mima, A.; King, G.L. Molecular mechanisms of diabetic vascular complications. J. Diabetes Investig. 2010, 1, 77–89. [Google Scholar] [CrossRef]
- Lee, S.C.; Pervaiz, S. Apoptosis in the pathophysiology of diabetes mellitus. Int. J. Biochem. Cell Biol. 2007, 39, 497–504. [Google Scholar] [CrossRef]



| Group | Dose (mg·kg−1) | N | Blood Glucose/(mmol·L−1) | AUC (mmol·h/L) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 1 h | 2 h | ||||
| Control group | 20 | 5.4 ± 0.4 ** | 14.7 ± 4.3 ** | 9.7 ± 1.9 ** | 5.6 ± 1.0 ** | 18.8 ± 4.0 ** | |
| Model group | 20 | 24.9 ± 2.8 | 31.6 ± 1.2 | 29.6 ± 1.4 | 27.6 ± 1.6 | 57.9 ± 2.4 | |
| Metformin group | 270 | 20 | 25.4 ± 3.0 | 27.4 ± 2.6 ** | 21.7 ± 2.5 ** | 19.1 ± 2.2 ** | 45.9 ± 4.7 ** |
| Treatment groups (High-dose, Medium-dose, Low-dose) | 1200 | 20 | 25.3 ± 2.5 | 30.0 ± 1.5 | 26.0 ± 3.0 ** | 23.0 ± 2.5 ** | 52.4 ± 4.5 ** |
| 600 | 20 | 24.3 ± 3.8 | 30.0 ± 1.6 | 26.8 ± 2.1 * | 24.7 ± 2.1 * | 53.6 ± 4.1 * | |
| 300 | 20 | 24.9 ± 2.0 | 30.4 ± 2.0 | 27.5 ± 1.7 | 25.1 ± 1.8 * | 54.7 ± 3.4 | |
| Group | Dose (mg·kg−1) | N | SOD (U·mL−1) | MDA (nmol·mL−1) |
|---|---|---|---|---|
| Control group | 20 | 154.59 ± 36.66 ** | 4.59 ± 2.67 ** | |
| Model group | 20 | 113.78 ± 22.20 | 8.14 ± 1.62 | |
| Metformin group | 270 | 20 | 144.53 ± 21.90 ** | 5.00 ± 2.73 ** |
| Treatment groups (High-dose, Medium-dose, Low-dose) | 1200 | 20 | 148.87 ± 18.36 ** | 4.42 ± 1.96 ** |
| 600 | 20 | 142.99 ± 20.41 ** | 4.99 ± 2.12 ** | |
| 300 | 20 | 139.19 ± 16.11 * | 5.29 ± 2.60 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Fan, W.; Qin, J.; Guo, S.; Xiao, H.; Tang, Z. Pharmacological and Pathological Effects of Mulberry Leaf Extract on the Treatment of Type 1 Diabetes Mellitus Mice. Curr. Issues Mol. Biol. 2023, 45, 5403-5421. https://doi.org/10.3390/cimb45070343
Luo L, Fan W, Qin J, Guo S, Xiao H, Tang Z. Pharmacological and Pathological Effects of Mulberry Leaf Extract on the Treatment of Type 1 Diabetes Mellitus Mice. Current Issues in Molecular Biology. 2023; 45(7):5403-5421. https://doi.org/10.3390/cimb45070343
Chicago/Turabian StyleLuo, Liru, Wei Fan, Jingping Qin, Shiyin Guo, Hang Xiao, and Zhonghai Tang. 2023. "Pharmacological and Pathological Effects of Mulberry Leaf Extract on the Treatment of Type 1 Diabetes Mellitus Mice" Current Issues in Molecular Biology 45, no. 7: 5403-5421. https://doi.org/10.3390/cimb45070343
APA StyleLuo, L., Fan, W., Qin, J., Guo, S., Xiao, H., & Tang, Z. (2023). Pharmacological and Pathological Effects of Mulberry Leaf Extract on the Treatment of Type 1 Diabetes Mellitus Mice. Current Issues in Molecular Biology, 45(7), 5403-5421. https://doi.org/10.3390/cimb45070343





