Abstract
Kidney tumors comprise a broad spectrum of different histopathological entities, with more than 0.4 million newly diagnosed cases each year, mostly in middle-aged and older men. Based on the description of the 2022 World Health Organization (WHO) classification of renal cell carcinoma (RCC), some new categories of tumor types have been added according to their specific molecular typing. However, studies on these types of RCC are still superficial, many types of these RCC currently lack accurate diagnostic standards in the clinic, and treatment protocols are largely consistent with the treatment guidelines for clear cell RCC (ccRCC), which might result in worse treatment outcomes for patients with these types of molecularly defined RCC. In this article, we conduct a narrative review of the literature published in the last 15 years on molecularly defined RCC. The purpose of this review is to summarize the clinical features and the current status of research on the detection and treatment of molecularly defined RCC.
1. Introduction
Kidney cancer is the 14th most common cancer worldwide, and its incidence has continued to increase in recent years []. To date, more than 0.4 million new cases of kidney cancer are diagnosed each year [,]. Among them, more than 85% of patients present with renal cell carcinoma (RCC) []. Based on the traditional histopathological classification, RCC can be divided into three main categories: clear cell carcinoma (ccRCC, 75%), papillary renal cell carcinoma (PRCC, 15–20%), and chromophobe cell renal carcinoma (chRCC, 5%) []. Studies in recent years have found that RCC mostly occurs in older men [] and most cases are localized tumors, with only 17% of RCC patients having distant metastases at the time of diagnosis, which are mainly found in lung, bone, liver, lymph nodes, and adrenal gland [,]. In 2020, a statistic by Padala SA showed that the 5-year survival rate for kidney cancer patients with metastatic disease was only 12% []. Currently, there are more and more treatment modalities for patients with metastatic RCC (mRCC), with targeted therapies and immune checkpoint inhibitor-based immunotherapy gradually proving to be effective in the treatment of patients with mRCC and the survival rate of those patients greatly improving recently [,].
Epigenetic alterations are considered to be a hallmark of cancer []. However, recent studies found that RCC has multiple molecular alterations, such as DNA methylation and micro-RNA alterations in ccRCC, which could greatly affect the biological progression of these tumors [,]. The 2022 World Health Organization (WHO) classification of pathological kidney tumors added new histopathological subtypes, including molecularly defined RCC [,]. It includes transcription factor binding to IGHM enhancer 3 (TFE3)-rearranged renal cell carcinomas, transcription factor EB (TFEB)-altered renal cell carcinomas, elongin C (ELOC)-mutated renal cell carcinoma, fumarate hydratase (FH)-deficient renal cell carcinoma, succinate dehydrogenase (SDH)-deficient renal cell carcinoma, anaplastic lymphoma kinase (ALK)-rearranged renal cell carcinomas, and SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)-deficient renal medullary carcinoma (see Table 1) []. These different molecularly defined histopathological subtypes of RCC are easily confused and may lead to suboptimal treatment outcomes as a result of misdiagnoses []. In this article, we summarize the pathological and clinical characteristics of each molecularly defined RCC subtypes and present their molecular features and the current treatment strategy status. We hope that this will be helpful for physicians to develop accurate diagnostic and therapeutic options for those RCC patients in clinical practice.

Table 1.
Genes of molecularly defined renal cell carcinoma and associated clinical syndromes.
2. TFE3-Rearranged Renal Cell Carcinomas
Transcription factor binding to IGHM enhancer 3 (TFE3) is an important regulator of the immune system and has now been shown to cooperate with transcription factor EB (TFEB) to control and regulate carbohydrate and lipid metabolism and mitochondrial homeostasis []. The TFE3/TFEB rearrangement renal cell carcinoma is characterized by translocations involving the TFE3 and TFEB genes. They are both derived from the microphthalmia transcription (MiT) family of heterotopic RCC according to the 2016 version of the WHO classification. The MiT subfamily of transcription factors includes TFE3, TFEB, TFEC, and MITF []. TFE3- and TFEB-rearranged RCC accounts for 1–4% of the newly diagnosed adult patients []. Recent studies have shown that TFE3/TFEB-rearranged RCC can be frequently detected in children []. In adults RCC patients, TFE3 ectopic fusions with chaperone genes are more commonly seen [], and there are no significant prognostic gender differences [] (Figure 1). This ectopic fusion with a chaperone gene and the decreased immunity in adults TFE3-rearranged RCC patients cause them to have a potentially more aggressive course compared to the pediatric patients []. Current studies suggest that previous exposure to cytotoxic chemotherapy might be a predisposing factor [].
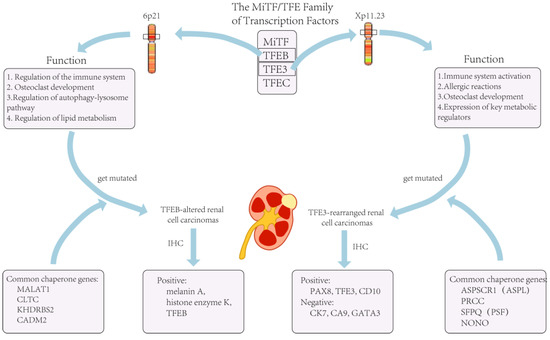
Figure 1.
The role of TFE3 in the organism and tumors caused by its mutation.
The list of chaperone genes has been growing and evolving, with more than a dozen having been reported []. The three most common translocations currently include a fusion of the PRCC and TFE3 genes, a fusion of the ASPL (ASPSCR1) and TFE3 genes, and a fusion of the SFPQ and TFE3 genes []. In addition to this, there are also genes such as CLTC, PARP14, RBM10, NONO, and MED15 that can be fused with ectopic TFE3 []. However, current studies suggest that different chaperone genes may exhibit different oncological behaviors and tumor morphologies, and these features vary depending on the type of the involved chaperone genes []. For example, TFE3 is more likely to exhibit lymph node metastasis when fused with PRCC than when fused with ASPSCR3 [].
In terms of histopathological morphology, the characteristics of TFE3 fusion usually presents with transparent eosinophils, a papillary architecture, and psammoma bodies under the microscope [,]. However, due to chaperone genes, RCC with TFE3 rearrangement may also resemble other types of RCC, including ccRCC, PRCC, and epithelioid vascular smooth muscle lipoma []. Therefore, attention should be paid and the impact of the genes that are fused with should be determined as much as possible both in the diagnosis and in the treatment of TFE3-rearranged renal cell carcinoma.
When facing TFE3-rearranged renal cell carcinoma, immunohistochemistry (IHC) is the most commonly used examination for diagnosis []. If IHC is not used at the time of diagnosis, a large proportion of TFE3-rearranged renal cell carcinomas are likely to be misdiagnosed as ccRCC []. For most other types of RCC, the positive IHC markers are cytokeratin 7 (CK7), carbonic anhydrase 9 (CA9), and GATA3. However, these are not expressed in TFE3-rearranged RCC and are usually positive for histone K []. In a recent review article, IHC data from nearly 400 cases of TFE3-rearranged RCC patients were analyzed, and the biomarkers with the highest probability of positivity were found to be PAX8 (100%), TFE3 (95%), CD10 (89%), and achromatase (82%) []. However, TFE3-rearranged RCC did not always exhibit TFE3 overexpression, and lower TFE3 expression at the time of detection often resulted in false-positive or false-negative results; thus, this could limit the sensitivity and specificity of IHC for detecting TFE3-rearranged RCC []. In addition to this, the accuracy of IHC might be affected by the technique and be influenced by the formalin fixation time []. On the other hand, there has been no consensus or standardized guidelines regarding the judgment of TFE3 staining results [], and different pathologists might give completely opposite judgments if specimens show heterogeneous or focal staining. Lee HJ et al. used tissue specimens from 303 RCC patients for IHC testing and found that 23.2% of IHC-negative TFE3 tumors were eventually diagnosed as TFE3-rearranged RCC []. Therefore, in clinical practice, a negative TFE3 IHC result alone did not exclude the possibility of a TFE3-rearranged RCC case. Thus, in some cases, a combination of clinical presentation and other examination results might be needed.
The current literature suggests that the detection of TFE3 gene rearrangements by fluorescence in situ hybridization (FISH) is more sensitive and advantageous in experimental manipulation than traditional IHC [], and its results are more stable in formalin-fixed tissues []. Therefore, FISH is currently considered as the gold standard for the diagnosis of TFE3-rearranged RCC []. However, some chaperone genes, such as NONO, RBM10, and GRIPAP1, after fusing with TFE3, may not be detected by traditional FISH assays for significant TFE3-positive results. []. In addition, similar to IHC testing, the current standard definition of a positive FISH result varies widely among laboratories, from as low as 10% up to 30% []. These results suggest that, although the FISH test is currently the gold standard for the diagnosis of TFE3-rearranged RCC, in clinical practice, it should be carefully used together with other test results. For example, the previously mentioned IHC and FISH tests should be considered along with the option of gene probes or alternative molecular techniques []. In fact, FISH cannot provide information about fused genes, so in order to further confirm the diagnosis in clinical practice, RNA sequencing is often used to identify the gene involved in the translocation []. Recently, TRIM63 determination by RNA in situ hybridization (RNA-ISH) was proposed as an alternative diagnostic tool for TFE3- and TFEB-rearranged RCC [], but no strong evidence is available from in vitro studies.
Due to the rarity of TFE3-rearranged RCC and the fact that it has not been previously considered as a specific tumor subtype, there are no treatment recommendations for it to date []. Most previous treatment regimens are consistent with those for patients with ccRCC; however, due to recent developments in detection technology, its diagnosis has become more accurate, similarly to the detection of ccRCC. More importantly, drugs that normally treat ccRCC may not be effective against TFE3-rearranged RCC []. Additionally, Aldera AP et al. found that patients with TFE3-rearranged RCC may develop metastases within 20–30 years after diagnosis, so such patients may also need long-term clinical follow-up [].
3. TFEB-Altered Renal Cell Carcinomas
As previously stated, TFEB-altered RCC has been included in the MiTF-translocated carcinoma family and TFEB-overexpressing renal tumors were initially identified in pediatric patients. Nowadays, with the availability of accurate examinations, more and more adult RCC patients are diagnosed with TFEB-altered RCC []. Nevertheless, the number of TFEB-altered RCC cases is still much lower than for TFE3-rearranged RCC []. There are two types of TFEB-altered RCC, including TFEB-rearranged RCC and TFEB-amplified RCC. The TFEB gene in TFEB-rearranged RCC is located on chromosome 6 and is most often translocated into chromosome 11, fusing with the MALAT1 gene. Therefore, it was previously called t(6;11) RCC []. In the last few years, researchers have identified cases of RCC related to TFEB amplification, and after further testing and analysis, it was found that both genetic alteration patterns could co-exist in one case []. Due to the rarity of the disease, there are few studies on the distinction between different subtypes of TFEB-altered RCC, and current case studies show that the mean age of diagnosis for TFEB-amplified RCC is 62.5–64 years, while the mean age of diagnosis for TFEB translocated RCC is 32.8–34 years [,].
Similar to TFE3-rearranged RCC, TFEB can also be ectopically fused to chaperone genes [] (Figure 1). Furthermore, for TFEB-amplified RCC, in addition to the possible elevated expression of TFEB, they are often accompanied by the amplification of other oncogenes, such as vascular endothelial growth factor A (VEGFA) and G1 S specific cyclin D3 (CCND3) []. It has been shown that these two genes are associated with aggressive oncological behavior [], which would precisely explain the severe clinical symptoms and poor prognosis of patients with TFEB-amplified RCC.
Although TFEB genes are altered in TFEB-altered RCC, the characteristics of tumor growth vary considerably between different patterns of alteration. It has been widely reported that in TFEB-translocated RCC, the most commonly found morphology is a biphasic growth pattern consisting of large and small tumor cells [], with smaller cells around the basement membrane-like structures. In addition to this, extensive hyalinization, a papillary architecture, and a clear cell morphology can be seen []. However, in TFEB-amplified RCC, this pattern is less common. Gupta S et al. investigated 37 patients with TFEB-altered RCC and found that nearly half of the patients had renal tubular structures and prominent cytoplasmic eosinophilia of tumor cells in their tumor specimens [].
IHC and FISH are commonly used tests to detect TFEB-altered RCC; however, when assessing whether the TFEB gene is amplified or translocated, the markers used in the detection are quite similar. For TFEB-altered RCC, it has been shown that the staining results for both histone K and Melan-A are positive []. Similarly, Gupta S et al. and Wyvekens N et al. studied TFEB-amplified RCC and TFEB-translocated RCC, respectively, and they found that both types of tumors typically express melanin A and histone enzyme K. The difference was that tumor cells in TFEB-amplified RCC were usually diffusely or patchily positive when tested for TFEB levels []. However, there was also a subset of TFEB-amplified RCC that had lower TFEB expression levels than TFEB-translocated RCC []. Therefore, the type of TFEB gene alteration cannot be distinguished by a TFEB-specific assay alone. If a type of TFEB gene alteration is suspected, it should also be demonstrated using a FISH breakdown test or identified by RNA sequencing with a gene fusion examination []. In clinical practice, such detailed testing and diagnosis is not always necessary for all patients because of the very low incidence of the disease, the high cost of FISH, and the use of sequencing tests.
In addition to this, it has also been found that TFEB-amplified RCC exhibits a higher tumor aggressiveness than TFEB-rearranged tumors, and the 5-year survival rate for TFEB-amplified RCC is only 48% [], while TFEB-translocated RCC progresses more slowly than TFE3-rearranged RCC. Therefore, in clinical practice, physicians should distinguish TFEB-amplified RCC from TFEB-translocated RCC. Since TFEB-altered RCC has often been previously diagnosed as ccRCC, its current treatment modality still differs little from the standard treatment for patients with ccRCC, which may also contribute to the poor prognostic outcome for patients with TFEB-altered RCC. We hope that more appropriate targeted drugs and treatment strategies for TFEB-altered RCC will become available in the future.
4. Elongin C (ELOC, Formerly TCEB1)-Mutated Renal Cell Carcinoma
Elongin C (ELOC) is a transcription factor in the human body and the product of this gene expression is ELOC, which is part of the von Hippel–Lindau (VHL) protein complex and is responsible for the ubiquitination of hypoxia inducible factor 1 alpha subunit (HIF1α) and its subsequent degradation []. Previous studies have shown that HIF can activate the transcription of a large number of oncogenes, leading to tumorigenesis []. ELOC-mutated RCC was classified as ccRCC in previous WHO classifications [], accounting for 0.5% to 5% of ccRCC []. However, in recent years, ELOC-mutated RCC has been found to present as wild-type VHL, exhibiting somatic mutations in the ELOC gene and deletion of the alternative allele (8q21) []. In addition to this, the microscopic morphology of ELOC-mutated RCC also differs in many ways from ccRCC []. Therefore, in the latest WHO classification for RCC, it was assigned to the molecularly defined tumors as a separate pathological type. ELOC-mutated RCC is a rare form of RCC [] that usually develops in middle-aged and elderly male patients, most of whom are around 50 years of age [].
Unlike the previous tumor types, ELOC-mutated RCC can be seen under the microscope with a clear cellular morphology []. It is usually similar to ccRCC [], which has a transparent cellular appearance []. This is one of the reasons why it was assigned to the ccRCC category in the previous WHO classification. However, recent studies have shown that ELOC-mutated RCC also have thick fibromuscular bands and branching glandular vesicular or tubular structures similar to the morphology of ccRCC [,], and these manifestations can be distinguished from ccRCC. When tested using IHC, ELOC-mutated RCC can show the same aspects as ccRCC in that it is positive for both CA9 and CD10 []. However, in the study by Wang Y et al., IHC testing was performed in four patients with ELOC-mutated RCC and it was found that they all showed strong positive expression for CA9 and three patients showed positive results for CK7 and CD10. In addition, the authors observed ELOC positivity localized only in the nucleus of all four patients []. Despite the small number of cases selected, this result might also indicate that ELOC positivity in the nucleus was a characteristic manifestation of ELOC-mutated RCC. Similarly, Shah RB et al. conducted a study including 21 RCC patients with ELOC mutations and found that 16 of them had IHC staining results expressing diffuse positivity for CK7 []. In summary, in addition to observing the characteristic structure of ELOC-mutated RCC under a microscope, the use of IHC to detect CK7, ELOC, CA9, and CD10 could further help to confirm the diagnosis.
Previous studies have shown that ELOC-mutated RCC tends to be inert compared to ccRCC [], but recently there have been some case studies demonstrating that certain cases could exhibit an aggressive oncological behavior. For example, DiNatale RG et al. investigated clinical data from five patients with ELOC-mutated RCC and found that four of them had advanced tumors (stage III-IV) and four had developed distant metastases []. This aggressiveness might be related to oncogene activation due to mutations in ELOC. Since ELOC-mutated RCC was previously widely considered to be one type of ccRCC, the current treatment is largely consistent with the treatment guidelines for ccRCC.
5. Fumarate Hydratase-Deficient Renal Cell Carcinoma
Fumarate hydratase (FH) is an indispensable enzyme in the tricarboxylic acid cycle that produces cellular energy in the form of ATP through oxidative phosphorylation (OXPHOS) in mitochondria []. Mutations in the gene where FH is located can lead to fumarate accumulation, which not only causes an imbalance in the energy supply but also impairs the function of histones and DNA demethylases, thus causing abnormal gene expression []. Singh NP et al. analyzed the TCGA database and found that alterations in the FH gene were associated with the immune function of PRCC [], in addition to the accumulation of metabolites, such as fumarate, which promote the expression of inflammatory factors and suppress the body’s tumor immunity [,]. Fumarate hydratase (FH)-deficient RCC is a rare subtype of renal cancer that was considered a subtype of PRCC in the previous classification of RCC []. In the fifth edition of the WHO cancer classification, FH-deficient RCC has replaced hereditary leiomyomatosis and renal cell carcinoma (HLRCC) as a separate molecular subtype. It is characterized by germline mutations or somatic mutations in the FH gene, resulting in decreased expression of FH []. In addition to this, several studies have shown that methylation of genes, such as cyclin-dependent kinase inhibitor 2A (CDKN2A), O-6-methylguanine DNA methyltransferase (MGMT), adenomatous polyposis coli (APC), and tumor protein P53 (TP53), all of which are associated with tumorigenesis and progression, have been observed in FH-deficient RCC [], which may explain the aggressiveness and poor prognostic outcome for patients observed in the clinic. HLRCC is an inherited syndrome caused by congenital mutations in FH gene and it is inherited in an autosomal dominant fashion. In clinical practice, HLRCC often presents as uterine tumors and smooth muscle tumors of the skin [,]. Indeed, it has long been shown that HLRCC increases the susceptibility to aggressive RCC []. However, there is no very precise treatment modality for patients with FH-deficient RCC.
Due to the rarity of FH-deficient RCC, the current knowledge of its disease characteristics and course is not very accurate. Yu YF et al. found that the mean age of onset was 36.7 years through a survey of 11 patients with FH-deficient RCC, which is lower than that of RCC patients without FH defects []. FH-deficient RCC could also exhibit many pathological structures, thus increasing its probability of being misdiagnosed []. Often, patients are much younger compared to other types of renal tumors when firstly diagnosed.
FH-deficient RCC can exhibit a variety of growth patterns and is, therefore, difficult to differentiate histologically []. The papillary type is the most common structure, and other common types include solid, tubulocystic, and sieve-like []. Microscopically, FH-deficient RCC also has characteristic histological manifestations, such as a papillary architecture with tubule cystic growth patterns, abundant eosinophilic granulocytes, and a perinuclear halo []. However, microscopic observation alone is not enough; more tests, such as IHC and imaging, are required to confirm the diagnosis []. In the clinical setting, genetic detection of mutations in FH is the gold standard for the diagnosis of FH-deficient RCC []. The imaging manifestations of FH-deficient RCC are very diverse, and it can present as a solid enhancing mass or as a mildly enhancing cystic mass, etc. These presentations cannot be distinguished from other types of RCC; therefore, diagnosis by imaging alone is incomplete []. Magnetic resonancespectroscopy (MRS) has also recently been proposed to be helpful in confirming the diagnosis of FH-deficient RCC. Wu G et al. used MRS in six patients with FH-deficient RCC and showed that the sensitivity, specificity, and accuracy were 69%, 100%, and 91%, respectively []. In IHC testing, the characteristic presentation of FH-deficient RCC is the lack of FH staining []; however, a recent study reported that there were isolated cases of FH-deficient RCC in which positive FH could still be detected []. Therefore, a positive result for FH does not completely exclude the possibility of FH-deficient RCC. In addition to detecting FH, studies in recent years suggested that some other biomarkers might play a key role in the detection of this disease. For example, CK7 and TFE3 usually show negative results, while PAX8 and succinate dehydrogenase B abnormal succinate semicarbonate (2SC) S-(2-succino)-cysteine usually show positive results in the detection of patients with FH-deficient RCC [,,].
Clinically, most FH-deficient RCC exhibit highly aggressive tumors, and patients are often found to have highly staged or distant metastases when they are diagnosed [], with the most common sites of metastasis being the lymph nodes in the chest and abdomen, bone, and liver []. In addition, there is no clear standard treatment strategy for patients with FH-deficient RCC [], and its highly aggressive course often makes treatment more difficult []. Most treatment stratigies for patients with FH-deficient RCC are quite similar to the treatment guidelines for patients withccRCC; however, due to the different pathogenesis and oncologic behavior, treatments that mimic ccRCC often result in an increased chance of distant metastasis and death for patients with FH-deficient RCC []. In the past years, several new drugs have been explored for the treatment of this disease, such as sunitinib, pazopanib and immune checkpoint inhibitors (ICIs), including ipilimumab and nivolumab []. However, the efficacy of these drugs is not yet supported by clear positive evidence. In a recent study comparing treatment outcomes in 55 patients with FH-deficient RCC, the analysis found that the treatment with ICIs in combination with tyrosine kinase inhibitor (TKI) may have a better clinical outcome compared to monotherapy []. In addition to this, Gleeson JP et al. analyzed 26 patients with FH-deficient RCC to assess the efficacy of combined treatment with vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR), and the study demonstrated that the objective response rate of this combination therapy was 44% []. In addition to this, recent reports demonstrated that bevacizumab in combination with erlotinib had entered phase II clinical trials and was currently showing positive results []. In the future, more targeted agents and more standard treatments will be available to help patients with FH-deficient RCC.
6. Succinate Dehydrogenase-Deficient Renal Cell Carcinoma
Succinate dehydrogenase (SDH), a complex that functions in mitochondria, is composed of several subunits (SDHA, SDHB, SDHC, and SDHD) []. It plays an important role in cellular respiration and energy metabolism, catalyzing the conversion of succinate to fumarate []. In tumorigenesis, SDH is considered as a class of cancer suppressor gene [], and current studies demonstrate that when the SDH gene germline is altered, it often results in the development of paragangliomas, gastrointestinal mesenchymal tumors, and pituitary adenomas [,]. In addition, SDH-deficient RCC has also been shown to be associated with SDH germline mutations, and by far the most commonly found are mutations in SDHB, while SDHC, SDHA, and SDHD mutations are rare. SDH-deficient RCC is rare, accounting for an estimated 0.05% to 0.2% of all RCC cases [].
SDH-deficient RCC can be seen in a wide variety of age groups and, in a survey by Gill AJ et al., they found that the age of diagnosis of SDH-deficient RCC can range from 14 to 76 years and is predominate in male patients []. Unlike the previously described RCC, most SDH-deficient RCC cases are low grade and have a good prognosis with a low probability of metastasis []. However, some SDH-deficient RCC cases with high-grade nuclei, sarcomatoid changes, or coagulative necrosis can have an aggressive oncological behavior with a poor prognosis []. Therefore, in facing RCC patients with the above pathological features, an aggressive molecular diagnosis should be clarified and early therapeutic measures should be taken to improve the quality of life and life expectancy of these patients.
For SDH-deficient RCC, its tumor cells are usually cuboidal, with nested or tubular growth pattern. However, its most characteristic morphology compared to other RCCs is the presence of vesicles or flocculent inclusions in the cytoplasm [], which is often due to the enlargement of mitochondria as a result of an altered respiratory chain []. In terms of IHC, the negative result of SDHB staining is currently considered important for the definitive confirmation of the diagnosis []. However, recent studies have shown that decreased SDH expression is also observed in some non-SDH germline-deficient tumors [], a condition that may be somewhat misleading in IHC, and, therefore, it may be inaccurate to solely rely on the decreased SDH expression to make the diagnosis. SDH-deficient RCC usually shows negativity for CK7, CD117, histone K, TFE3, and HMB45, but positivity for biomarkers such as PAX8 and epithelial membrane antigen (EMA) [,,]. Another recent study indicated that tumor cells in SDHA-deficient RCC showed negativity for both SDHA and SDHB, while RCC caused by defects in the SDHB, SDHC, or SDHD genes only showed negativity for SDHB []. This is also a possible way to diagnose SDH-deficient RCC accurately.
Clinically, most SDH-deficient RCC patients present as low-grade tumors; however, in some rare cases, distant metastases may be present []. In this regard, most SDH-deficient RCC can usually be easily cured by surgical resection [], and for early-stage tumors, even partial nephrectomy can be performed to preserve the kidney []. For patients with advanced-grade or with distant metastases, some studies have shown that targeted therapy with tyrosine kinase inhibitors, VEGF-targeted drugs, or mTOR-targeted drugs has shown positive therapeutic effects in patients with SDH-deficient RCC [,].
7. ALK-Rearranged Renal Cell Carcinomas
Anaplastic lymphoma kinase (ALK) is a membrane-associated tyrosine kinase that belongs to the insulin receptor family [,]. ALK functions to regulate cell proliferation and promote cell motility []. When ALK gene rearrangement occurs, it may lead to tumorigenesis. In 2011, two cases of ALK-rearranged RCC were first identified and diagnosed [], and until now, it is still a very rare tumor [], which accounts for 0.12–0.56% of all RCC cases []. Generally, a high expression of ALK can be observed in patients with ALK-rearranged RCC []. Similar to TFE3-rearraged RCC, it has many accompanying fusion genes. Various fusion genes have been identified in recent years, such as VCL, TPM3, EML4, STRN, and HOOK1 [], with renal tumors of VCL and HOOK1 rearranged with ALK only described in pediatric patients [].
Due to its rarity, there is no standard characteristic description of the clinical presentation of patients with ALK-rearranged RCC, which remains similar to PRCC and ccRCC in this sense []. ALK-rearranged RCC has many pathological manifestations, most of which display a shaped structure, in addition to solid and tubular patterns []. Among them, they can be roughly divided into two categories according to the morphology: One is ALK-rearranged RCC with VCL as a fusion gene, which occurs mostly in childhood and has a sickle-cell trait, eosinophilic granulocytic stroma, and cytoplasmic lumen [,]; the other category comprises other ALK-rearranged RCCs, most of which have a morphology similar to PRCC and also consist of abundant intracellular and extracellular mucins with eosinophilic granuloplasm [,]. In terms of IHC, the detection of ALK expressed in abundance in ALK-rearranged RCC using IHC has proven to be a valuable tool for the diagnosis of ALK []. In addition to this, several recent studies have found that the majority of ALK-rearranged RCC cases showed positive results for biomarkers such as PAX7, CK10, AMACR, CD3, and cytokeratin; negative results for biomarkers such as carbonic anhydrase IX, TFE45, histone enzyme K, Melan A, and HMB45 [,]. These results can further help physicians to differentiate ALK-rearranged RCC from other types of RCC.
There is no standard treatment for patients with ALK-rearranged RCC; however, a recent study found that ALK-rearranged RCC with VCL as a fusion gene did not generally exhibit recurrence or distant metastasis [], while ALK-rearranged RCC accompanied by other fusion genes showed a more aggressive clinical course []. As targeted agents continue to be developed, there is evidence that inhibitors of ALK, such as crizotinib and alectinib, can demonstrate efficacy in the treatment of nonsmall cell lung cancer and myofibroblastic tumors due to ALK rearrangements [,,]. Although evidence for the treatment of ALK-rearranged RCC is still lacking, it is hoped that more clinical trials will be conducted in the future to demonstrate the efficacy of targeted agents for the treatment of patients with ALK-rearranged RCC.
8. SMARCB1-Deficient Renal Medullary Carcinoma
SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1) is a SWI/SNF protein complex that was considered to be a tumor suppressor in past studies and plays an important regulatory role in the organism []. In recent years, researchers have discovered that the SMARCB1 gene is located on chromosome 22 and, when it is altered, SMARCB1 expression is decreased or even absent [], and a series of tumors are rapidly developed, such as malignant rhabdoid tumors of the central nervous system, renal medullary RCC, and epithelioid sarcoma []. In the 2022 edition of the WHO classification for RCC, this class of renal medullary carcinoma (RMC) with mutations in the SMARCB1 gene is classified as a new molecular category called SMARCB1-deficient RMC []. SMARCB1-deficient RMC is a rare cancer [], which usually develops in patients with the sickle-cell trait (SCT) or sickle-cell disease (SCD).
SMARCB1-deficient RMC is an aggressive tumor that commonly affects males and is predominantly right sided []. It is often found at an advanced stage or with distant metastases, and recent studies have shown that SMARCB1-deficient RMC is also associated with the sickle-cell trait []. Specific symptoms are usually abdominal pain, hematuria, and weight loss [], while distant metastases can be found in the renal lymph nodes, adrenal glands, lungs, and liver []. Due to the prevalence of SMARCB1-deficient RMC in children and adolescents and the aggressive nature of the tumor, early recognition and diagnosis are a priority for physicians.
In previous clinical practice, patients with SMARCB1-deficient RMC were often misdiagnosed as ccRCC []. With the advancement of detection technology in recent years, some characteristic manifestations of SMARCB1-deficient RMC have been gradually proposed. First, in addition to the previously mentioned clinical symptoms and prodromal nature during adolescence, SMARCB1-deficient RMC usually develops with SCT and SCD []. Secondly, the tumor is often already at a high grade at the time of detection, showing infiltrative growth and exhibiting a sieve or reticular appearance [,]. In addition to this, and most importantly, all SMARCB1-deficient RMC showed negative staining for SMARCB1 when IHC for the detection of the SMARCB1 protein was performed []. Therefore, when adolescent RCC patients with hematologic disorders such as SCT are identified in the clinic, physicians should perform IHC testing as early as possible to determine whether SMARCB1-deficient RMC is present.
Due to the rarity of the disease and the highly aggressive nature of the tumor, the current treatment options for SMARCB1-deficient RMC are not effective, with one study published in 2015 showing that the average overall survival of patients with SMARCB1-deficient RMC was only 6–8 months, with only one patient reaching 1 year []. Moreover, there is no standard treatment strategy for the disease. Due to the rapid progression of the disease, the predominant recommended treatment modality in the clinic is platinum-based chemotherapy []. In recent years, in addition to conventional treatments for kidney cancer, investigators have tried to explore the efficacy of various targeted agents for SMARCB1-deficient RMC. Examples include VEGF inhibitors, mTOR inhibitors (e.g., everolimus), etc. []; however, none of the patient outcomes have been very satisfactory. Immunosuppressive agents have been popular for oncology treatment, and Forrest SJ et al. tested 30 patients with SMARCB1-deficient RMC and found that 47% of them were positive for PD-L1 expression []. Furthermore, it has also been shown that, for SMARCB1-deficient RMC, differences in the tumor cell origin make it difficult for physicians to grasp the immune profile of the tumor []. Therefore, immunotherapy for SMARCB1-deficient RMC requires more in-depth studies in the future.
9. Conclusions
RCC is a common tumor that occurs mostly in men and most of them are low-grade tumors. However, in recent years, it has been discovered that RCC also has many specific molecular types, and the different molecular types may determine different clinical features and treatment outcomes. However, for many years, due to limited testing technology, many RCC patients were not diagnosed with a clear molecular type and most were managed according to the standard treatment protocol of ccRCC, resulting in poor outcomes and prognosis for many patients. This article presents the molecular types in the 2022 WHO classification of renal cancers, including the genetic alterations and clinical manifestations of each tumor type, followed by a summary of the current molecular testing results and current treatment status for each tumor type. Here, we suggest that urological clinicians should individualize the genetic level of testing when presented with RCC patients based on clinical manifestations and laboratory tests and should give targeted treatment after diagnosis. For certain congenital genetic defective RCCs, attention should also be paid to the effect of the genetic defect at other sites. However, because physicians did not previously pay much attention to the molecular types of kidney cancer, and because of the rarity of the onset of certain RCC types, the existing clinical studies are inevitably limited in terms of sample size, observation angle, and treatment bias, and there are still many inconsistent conclusions on the characteristic manifestations of molecular detection and clinical treatment criteria. In recent years, research on molecular detection technologies and targeted drugs or immune checkpoint inhibitors has progressed very rapidly, physicians’ knowledge of the disease has become more and more mature, and significant progress has been made in the diagnosis and treatment of RCC. In the future, we hope that there will be more tests and detection standards for RCC in molecular science and effective drugs to help RCC patients have a better prognosis and higher quality of life.
Author Contributions
Conceptualization, G.Z.; original draft writing, X.H.; figures preparation and table making, C.T.; manuscript review and editing, G.Z.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The Fundamental Research Funds for the Central Universities of China (No. xjj2018zyts34) and the Research Funds on Social Development from the Department of Science and Technology of Shaanxi Province of China (No. 2020SF-119) to Guodong Zhu are acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 10021, 894–906. [Google Scholar] [CrossRef]
- Dibajnia, P.; Cardenas, L.M.; Lalani, A.A. The emerging landscape of neo/adjuvant immunotherapy in renal cell carcinoma. Hum. Vaccin. Immunother. 2023, 1, 2178217. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 6, 394–424. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 1, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 5, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 3, 179–184. [Google Scholar]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 3, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Stewart, G.D.; Frezza, C. Oncometabolites in renal cancer. Nat. Rev. Nephrol. 2020, 3, 156–172. [Google Scholar] [CrossRef]
- Navani, V.; Heng., D.Y.C. Treatment Selection in First-line Metastatic Renal Cell Carcinoma-The Contemporary Treatment Paradigm in the Age of Combination Therapy: A Review. JAMA Oncol. 2022, 8, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.; Esteller, M. Cancer epigenomics: Beyond genomics. Curr. Opin. Genet. Dev. 2012, 1, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Turajlic, S.; Rowan, A.; Nicol, D.; Farmery, J.H.R.; O’Brien, T.; Martincorena, I.; Tarpey, P.; Angelopoulos, N.; Yates, L.R.; et al. TRACERx Renal Consortium. Timing the Landmark Events in the Evolution of Clear Cell Renal Cell Cancer: TRACERx Renal. Cell 2018, 3, 611–623.e17. [Google Scholar] [CrossRef]
- Xing, T.; He, H. Epigenomics of clear cell renal cell carcinoma: Mechanisms and potential use in molecular pathology. Chin. J. Cancer Res. 2016, 1, 80–91. [Google Scholar]
- Pastore, N.; Vainshtein, A.; Klisch, T.J.; Armani, A.; Huynh, T.; Herz, N.J.; Polishchuk, E.V.; Sandri, M.; Ballabio, A. TFE3 Regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol. Med. 2017, 5, 605–621. [Google Scholar] [CrossRef]
- Argani, P. Translocation carcinomas of the kidney. Genes. Chromosomes Cancer 2022, 5, 219–227. [Google Scholar] [CrossRef]
- Caliò, A.; Segala, D.; Munari, E.; Brunelli, M.; Martignoni, G. MiT Family Translocation Renal Cell Carcinoma: From the Early Descriptions to the Current Knowledge. Cancers 2019, 8, 1110. [Google Scholar] [CrossRef]
- Sukov, W.R.; Hodge, J.C.; Lohse, C.M.; Leibovich, B.C.; Thompson, R.H.; Pearce, K.E.; Wiktor, A.E.; Cheville, J.C. TFE3 rearrangements in adult renal cell carcinoma: Clinical and pathologic features with outcome in a large series of consecutively treated patients. Am. J. Surg. Pathol. 2012, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Akgul, M.; Williamson, S.R.; Ertoy, D.; Argani, P.; Gupta, S.; Caliò, A.; Reuter, V.; Tickoo, S.; Al-Ahmadie, H.A.; Netto, G.J.; et al. Diagnostic approach in TFE3-rearranged renal cell carcinoma: A multi-institutional international survey. J. Clin. Pathol. 2021, 5, 291–299. [Google Scholar] [CrossRef]
- Kmeid, M.; Akgul, M. TFE3 Rearrangement and Expression in Renal Cell Carcinoma. Int. J. Surg. Pathol. 2022, 1, 10668969221108517. [Google Scholar] [CrossRef]
- Caliò, A.; Marletta, S.; Brunelli, M.; Pedron, S.; Portillo, S.C.; Segala, D.; Bariani, E.; Gobbo, S.; Netto, G.; Martignoni, G. TFE3 and TFEB-rearranged renal cell carcinomas: An immunohistochemical panel to differentiate from common renal cell neoplasms. Virchows Arch. 2022, 6, 877–891. [Google Scholar] [CrossRef]
- Argani, P.; Zhong, M.; Reuter, V.E.; Fallon, J.T.; Epstein, J.I.; Netto, G.J.; Antonescu, C.R. TFE3-Fusion Variant Analysis Defines Specific Clinicopathologic Associations Among Xp11 Translocation Cancers. Am. J. Surg. Pathol. 2016, 6, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Argani, P. MiT family translocation renal cell carcinoma. Semin. Diagn. Pathol. 2015, 2, 103–113. [Google Scholar] [CrossRef]
- Tretiakova, M.S. Chameleon TFE3-translocation RCC and How Gene Partners Can Change Morphology: Accurate Diagnosis Using Contemporary Modalities. Adv. Anat. Pathol. 2022, 3, 131–140. [Google Scholar] [CrossRef]
- Green, W.M.; Yonescu, R.; Morsberger, L.; Morris, K.; Netto, G.J.; Epstein, J.I.; Illei, P.B.; Allaf, M.; Ladanyi, M.; Griffin, C.A.; et al. Utilization of a TFE3 break-apart FISH assay in a renal tumor consultation service. Am. J. Surg. Pathol. 2013, 8, 1150–1163. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, D.H.; Kim, S.Y.; Hwang, C.S.; Lee, J.H.; Park, W.Y.; Choi, K.U.; Kim, J.Y.; Lee, C.H.; Sol, M.Y.; et al. TFE3 translocation and protein expression in renal cell carcinoma are correlated with poor prognosis. Histopathology 2018, 5, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Y.; Mannan, R.; Skala, S.L.; Rangaswamy, R.; Chinnaiyan, A.; Su, F.; Cao, X.; Zelenka-Wang, S.; McMurry, L.; et al. TRIM63 is a sensitive and specific biomarker for MiT family aberration-associated renal cell carcinoma. Mod. Pathol. 2021, 8, 1596–1607. [Google Scholar] [CrossRef]
- Aldera, A.P.; Ramburan, A.; John, J. TFE3-rearranged renal cell carcinoma with osseous metaplasia and indolent behaviour. Urol. Case Rep. 2022, 42, 102041. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Argani, P.; Jungbluth, A.A.; Chen, Y.B.; Tickoo, S.K.; Fine, S.W.; Gopalan, A.; Al-Ahmadie, H.A.; Sirintrapun, S.J.; Sanchez, A.; et al. TFEB Expression Profiling in Renal Cell Carcinomas: Clinicopathologic Correlations. Am. J. Surg. Pathol. 2019, 11, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Reuter, V.E.; Zhang, L.; Sung, Y.S.; Ning, Y.; Epstein, J.I.; Netto, G.J.; Antonescu, C.R. TFEB-amplified Renal Cell Carcinomas: An Aggressive Molecular Subset Demonstrating Variable Melanocytic Marker Expression and Morphologic Heterogeneity. Am. J. Surg. Pathol. 2016, 11, 1484–1495. [Google Scholar] [CrossRef]
- Wyvekens, N.; Rechsteiner, M.; Fritz, C.; Wagner, U.; Tchinda, J.; Wenzel, C.; Kuithan, F.; Horn, L.C.; Moch, H. Histological and molecular characterization of TFEB-rearranged renal cell carcinomas. Virchows Arch. 2019, 5, 625–631. [Google Scholar] [CrossRef]
- Williamson, S.R.; Grignon, D.J.; Cheng, L.; Favazza, L.; Gondim, D.D.; Carskadon, S.; Gupta, N.S.; Chitale, D.A.; Kalyana-Sundaram, S.; Palanisamy, N. Renal Cell Carcinoma with Chromosome 6p Amplification Including the TFEB Gene: A Novel Mechanism of Tumor Pathogenesis? Am. J. Surg. Pathol. 2017, 3, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Eble, J.N.; Palanisamy, N. Sclerosing TFEB-rearrangement renal cell carcinoma: A recurring histologic pattern. Hum. Pathol. 2017, 62, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Johnson, S.H.; Vasmatzis, G.; Porath, B.; Rustin, J.G.; Rao, P.; Costello, B.A.; Leibovich, B.C.; Thompson, R.H.; Cheville, J.C.; et al. TFEB-VEGFA (6p21.1) co-amplified renal cell carcinoma: A distinct entity with potential implications for clinical management. Mod. Pathol. 2017, 7, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, R.G.; Gorelick, A.N.; Makarov, V.; Blum, K.A.; Silagy, A.W.; Freeman, B.; Chowell, D.; Marcon, J.; Mano, R.; Sanchez, A.; et al. Putative Drivers of Aggressiveness in TCEB1-mutant Renal Cell Carcinoma: An Emerging Entity with Variable Clinical Course. Eur. Urol. Focus. 2021, 2, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, P.; Wang, L.; Wang, J.; Ji, X.; Li, Y.; Shi, H.; Li, Y.; Zhang, W.; Jiang, Y. Analysis of clinicopathological and molecular features of ELOC(TCEB1)-mutant renal cell carcinoma. Pathol. Res. Pract. 2022, 235, 153960. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, J. Molecular genetics of clear-cell renal cell carcinoma. J. Clin. Oncol. 2014, 18, 1968–1976. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 8, 860–867. [Google Scholar] [CrossRef]
- Shah, R.B. Renal Cell Carcinoma with Fibromyomatous Stroma-The Whole Story. Adv. Anat. Pathol. 2022, 3, 168–177. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Tickoo, S.K.; Jacobsen, A.; Sarungbam, J.; Sfakianos, J.P.; Sato, Y.; Morikawa, T.; Kume, H.; Fukayama, M.; Homma, Y.; et al. TCEB1-mutated renal cell carcinoma: A distinct genomic and morphological subtype. Mod. Pathol. 2015, 6, 845–853. [Google Scholar] [CrossRef]
- Shah, R.B.; Stohr, B.A.; Tu, Z.J.; Gao, Y.; Przybycin, C.G.; Nguyen, J.; Cox, R.M.; Rashid-Kolvear, F.; Weindel, M.D.; Farkas, D.H.; et al. “Renal Cell Carcinoma with Leiomyomatous Stroma” Harbor Somatic Mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and Molecular Characterization of 18 Sporadic Tumors Supports a Distinct Entity. Am. J. Surg. Pathol. 2020, 5, 571–581. [Google Scholar] [CrossRef]
- Lindner, A.K.; Tulchiner, G.; Seeber, A.; Siska, P.J.; Thurnher, M.; Pichler, R. Targeting strategies in the treatment of fumarate hydratase deficient renal cell carcinoma. Front. Oncol. 2022, 12, 906014. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 12, 1326–1338. [Google Scholar] [CrossRef]
- Singh, N.P.; Vinod, P.K. Integrative analysis of DNA methylation and gene expression in papillary renal cell carcinoma. Mol. Genet. Genom. 2020, 3, 807–824. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 6, 807–819. [Google Scholar] [CrossRef]
- Ge, X.; Li, M.; Yin, J.; Shi, Z.; Fu, Y.; Zhao, N.; Chen, H.; Meng, L.; Li, X.; Hu, Z.; et al. Fumarate inhibits PTEN to promote tumorigenesis and therapeutic resistance of type2 papillary renal cell carcinoma. Mol. Cell 2022, 7, 1249–1260.e7. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Liang, J.; Pan, X.; Zhu, S.; Liu, Z.; Armstrong, C.M.; Chen, J.; Lin, W.; Liao, B.; et al. Integrated Molecular Characterization of Fumarate Hydratase-deficient Renal Cell Carcinoma. Clin. Cancer Res. 2021, 6, 1734–1743. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Nikolovski, I.; Dinatale, R.; Zucker, M.; Knezevic, A.; Patil, S.; Ged, Y.; Kotecha, R.R.; Shapnik, N.; Murray, S.; et al. Comprehensive Molecular Characterization and Response to Therapy in Fumarate Hydratase-Deficient Renal Cell Carcinoma. Clin. Cancer Res. 2021, 10, 2910–2919. [Google Scholar] [CrossRef]
- Yu, Y.F.; He, S.M.; Wu, Y.C.; Xiong, S.W.; Shen, Q.; Li, Y.Y.; Yang, F.; He, Q.; Li, X.S. Clinicopathological features and prognosis of fumarate hydratase deficient renal cell carcinoma. Beijing Da Xue Xue Bao Yi Xue Ban 2021, 4, 640–646. [Google Scholar]
- Lau, H.D.; Chan, E.; Fan, A.C.; Kunder, C.A.; Williamson, S.R.; Zhou, M.; Idrees, M.T.; Maclean, F.M.; Gill, A.J.; Kao, C.S. A Clinicopathologic and Molecular Analysis of Fumarate Hydratase-deficient Renal Cell Carcinoma in 32 Patients. Am. J. Surg. Pathol. 2020, 1, 98–110. [Google Scholar] [CrossRef]
- Muller, M.; Guillaud-Bataille, M.; Salleron, J.; Genestie, C.; Deveaux, S.; Slama, A.; de Paillerets, B.B.; Richard, S.; Benusiglio, P.R.; Ferlicot, S. Pattern multiplicity and fumarate hydratase (FH)/S-(2-succino)-cysteine (2SC) staining but not eosinophilic nucleoli with perinucleolar halos differentiate hereditary leiomyomatosis and renal cell carcinoma-associated renal cell carcinomas from kidney tumors without FH gene alteration. Mod. Pathol. 2018, 6, 974–983. [Google Scholar]
- Chen, Y.B.; Brannon, A.R.; Toubaji, A.; Dudas, M.E.; Won, H.H.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Frizzell, N.; Voss, M.H.; et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: Recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am. J. Surg. Pathol. 2014, 5, 627–637. [Google Scholar] [CrossRef]
- Nikolovski, I.; Carlo, M.I.; Chen, Y.B.; Vargas, H.A. Imaging features of fumarate hydratase-deficient renal cell carcinomas: A retrospective study. Cancer Imaging 2021, 1, 24. [Google Scholar] [CrossRef]
- Wu, G.; Liu, G.; Wang, J.; Pan, S.; Luo, Y.; Xu, Y.; Kong, W.; Sun, P.; Xu, J.; Xue, W.; et al. MR Spectroscopy for Detecting Fumarate Hydratase Deficiency in Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Radiology 2022, 3, 631–639. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Gu, Y.; Xu, H.; Fan, Y.; Li, X.; Dong, L.; Zhou, L.; Yang, X.; Wang, C. GATA3 aids in distinguishing fumarate hydratase-deficient renal cell carcinoma from papillary renal cell carcinoma. Ann. Diagn. Pathol. 2022, 60, 152007. [Google Scholar] [CrossRef]
- Grubb, R.L.; Franks, M.E.; Toro, J.; Middelton, L.; Choyke, L.; Fowler, S.; Torres-Cabala, C.; Glenn, G.M.; Choyke, P.; Merino, M.J.; et al. Hereditary leiomyomatosis and renal cell cancer: A syndrome associated with an aggressive form of inherited renal cancer. J. Urol. 2007, 6, 2074–2079. [Google Scholar] [CrossRef]
- Choi, Y.; Keam, B.; Kim, M.; Yoon, S.; Kim, D.; Choi, J.G.; Seo, J.Y.; Park, I.; Lee, J.L. Bevacizumab Plus Erlotinib Combination Therapy for Advanced Hereditary Leiomyomatosis and Renal Cell Carcinoma-Associated Renal Cell Carcinoma: A Multicenter Retrospective Analysis in Korean Patients. Cancer Res. Treat. 2019, 4, 1549–1556. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, W.; Cao, M.; Wang, J.; Wang, Z.; Zheng, L.; Wu, X.; Cheng, R.; He, W.; Yang, B.; et al. Genomic Profiling and Response to Immune Checkpoint Inhibition plus Tyrosine Kinase Inhibition in FH-Deficient Renal Cell Carcinoma. Eur. Urol. 2023, 2, 163–172. [Google Scholar] [CrossRef]
- Williamson, S.R.; Eble, J.N.; Amin, M.B.; Gupta, N.S.; Smith, S.C.; Sholl, L.M.; Montironi, R.; Hirsch, M.S.; Hornick, J.L. Succinate dehydrogenase-deficient renal cell carcinoma: Detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod. Pathol. 2015, 1, 80–94. [Google Scholar] [CrossRef]
- Wang, G.; Rao, P. Succinate Dehydrogenase-Deficient Renal Cell Carcinoma: A Short Review. Arch. Pathol. Lab. Med. 2018, 10, 1284–1288. [Google Scholar] [CrossRef]
- Tsai, T.H.; Lee, W.Y. Succinate Dehydrogenase-Deficient Renal Cell Carcinoma. Arch. Pathol. Lab. Med. 2019, 5, 643–647. [Google Scholar] [CrossRef]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018, 1, 106–116. [Google Scholar] [CrossRef]
- Gill, A.J.; Hes, O.; Papathomas, T.; Šedivcová, M.; Tan, P.H.; Agaimy, A.; Andresen, P.A.; Kedziora, A.; Clarkson, A.; Toon, C.W.; et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: A morphologically distinct entity: A clinicopathologic series of 36 tumors from 27 patients. Am. J. Surg. Pathol. 2014, 12, 1588–1602. [Google Scholar] [CrossRef]
- Aggarwal, R.K.; Luchtel, R.A.; Machha, V.; Tischer, A.; Zou, Y.; Pradhan, K.; Ashai, N.; Ramachandra, N.; Albanese, J.M.; Yang, J.I.; et al. Functional succinate dehydrogenase deficiency is a common adverse feature of clear cell renal cancer. Proc. Natl. Acad. Sci. USA 2021, 39, e2106947118. [Google Scholar] [CrossRef]
- Sun, A.; Liu, Z.; Wang, T.; Xing, J. Succinate dehydrogenase-deficient renal cell carcinoma: A case report and review of the literature. Asian J. Surg. 2021, 4, 692–693. [Google Scholar] [CrossRef]
- Gill, A.J.; Pachter, N.S.; Chou, A.; Young, B.; Clarkson, A.; Tucker, K.M.; Winship, I.M.; Earls, P.; Benn, D.E.; Robinson, B.G.; et al. Renal tumors associated with germline SDHB mutation show distinctive morphology. Am. J. Surg. Pathol. 2011, 10, 1578–1585. [Google Scholar] [CrossRef]
- Paik, J.Y.; Toon, C.W.; Benn, D.E.; High, H.; Hasovitz, C.; Pavlakis, N.; Clifton-Bligh, R.J.; Gill, A.J. Renal carcinoma associated with succinate dehydrogenase B mutation: A new and unique subtype of renal carcinoma. J. Clin. Oncol. 2014, 6, e10–e13. [Google Scholar] [CrossRef]
- Linehan, W.M.; Ricketts, C.J. The metabolic basis of kidney cancer. Semin. Cancer Biol. 2013, 1, 46–55. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Jiang, Y.; Zhang, W.; Li, Y. Genetic analysis and clinicopathological features of ALK-rearranged renal cell carcinoma in a large series of resected Chinese renal cell carcinoma patients and literature review. Histopathology 2017, 1, 53–62. [Google Scholar] [CrossRef]
- Jeanneau, M.; Gregoire, V.; Desplechain, C.; Escande, F.; Tica, D.P.; Aubert, S.; Leroy, X. ALK rearrangements-associated renal cell carcinoma (RCC) with unique pathological features in an adult. Pathol. Res. Pract. 2016, 11, 1064–1066. [Google Scholar] [CrossRef]
- Gorczynski, A.; Czapiewski, P.; Korwat, A.; Budynko, L.; Prelowska, M.; Okon, K.; Biernat, W. ALK-rearranged renal cell carcinomas in Polish population. Pathol. Res. Pract. 2019, 12, 152669. [Google Scholar] [CrossRef]
- Hang, J.F.; Chung, H.J.; Pan, C.C. ALK-rearranged renal cell carcinoma with a novel PLEKHA7-ALK translocation and metanephric adenoma-like morphology. Virchows Arch. 2020, 6, 921–929. [Google Scholar] [CrossRef]
- Kuroda, N.; Sugawara, E.; Kusano, H.; Yuba, Y.; Yorita, K.; Takeuchi, K. A review of ALK-rearranged renal cell carcinomas with a focus on clinical and pathobiological aspects. Pol. J. Pathol. 2018, 2, 109–113. [Google Scholar] [CrossRef]
- Debelenko, L.V.; Daw, N.; Shivakumar, B.R.; Huang, D.; Nelson, M.; Bridge, J.A. Renal cell carcinoma with novel VCL-ALK fusion: New representative of ALK-associated tumor spectrum. Mod. Pathol. 2011, 3, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Sukov, W.R.; Hodge, J.C.; Lohse, C.M.; Akre, M.K.; Leibovich, B.C.; Thompson, R.H.; Cheville, J.C. ALK alterations in adult renal cell carcinoma: Frequency, clinicopathologic features and outcome in a large series of consecutively treated patients. Mod. Pathol. 2012, 11, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H.; Bazhenova, L.; Camidge, D.R.; Solomon, B.J.; Herman, J.; Kain, T.; Bang, Y.J.; Kwak, E.L.; Shaw, A.T.; Salgia, R.; et al. Rapid and dramatic radiographic and clinical response to an ALK inhibitor (crizotinib, PF02341066) in an ALK translocation-positive patient with non-small cell lung cancer. J. Thorac. Oncol. 2010, 12, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nakajima, T.; Takeuchi, K.; Soda, M.; Mano, H.; Iizasa, T.; Matsui, Y.; Yoshino, M.; Shingyoji, M.; Itakura, M.; et al. ALK fusion gene positive lung cancer and 3 cases treated with an inhibitor for ALK kinase activity. Lung Cancer 2012, 1, 66–72. [Google Scholar] [CrossRef]
- Pal, S.K.; Bergerot, P.; Dizman, N.; Bergerot, C.; Adashek, J.; Madison, R.; Chung, J.H.; Ali, S.M.; Jones, J.O.; Salgia, R. Responses to Alectinib in ALK-rearranged Papillary Renal Cell Carcinoma. Eur. Urol. 2018, 1, 124–128. [Google Scholar] [CrossRef]
- Wang, X.; Haswell, J.R.; Roberts, C.W. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer--mechanisms and potential therapeutic insights. Clin. Cancer Res. 2014, 1, 21–27. [Google Scholar] [CrossRef]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 5, 720–734.e13. [Google Scholar] [CrossRef]
- Agaimy, A. The expanding family of SMARCB1(INI1)-deficient neoplasia: Implications of phenotypic, biological, and molecular heterogeneity. Adv. Anat. Pathol. 2014, 6, 394–410. [Google Scholar] [CrossRef]
- Hong, A.L.; Tseng, Y.Y.; Wala, J.A.; Kim, W.J.; Kynnap, B.D.; Doshi, M.B.; Kugener, G.; Sandoval, G.J.; Howard, T.P.; Li, J.; et al. Renal medullary carcinomas depend upon SMARCB1 loss and are sensitive to proteasome inhibition. Elife 2019, 8, e44161. [Google Scholar] [CrossRef]
- Al-Daghmin, A.; Gaashan, M.; Haddad, H. Atypical presentation of renal medullary carcinoma: A case report and review of the literature. Urol. Case Rep. 2018, 22, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.; Merrimen, J.; Pringle, C.; Wood, L.A. Renal medullary carcinoma and its association with sickle cell trait: A case report and literature review. Curr. Oncol. 2020, 1, e53–e56. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hong, A.L. Recent Advances in Renal Medullary Carcinoma. Int. J. Mol. Sci. 2022, 13, 7097. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, K.E.; Sharma, D.; Chaturvedi, S.; Msaouel, P.; Abboud, M.R.; Allory, Y.; Bourdeaut, F.; Calderaro, J.; de Cubas, A.A.; Derebail, V.K.; et al. Renal Medullary Carcinoma: Establishing Standards in Practice. J. Oncol. Pract. 2017, 7, 414–421. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cheng, L.; Raspollini, M.R.; Montironi, R. SMARCB1/INI1 Genetic Alterations in Renal Medullary Carcinomas. Eur. Urol. 2016, 6, 1062–1064. [Google Scholar] [CrossRef]
- Scarpelli, M.; Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; De Nictolis, M.; Santoni, M.; Montironi, R. Renal cell carcinoma with rhabdoid features and loss of INI1 expression in an individual without sickle cell trait. Pathology 2014, 7, 653–655. [Google Scholar] [CrossRef]
- Iacovelli, R.; Modica, D.; Palazzo, A.; Trenta, P.; Piesco, G.; Cortesi, E. Clinical outcome and prognostic factors in renal medullary carcinoma: A pooled analysis from 18 years of medical literature. Can. Urol. Assoc. J. 2015, 3–4, E172–E177. [Google Scholar] [CrossRef]
- Wiele, A.J.; Surasi, D.S.; Rao, P.; Sircar, K.; Su, X.; Bathala, T.K.; Shah, A.Y.; Jonasch, E.; Cataldo, V.D.; Genovese, G.; et al. Efficacy and Safety of Bevacizumab Plus Erlotinib in Patients with Renal Medullary Carcinoma. Cancers 2021, 9, 2170. [Google Scholar] [CrossRef]
- Forrest, S.J.; Al-Ibraheemi, A.; Doan, D.; Ward, A.; Clinton, C.M.; Putra, J.; Pinches, R.S.; Kadoch, C.; Chi, S.N.; DuBois, S.G.; et al. Genomic and Immunologic Characterization of INI1-Deficient Pediatric Cancers. Clin. Cancer Res. 2020, 12, 2882–2890. [Google Scholar] [CrossRef]
- Ngo, C.; Postel-Vinay, S. Immunotherapy for SMARCB1-Deficient Sarcomas: Current Evidence and Future Developments. Biomedicines 2022, 3, 650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).