Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Fungal Pathogens, and Growth Conditions
2.2. Strain Construction
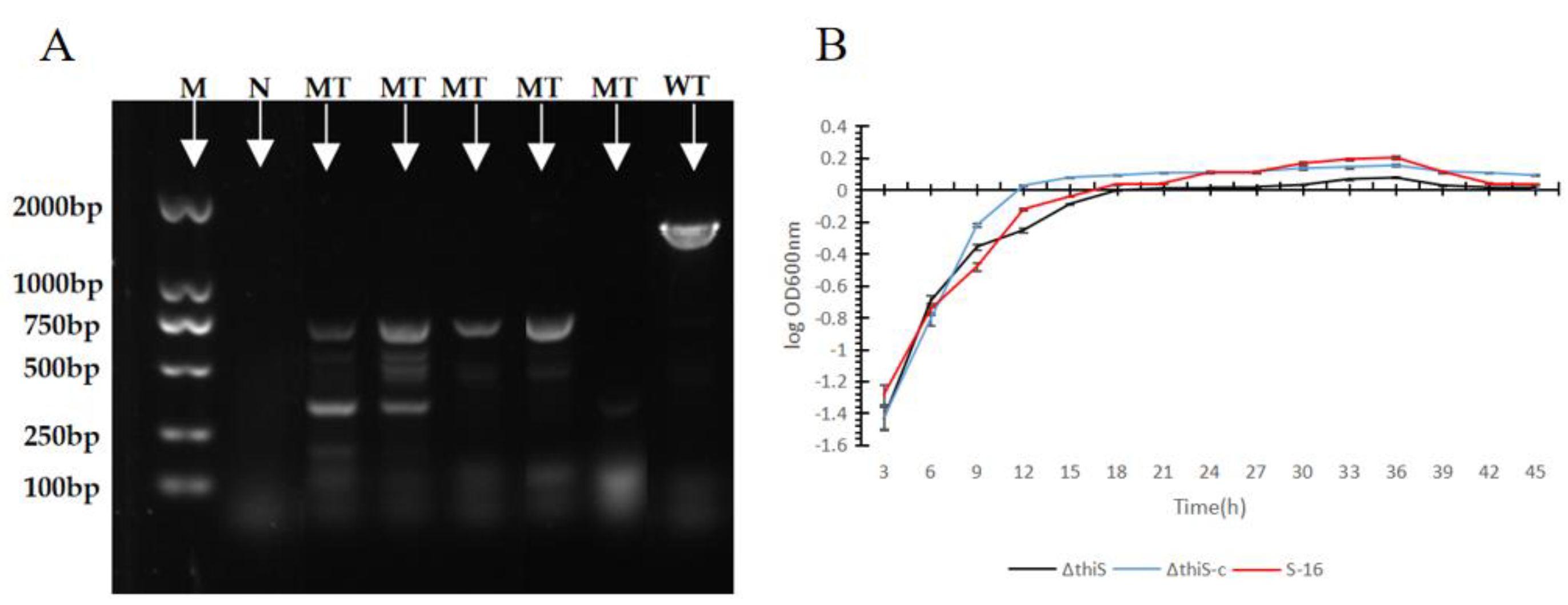
2.3. Measurements of the Growth Curve
2.4. In Vitro Antifungal Activities of the VOCs Produced by B. subtilis S-16 and Its Mutants
2.5. Scanning Electron Microscopy
2.6. In Vivo Antifungal Activities of the VOCs Produced by B. subtilis S-16 and Its Mutants
2.7. Collection of VOCs via SPME and GC-MS Analysis
2.8. Statistical Analyses
3. Results
3.1. Knockout of thiS from the Genome of B. subtilis S-16
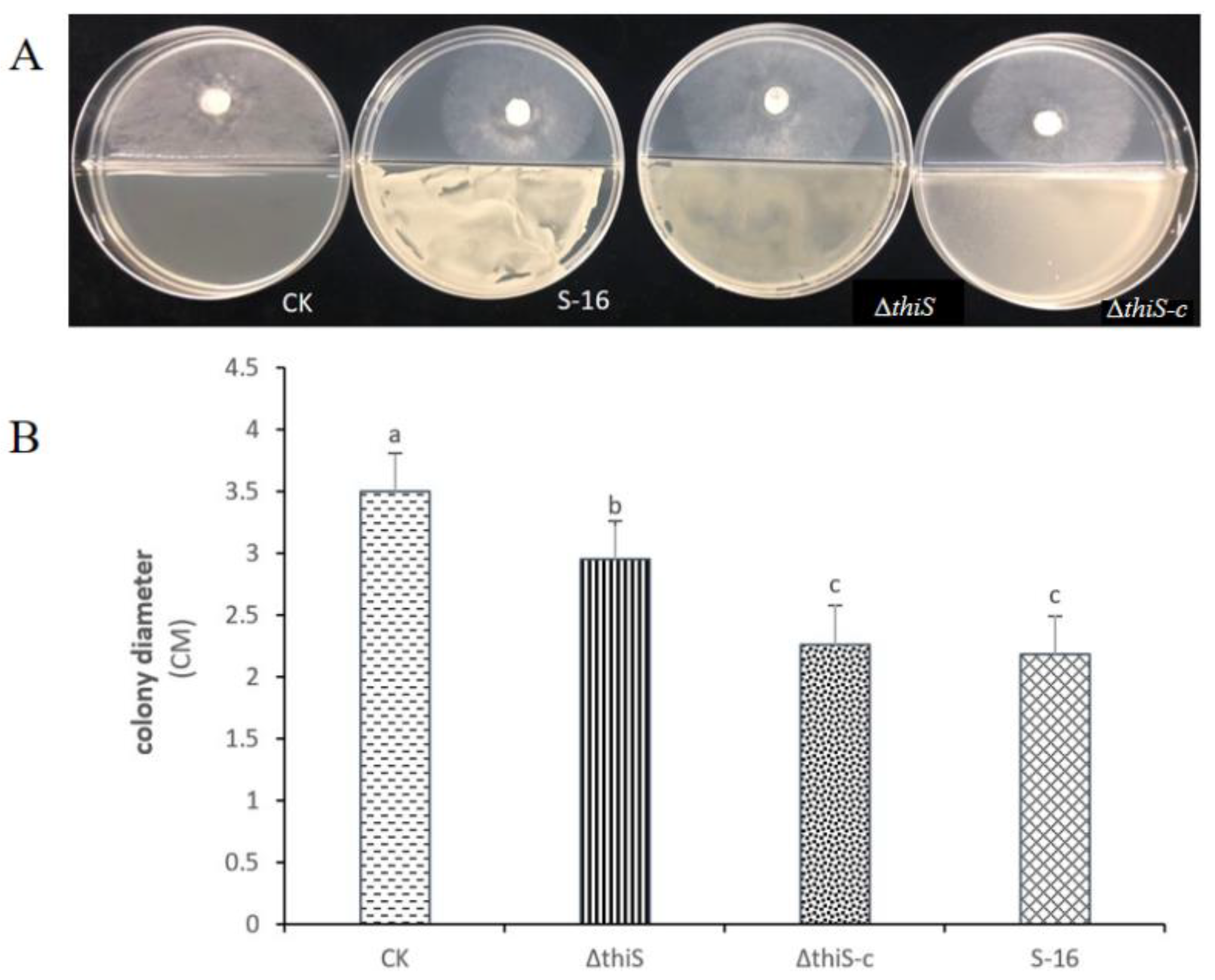
3.2. In Vitro Antifungal Activities of the VOCs from B. subtilis S-16 and Its Mutants
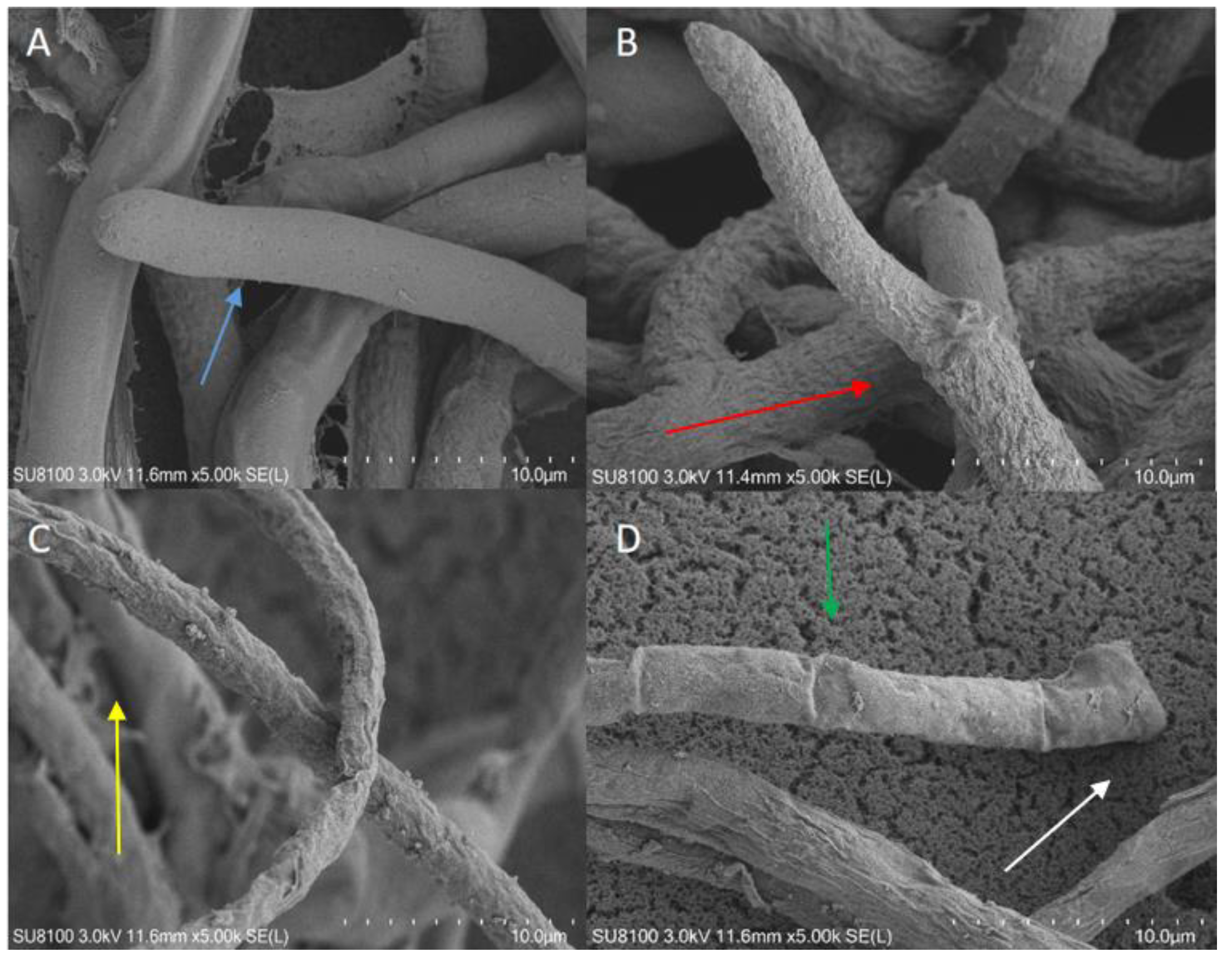
3.3. Determination of the Morphological Characteristics of the Fungal Hyphae Treated with the VOCs from Mutant Strains
3.4. Effects of Volatile Compounds from the Mutant Strains on the Formation of Sclerotia
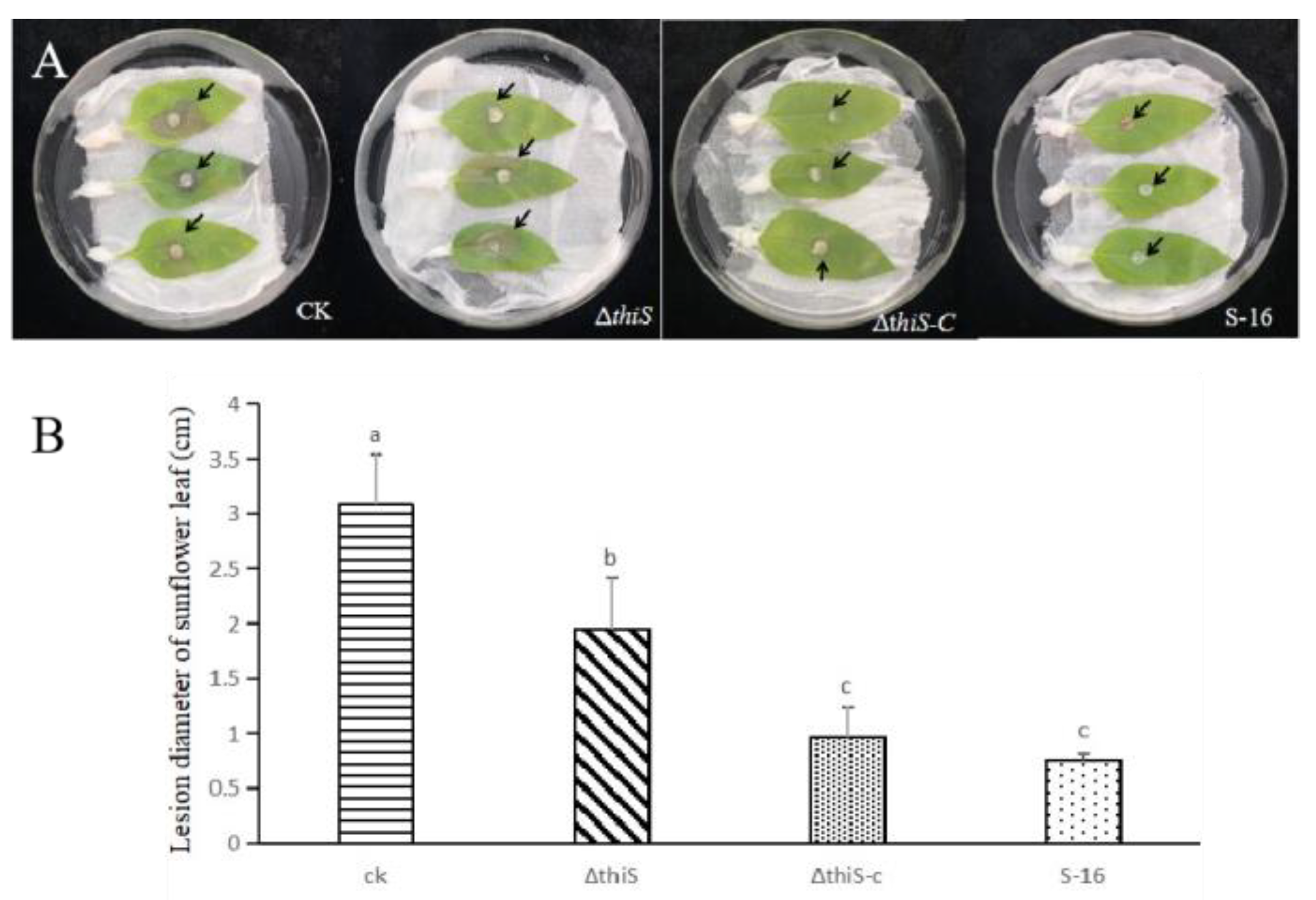
3.5. Test on Detached Sunflower Leaves
3.6. Analysis of the 2-MBTH Content in the Mutant Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, W.; Jianru, Z.; Chaomin, C.; Xia, C.; Zhiyong, C.; Xiaoshuo, L.; Xi, C.; Shuyin, W. Research progress of sunflower Sclerotinia sclerotiorum. J. North. Agric. 2006, 25–28. [Google Scholar]
- Bloomfield, B.J.; Alexander, M. Melanins and Resistance of Fungi to Lysis. J. Bacteriol. 1967, 93, 1276. [Google Scholar] [CrossRef]
- Dean, R.; Kan, J.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Guoqing, L.; Hongzhang, H. Study on diversity of sclerotia germination of Sclerotia of Sclerotiorum sclerotiorum. J. Plant Prot. 1997, 24, 59–64. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Minuto, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot. 2006, 25, 468–475. [Google Scholar] [CrossRef]
- Quansheng, L.; Zongming, X.; Zheng, L.; Guoli, Z.; Dongmei, W.; Ying, T. Screening and identification of antagonistic bacterium H14 against Verticillium dahliae Kleb. and its antagonistic mechanisms. J. Plant Prot. 2018, 45, 1204–1211. [Google Scholar]
- Afzal, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Arbi, A.E.; Rochex, A.; Chataigne, G.; Bechet, M.; Lecouturier, D.; Arnauld, S.; Gharsallah, N.; Jacques, P. The Tunisian oasis ecosystem is a source of antagonistic Bacillus spp. producing diverse antifungal lipopeptides. Res. Microbiol. 2016, 167, 46–57. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A Gold Mine of Antibiotic Candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215. [Google Scholar] [CrossRef]
- Minerdi, D.; Bossi, S.; Gullino, M.L.; Garibaldi, A. Volatile organic compounds: A potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA 35. Environ. Microbiol. 2010, 11, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Asghar, H.; Mohammad, P. A Review on Biological Control of Fungal Plant Pathogens Using Microbial Antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar]
- Tyc, O.; Garbeva, P. Poster OlafTyc FEMS2017 The Ecological Role of Volatile and Soluble Secondary Metabolites produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Breinig, S.; Schiltz, E.; Fuchs, G. Genes Involved in Anaerobic Metabolism of Phenol in the Bacterium Thauera aromatica. J. Bacteriol. 2000, 182, 5849–5863. [Google Scholar] [CrossRef]
- Zou, C.S.; Mo, M.H.; Gu, Y.Q.; Zhou, J.P.; Zhang, K.Q. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol. Biochem. 2007, 39, 2371–2379. [Google Scholar] [CrossRef]
- Alström, S. Characteristics of Bacteria from Oilseed Rape in Relation to their Biocontrol Activity against Verticillium dahliae. J. Phytopathol. 2010, 149, 57–64. [Google Scholar] [CrossRef]
- Koitabashi, M. New biocontrol method for parsley powdery mildew by the antifungal volatiles-producing fungus Kyu-W63. J. Gen. Plant Pathol. 2005, 71, 280–284. [Google Scholar] [CrossRef]
- Guevara-Avendano, E.; Carrillo, J.D.; Ndinga-Muniania, C.; Moreno, K.; Mendez-Bravo, A.; Guerrero-Analco, J.A.; Eskalen, A.; Reverchon, F. Antifungal activity of avocado rhizobacteria against Fusarium euwallaceae and Graphium spp., associated with Euwallacea spp. nr. fornicatus, and Phytophthora cinnamomi. Antonie Van Leeuwenhoek 2018, 111, 563–572. [Google Scholar] [CrossRef]
- Qi, W. Cloning of the Bacteriostat Related Gene and Identification of Antifungal Substances from Bacillus Subtilis S-16; Inner Mongolia Agricultural University: Hohhot, China, 2014. [Google Scholar]
- Jinghan, H.; Dong, W.; Baozhu, D.; Huanwen, M.; Jun, Z.; Hongyou, Z. The inhibitory effect of the volatile compound 2-methyl benzothiazole on pathogenic fungi Sclerotinia sclerotiorum and Botrytis cinerea. J. Plant Prot. 2021, 48, 781–788. [Google Scholar]
- Lijun, C.; Yanan, Z.; Chunsheng, W.; Jinzhu, C.; Yinli, J.; Yuehua, C. Analysis of the fumigation inhibition mechanism of essential oil from Chenopodium ambrosioides against the Botrytis cinerea. J. Plant Prot. 2020, v.47, 221–222. [Google Scholar]
- Ginsberg, G.; Toal, B.; Kurland, T. Benzothiazole Toxicity Assessment in Support of Synthetic Turf Field Human Health Risk Assessment. J. Toxicol. Environ. Health Part A 2011, 74, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Yiming, Z. Isolation and Identification of a Biocontrol Bacterium against Sclerotinia Sclerotinia of Sunflower and Preliminary Study on Its Antibacterial Mechanism; Inner Mongolia Agricultural University: Hohhot, China, 2010. [Google Scholar]
- Yaguang, H. The Study on the Pathogenicity Differentiation and Sclerotial Formation Mechanism of Sunflower Sclerotinia sclerotiorum; Inner Mongolia Agricultural University: Hohhot, China, 2016. [Google Scholar]
- Smith, K.; Youngman, P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 1992, 74, 705–711. [Google Scholar] [CrossRef]
- Xu, Y.B.; Mai, C.; Ying, Z.; Wang, M.; Ying, W.; Qiu-Bin, H.; Xue, W.; Wang, G. The phosphotransferase system gene ptsI in the endophytic bacterium Bacillus cereus is required for biofilm formation, colonization, and biocontrol against wheat sharp eyespot. Fems Microbiol. Lett. 2014, 354, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ishiwa, H.; Shibahara, H. New shuttle vectors for Escherichia coli and Bacillus subtilis. II: Plasmid pHY300PLK, a multipurpose cloning vector with a polylinker, derived from pHY460. Jpn. J. Genet. 1985, 60, 235–243. [Google Scholar] [CrossRef]
- Sahni, S.; Sarma, B.K.; Singh, K.P. Management of Sclerotium rolfsii with integration of non-conventional chemicals, vermicompost and Pseudomonas syringae. World J. Microbiol. Biotechnol. 2008, 24, 517–522. [Google Scholar] [CrossRef]
- Yurttaş, L.; Özkay, Y.; Duran, M.; Turan-Zitouni, G.; Özdemir, A.; Cantürk, Z.; Küçükoğlu, K.; Kaplancıklı, Z.A. Synthesis and antimicrobial activity evaluation of new dithiocarbamate derivatives bearing thiazole/benzothiazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1166–1173. [Google Scholar] [CrossRef]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 13506. [Google Scholar] [CrossRef]
- Soni, B.; Ranawat, M.S.; Sharma, R.; Bhandari, A.; Sharma, S. Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2938–2942. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzym. Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef] [PubMed]
- Franchini, C.; Muraglia, M.; Corbo, F.; Florio, M.A.; Mola, A.D.; Rosato, A.; Matucci, R.; Nesi, M.; Bambeke, F.V.; Vitali, C. Synthesis and biological evaluation of 2-mercapto-1,3-benzothiazole derivatives with potential antimicrobial activity. Archiv. Pharm. 2010, 342, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, V.; Reis, R.; Gomes, C.; Vasconcelos, T. Chemistry and biological activities of 1,3-benzothiazoles. Mini-Rev. Org. Chem. 2012, 9, 44–53. [Google Scholar] [CrossRef]
- Lad, N.P.; Manohar, Y.; Mascarenhas, M.; Pandit, Y.B.; Pandit, S.S. Methylsulfonyl benzothiazoles (MSBT) derivatives: Search for new potential antimicrobial and anticancer agents. Bioorganic Med. Chem. Lett. 2017, 27, 1319–1324. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, S1125–S1130. [Google Scholar] [CrossRef]
- Trapani, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Mercurio, A.; Rosato, A.; Mandracchia, D.; Tripodo, G.; Schiavone, B.I.P.; Franchini, C.; et al. Effect of Methyl-β-Cyclodextrin on the antimicrobial activity of a new series of poorly water-soluble benzothiazoles. Carbohydr. Polym. 2019, 207, 720–728. [Google Scholar] [CrossRef]
- Al-Talib, M.; Al-Soud, Y.A.; Abussaud, M.; Khshashneh, S. Synthesis and biological evaluation of new benzothiazoles as antimicrobial agents. Arab. J. Chem. 2011, 62, S926–S930. [Google Scholar] [CrossRef]
- Park, J.H.; Dorrestein, P.C.; Zhai, H.; Kinsland, C.; Mclafferty, F.W.; Begley, T.P. Biosynthesis of the Thiazole Moiety of Thiamin Pyrophosphate (Vitamin B1). Biochemistry 2003, 42, 12430–12438. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.; Begley, T.P.; Nicholson, L.K. Solution structure of thiS and implications for the evolutionary roots of ubiquitin. Nat. Struct. Biol. 2001, 8, 47–51. [Google Scholar]
- Settembre, E.C.; Dorrestein, P.C.; Park, J.; Augustine, A.; Begley, T.P.; Ealick, S.E. Structural and mechanistic studies on ThiO, a glycine oxidase essential for thiamin biosynthesis in Bacillus subtilis. Biochemistry 2003, 42, 2971–2981. [Google Scholar] [CrossRef]
- Lauhon, C.T.; Kambampati, R. The iscS Gene in Escherichia coli Is Required for the Biosynthesis of 4-Thiouridine, Thiamin, and NAD. J. Biol. Chem. 2000, 275, 20096–20103. [Google Scholar] [CrossRef] [PubMed]
- Dorrestein, P.C.; Zhai, H.; Taylor, S.V.; Mclafferty, F.W.; Begley, T.P. The biosynthesis of the thiazole phosphate moiety of thiamin (vitamin B1): The early steps catalyzed by thiazole synthase. J. Am. Chem. Soc. 2004, 126, 3091–3096. [Google Scholar] [CrossRef]
- Dorrestein, P.C.; Zhai, H.; Mclafferty, F.W.; Begley, T.P. The Biosynthesis of the Thiazole Phosphate Moiety of Thiamin: The Sulfur Transfer Mediated by the Sulfur Carrier Protein thiS. Cell Chem. Biol. 2004, 11, 1373–1381. [Google Scholar]
- Yanlei, Z. Study on experimental method of measuring bacterial growth curve. J. Microbiol. 2016, 36, 108–112. [Google Scholar]
- Jixian, M.; Zhigang, W.; Changhe, W. Comparison of common microbe number determination methods. Biol. Teach. 2012, 37, 42–43. [Google Scholar]
| Strain or Plasmid | Properties and Application | Source or Reference |
|---|---|---|
| B. subtilis | ||
| Bacillus subtilis S-16 | Wild-type strain in this study | Kept in our laboratory, isolated from sunflower root. |
| ΔthiS | The This gene’s defect mutant, ΔthiS | Construct in this study |
| ΔthiS-c | ΔthiS supplemented with its native thiS gene by allelic exchange | Construct in this study |
| E. coli | ||
| E. coli DH5α | Plasmid propagation | Purchased from Takara, Dalian |
| plasmid | ||
| pKSV7 | For gene knockout | Purchased from Takara, Dalian |
| pHY300PLK | For gene knockout | Purchased from Takara, Dalian |
| pKSV7-thiS | For gene knockout, Amp+; cm+ | Construct in this study |
| pHY300PLK-thiS | For gene complementation, Cm+ | Construct in this study |
| Sclerotinia sclerotiorum X-8 | For testing the inhibition of mycelial growth by VOCs | Kept in our laboratory, isolated from sunflower plants. |
| Gene Name | Primer (5′-3′) | Restriction Sites |
|---|---|---|
| thiS-1 | CGG AAT TCG ATG GAC GAT TTG GAC TCT G | EcoRI |
| thiS-2 | CGG GGT ACC ATA TCT GAA CCG CCT CCT TG | KpnI |
| thiS-3 | CGG GTA CCA GGC GGA TGA GCA TGT TAA C | KpnI |
| thiS-4 | CGG GAT CCG ACT GCT TGC CGT TCC GTA C | BamHI |
| thiS-promoter-F | CGGAATTCCTGCAAGCCGGTAGAAGAG | EcoRI |
| thiS-promoter-R | CGGGATCCGCACGCGGTGAATAGTAGAG | BamHI |
| thiS-comF | CGGGATCCAGGCGGTTCAGATATGATGC | BamHI |
| thiS-comR | CCCAAGCTTCTCATCCGCCTCCTAC | HindIII |
| Strain | The Number of Sclerotia (Single-Digit) | Sclerotium Weight (g) |
|---|---|---|
| ck | 4 ± 0.82 a | 0.22 ± 0.06 a |
| ΔthiS | 3.25 ± 0.82 a | 0.20 ± 0.03 a |
| ΔthiS-c | 1.75 ± 0.5 b | 0.17 ± 0.02 a |
| S-16 | 1.5 ± 0.58 b | 0.15 ± 0.07 a |
| Active VOCs | Retention Time (Min) | Height | Area | Area (%) | Compound |
|---|---|---|---|---|---|
| 2-methyl benzothiazole | 22.551 | 36,015,872 | 3,001,261,211 | 94.85 | 2-methyl benzothiazole |
| S-16 | 22.394 | 258,676 | 8,715,422 | 0.405 | 2-methyl benzothiazole |
| ΔthiS | 22.400 | 211,887 | 6,381,940 | 0.215 | 2-methyl benzothiazole |
| ΔthiS-c | 22.402 | 248,132 | 7,939,165 | 0.403 | 2-methyl benzothiazole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Su, Z.; Dong, B.; Wang, D.; Liu, X.; Meng, H.; Guo, Q.; Zhou, H. Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis. Curr. Issues Mol. Biol. 2023, 45, 4600-4611. https://doi.org/10.3390/cimb45060292
Hu J, Su Z, Dong B, Wang D, Liu X, Meng H, Guo Q, Zhou H. Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis. Current Issues in Molecular Biology. 2023; 45(6):4600-4611. https://doi.org/10.3390/cimb45060292
Chicago/Turabian StyleHu, Jinghan, Zhenhe Su, Baozhu Dong, Dong Wang, Xiaomeng Liu, Huanwen Meng, Qinggang Guo, and Hongyou Zhou. 2023. "Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis" Current Issues in Molecular Biology 45, no. 6: 4600-4611. https://doi.org/10.3390/cimb45060292
APA StyleHu, J., Su, Z., Dong, B., Wang, D., Liu, X., Meng, H., Guo, Q., & Zhou, H. (2023). Characterization of a Bacillus subtilis S-16 Thiazole-Synthesis-Related Gene thiS Knockout and Antimicrobial Activity Analysis. Current Issues in Molecular Biology, 45(6), 4600-4611. https://doi.org/10.3390/cimb45060292






