Neural Differentiation of Induced Pluripotent Stem Cells for a Xenogeneic Material-Free 3D Neurological Disease Model Neurulation from Pluripotent Cells Using a Human Hydrogel
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. iPS Cell Cultures
2.2. Neural Induction
2.3. Neural Stem Cell Expansion and Differentiation
2.4. Immunocytochemistry
2.5. Survival Assays
2.6. Quantification of Fluorescence Intensities
2.7. Statistics
3. Results
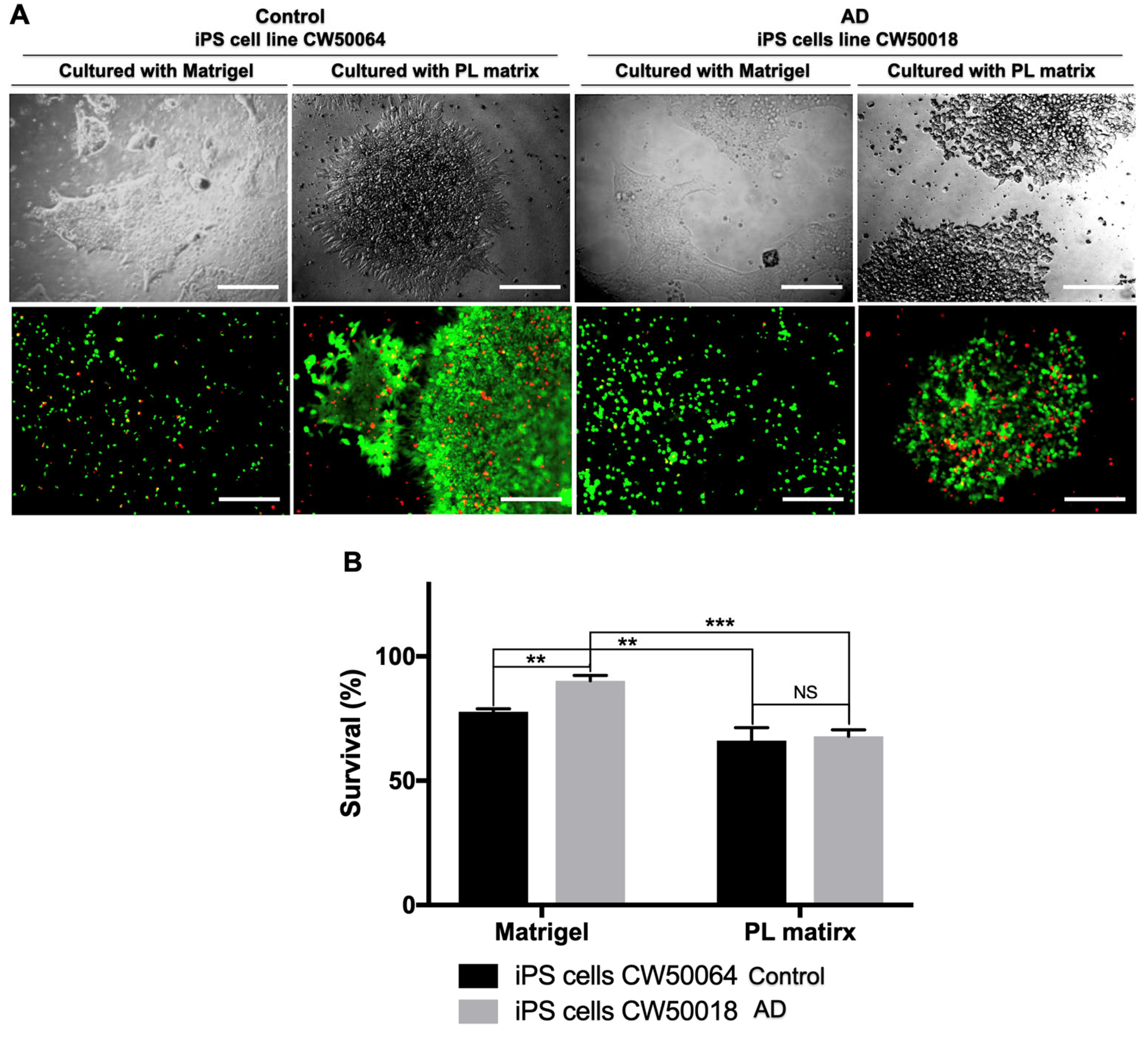
3.1. The Platelet-Derived Extracellular Matrix Allows the In Vitro Culture of Human iPS Cells
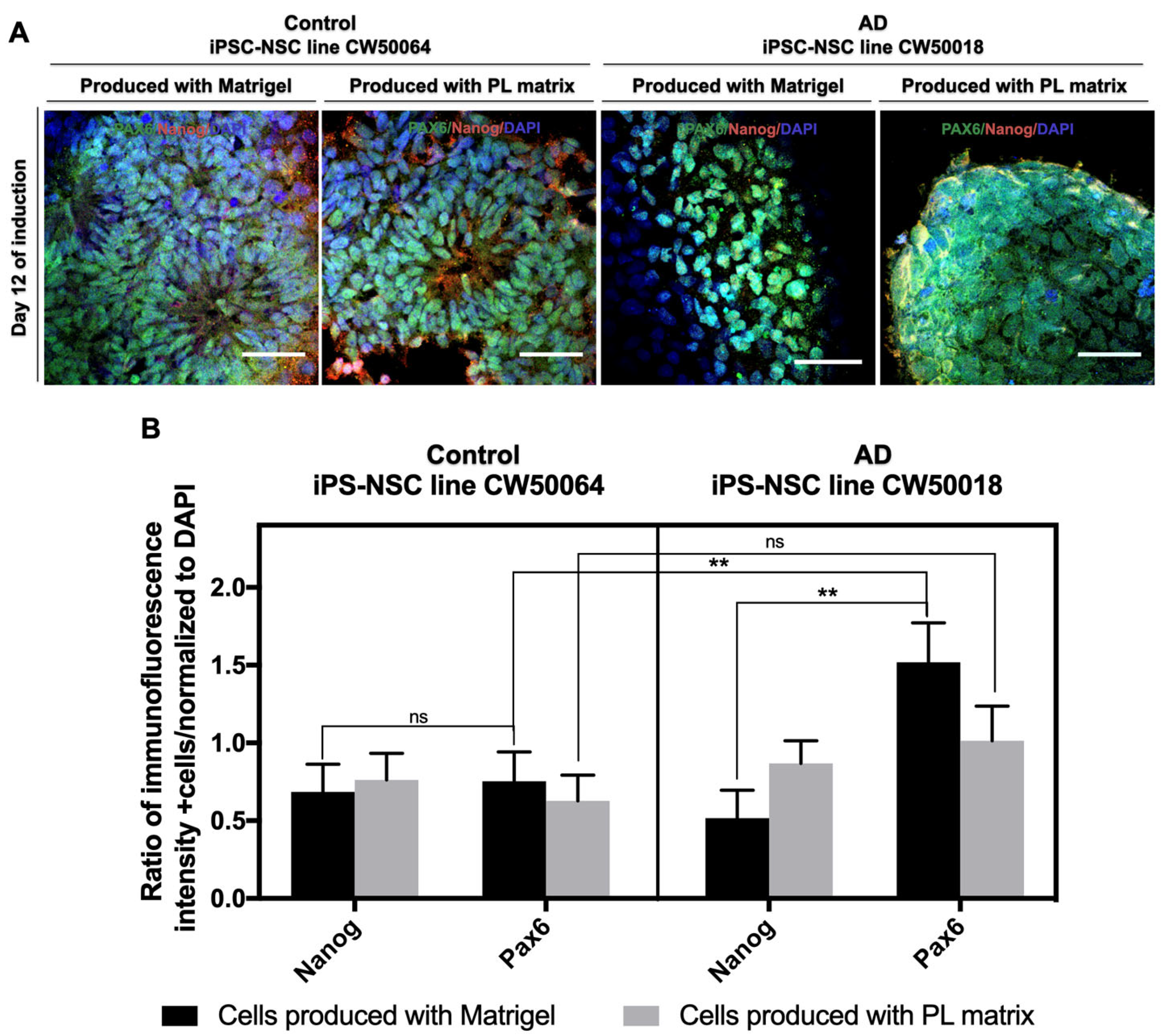
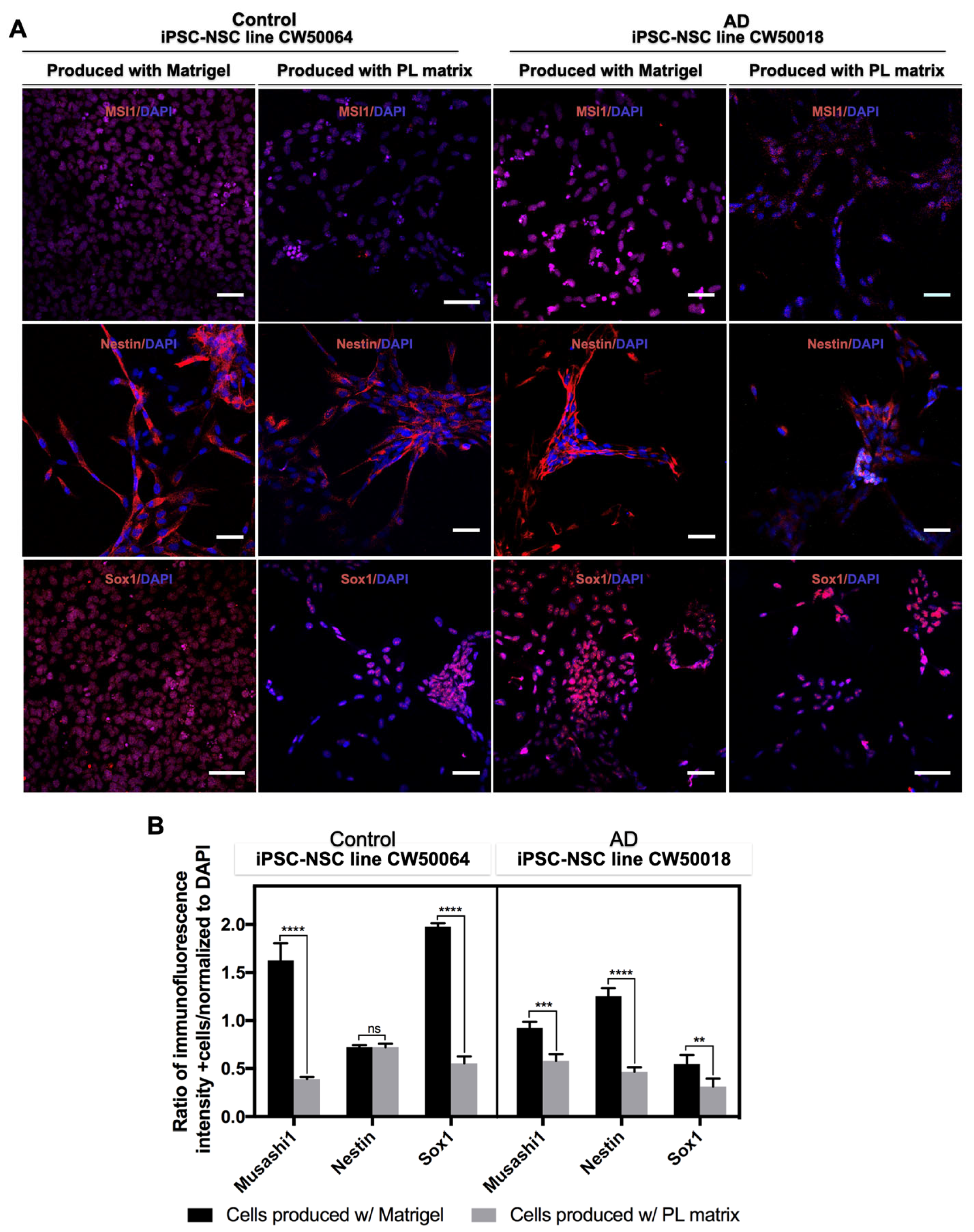
3.2. Expansion and Characterization of iPSC-NSCs Produced Using Platelet-Derived Extracellular Matrix
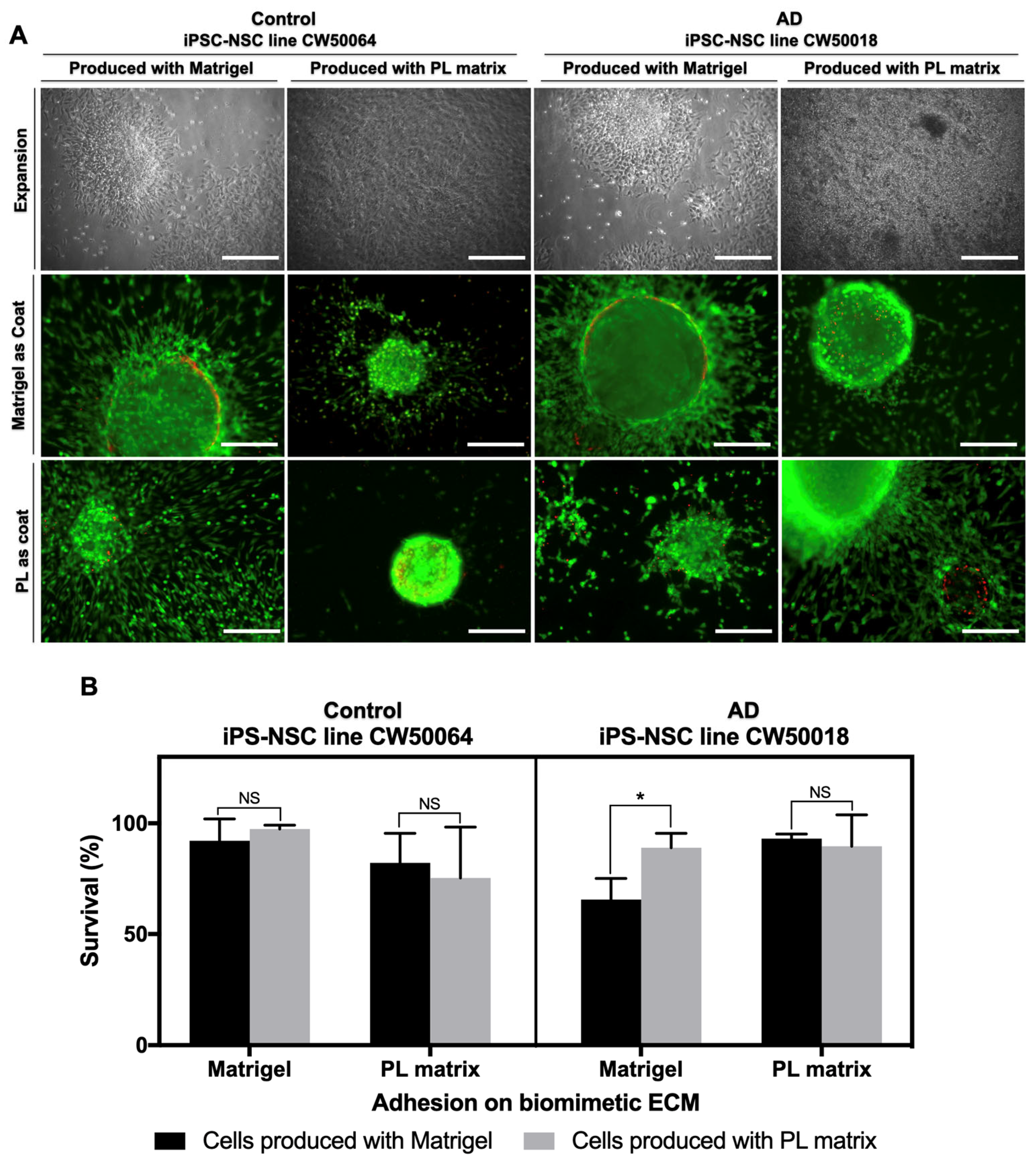
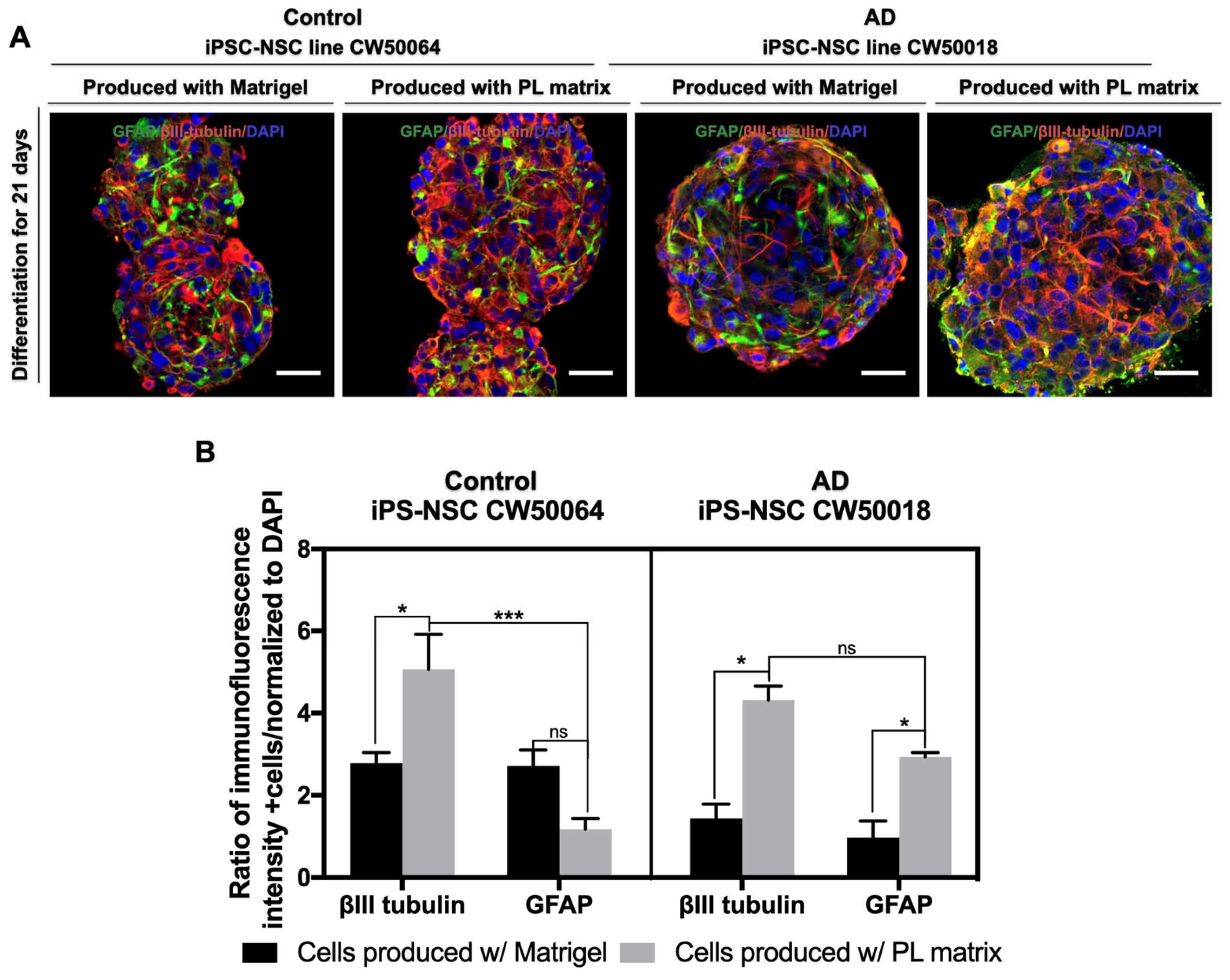
3.3. In Vitro Differentiation of the iPSC-NSCs Produced Using Platelet-Derived Extracellular Matrix
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugaya, K.; Alvarez, A.; Marutle, A.; Kwak, Y.D.; Choumkina, E. Stem cell strategies for Alzheimer’s disease therapy. Panminerva Med. 2006, 48, 87–96. [Google Scholar] [CrossRef]
- Sugaya, K.; Vaidya, M. Stem cell therapies for neurodegenerative diseases. Adv. Exp. Med. Biol. 2018, 1056, 61–84. [Google Scholar] [CrossRef]
- Aulston, B.; Liu, Q.; Mante, M.; Florio, J.; Rissman, R.A.; Yuan, S.H. Extracellular vesicles isolated from familial Alzheimer’s disease neuronal cultures induce aberrant tau phosphorylation in the wild-type mouse brain. J. Alzheimer’s Dis. 2019, 72, 575–585. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Okano, H. Modeling human neurological disorders with induced pluripotent stem cells. J. Neurochem. 2014, 129, 388–399. [Google Scholar] [CrossRef]
- Auboyer, L.; Monzo, C.; Wallon, D.; Rovelet-Lecrux, A.; Gabelle, A.; Gazagne, I.; Cacheux, V.; Lehmann, S.; Crozet, C. Generation of induced pluripotent stem cells (IRMBi001-A) from an Alzheimer’s disease patient carrying a G217D mutation in the PSEN1 gene. Stem Cell Res. 2019, 34, 101381. [Google Scholar] [CrossRef]
- Sugaya, K. Possible use of autologous stem cell therapies for Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 367–376. [Google Scholar] [CrossRef]
- Marutle, A.; Ohmitsu, M.; Nilbratt, M.; Greig, N.H.; Nordberg, A.; Sugaya, K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc. Natl. Acad. Sci. USA 2007, 104, 12506–12511. [Google Scholar] [CrossRef]
- Sugaya, K.; Kwak, Y.D.; Ohmitsu, O.; Marutle, A.; Greig, N.H.; Choumrina, E. Practical issues in stem cell therapy for Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 370–377. [Google Scholar] [CrossRef]
- Sugaya, K. Mechanism of glial differentiation of neural progenitor cells by amyloid precursor protein. Neurodegener. Dis. 2008, 5, 170–172. [Google Scholar] [CrossRef]
- Kwak, Y.-D.; Hendrix, B.J.; Sugaya, K. Secreted type of amyloid precursor protein induces glial differentiation by stimulating the BMP/Smad signaling pathway. Biochem. Biophys. Res. Commun. 2014, 447, 394–399. [Google Scholar] [CrossRef]
- Elkabetz, Y.; Panagiotakos, G.; Al Shamy, G.; Socci, N.D.; Tabar, V.; Studer, L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008, 22, 152–165. [Google Scholar] [CrossRef]
- Park, S.M.; Jung, J.S.; Jang, M.S.; Kang, K.S.; Kang, S.K. Transforming growth factor-beta1 regulates the fate of cultured spinal cord-derived neural progenitor cells. Cell Prolif. 2008, 41, 248–264. [Google Scholar] [CrossRef]
- Alarcón, C.; Zaromytidou, A.-I.; Xi, Q.; Gao, S.; Yu, J.; Fujisawa, S.; Barlas, A.; Miller, A.N.; Manova-Todorova, K.; Macias, M.J.; et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 2009, 139, 757–769. [Google Scholar] [CrossRef]
- Lu, J.; Tan, L.; Li, P.; Gao, H.; Fang, B.; Ye, S.; Geng, Z.; Zheng, P.; Song, H. All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol. 2009, 10, 57. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.S.; Leem, J.W.; Huh, Y.J.; Kim, J.Y.; Kim, H.-S.; Park, I.-H.; Daley, G.Q.; Hwang, D.-Y.; Kim, D.-W. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. Rep. 2010, 6, 270–281. [Google Scholar] [CrossRef]
- Kim, T.-G.; Yao, R.; Monnell, T.; Cho, J.-H.; Vasudevan, A.; Koh, A.; Peeyush, K.T.; Moon, M.; Datta, D.; Bolshakov, V.Y.; et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells 2014, 32, 1789–1804. [Google Scholar] [CrossRef]
- Chen, S.Q.; Huang, M.; Liu, C.L.; Shen, Y.Y.; Cai, Q.; Wang, P.J. Differentiation of induced pluripotent stem cells into neural stem cells induced by brain-derived neurotrophic factor via Wnt/β-catenin and extracellular signal-regulated kinase/mitogen-activated protein kinases signal pathway. Zhonghua Yi Xue Za Zhi 2017, 97, 3263–3268. [Google Scholar]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Pauly, M.G.; Krajka, V.; Stengel, F.; Seibler, P.; Klein, C.; Capetian, P. Adherent vs. Free-Floating Neural Induction by Dual SMAD Inhibition for Neurosphere Cultures Derived from Human Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2018, 6, 3. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Haque, A.; Yue, X.-S.; Motazedian, A.; Tagawa, Y.; Akaike, T. Characterization and neural differentiation of mouse embryonic and induced pluripotent stem cells on cadherin-based substrata. Biomaterials 2012, 33, 5094–5106. [Google Scholar] [CrossRef]
- Komura, T.; Kato, K.; Konagaya, S.; Nakaji-Hirabayashi, T.; Iwata, H. Optimization of surface-immobilized extracellular matrices for the proliferation of neural progenitor cells derived from induced pluripotent stem cells. Biotechnol. Bioeng. 2015, 112, 2388–2396. [Google Scholar] [CrossRef]
- Badenes, S.; Fernandes, T.G.; Cordeiro, C.S.M.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M. Defined Essential 8TM Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 2016, 11, e0151264. [Google Scholar] [CrossRef]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18, 3060–3072. [Google Scholar] [CrossRef]
- Edgar, J.M.; Robinson, M.; Willerth, S.M. Fibrin hydrogels induce mixed dorsal/ventral spinal neuron identities during differentiation of human induced pluripotent stem cells. Acta Biomater. 2017, 51, 237–245. [Google Scholar] [CrossRef]
- Yan, Y.; Martin, L.M.; Bosco, D.B.; Bundy, J.L.; Nowakowski, R.S.; Sang, Q.-X.A.; Li, Y. Differential effects of acellular embryonic matrices on pluripotent stem cell expansion and neural differentiation. Biomaterials 2015, 73, 231–242. [Google Scholar] [CrossRef]
- Lukovic, D.; Lloret, A.D.; Stojkovic, P.; Rodríguez-Martínez, D.; Arago, M.A.P.; Rodriguez-Jimenez, F.J.; González-Rodríguez, P.; López-Barneo, J.; Sykova, E.; Jendelova, P.; et al. Highly Efficient Neural Conversion of Human Pluripotent Stem Cells in Adherent and Animal-Free Conditions. Stem Cells Transl. Med. 2017, 6, 1217–1226. [Google Scholar] [CrossRef]
- Rajala, K.; Lindroos, B.; Hussein, S.M.; Lappalainen, R.S.; Pekkanen-Mattila, M.; Inzunza, J.; Rozell, B.; Miettinen, S.; Narkilahti, S.; Kerkelä, E.; et al. A defined and xeno-free culture method enabling the establishment of clinical-grade human embryonic, induced pluripotent and adipose stem cells. PLoS ONE 2010, 5, e10246. [Google Scholar] [CrossRef]
- Isoda, M.; Kohyama, J.; Iwanami, A.; Sanosaka, T.; Sugai, K.; Yamaguchi, R.; Matsumoto, T.; Nakamura, M.; Okano, H. Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions. Neurosci. Res. 2016, 110, 18–28. [Google Scholar] [CrossRef]
- Chen, M.-S.; Wang, T.-J.; Lin, H.-C.; Burnouf, T. Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton jelly mesenchymal stromal cells. New Biotechnol. 2019, 49, 151–160. [Google Scholar] [CrossRef]
- Cowper, M.; Frazier, T.; Wu, X.; Curley, J.L.; Ma, M.H.; Mohiuddin, O.A.; Dietrich, M.; McCarthy, M.; Bukowska, J.; Gimble, J.M. Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells. Cells 2019, 8, 724. [Google Scholar] [CrossRef]
- McGrath, M.; Tam, E.; Sladkova, M.; AlManaie, A.; Zimmer, M.; De Peppo, G.M. GMP-compatible and xeno-free cultivation of mesenchymal progenitors derived from human-induced pluripotent stem cells. Stem Cell Res. Ther. 2019, 10, 11. [Google Scholar] [CrossRef]
- Erlandsson, A.; Brännvall, K.; Gustafsdottir, S.; Westermark, B.; Forsberg-Nilsson, K. Autocrine/paracrine platelet-derived growth factor regulates proliferation of neural progenitor cells. Cancer Res. 2006, 66, 8042–8048. [Google Scholar] [CrossRef]
- Gouel, F.; Rolland, A.-S.; Devedjian, J.-C.; Burnouf, T.; Devos, D. Past and Future of Neurotrophic Growth Factors Therapies in ALS: From Single Neurotrophic Growth Factor to Stem Cells and Human Platelet Lysates. Front. Neurol. 2019, 10, 835. [Google Scholar] [CrossRef]
- Simakajornboon, N.; Kuptanon, T.; Jirapongsuwan, P. The effect of prenatal nicotine exposure on PDGFR-mediated anti-apoptotic mechanism in the caudal brainstem of developing rat. Neurosci. Lett. 2010, 478, 46–50. [Google Scholar] [CrossRef]
- Hayon, Y.; Dashevsky, O.; Shai, E.; Varon, D.; Leker, R.R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemost. 2013, 110, 323–330. [Google Scholar] [CrossRef]
- Chen, L.W.; Huang, C.-J.; Tu, W.-H.; Lu, C.-J.; Sun, Y.-C.; Lin, S.-Y.; Chen, W.-L. The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates. PLoS ONE 2018, 13, e0194345. [Google Scholar] [CrossRef]
- Fan, L.; Liu, C.; Chen, X.; Zou, Y.; Zhou, Z.; Lin, C.; Tan, G.; Zhou, L.; Ning, C.; Wang, Q. Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl. Mater. Interfaces 2018, 10, 17742–17755. [Google Scholar] [CrossRef]
- Crapo, P.M.; Medberry, C.J.; Reing, J.E.; Tottey, S.; van der Merwe, Y.; Jones, K.E.; Badylak, S.F. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 2012, 33, 3539–3547. [Google Scholar] [CrossRef]
- Medberry, C.J.; Crapo, P.M.; Siu, B.F.; Carruthers, C.A.; Wolf, M.T.; Nagarkar, S.P.; Agrawal, V.; Jones, K.E.; Kelly, J.; Johnson, S.A.; et al. Hydrogels derived from central nervous system extracellular matrix. Biomaterials 2013, 34, 1033–1040. [Google Scholar] [CrossRef]
- Kaiser, A.; Kale, A.; Novozhilova, E.; Olivius, P. The Effects of Matrigel® on the Survival and Differentiation of a Human Neural Progenitor Dissociated Sphere Culture. Anat. Rec. 2020, 303, 441–450. [Google Scholar] [CrossRef]
- Han, J.; Chen, L.; Luo, G.; Dai, B.; Wang, X.; Dai, J. Three-dimensional culture may promote cell reprogramming. Organogenesis 2013, 9, 118–120. [Google Scholar] [CrossRef]
- Macrí-Pellizzeri, L.; Pelacho, B.; Sancho, A.; Iglesias-García, O.; Simón-Yarza, A.M.; Soriano-Navarro, M.; González-Granero, S.; García-Verdugo, J.M.; De-Juan-Pardo, E.M.; Prosper, F. Substrate stiffness and composition specifically direct differentiation of induced pluripotent stem cells. Tissue Eng. Part A 2015, 21, 1633–1641. [Google Scholar] [CrossRef]
- Song, L.; Wang, K.; Li, Y.; Yang, Y. Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids Surf. B Biointerfaces 2016, 148, 49–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valerio, L.S.A.; Carrick, F.R.; Bedoya, L.; Sreerama, S.; Sugaya, K. Neural Differentiation of Induced Pluripotent Stem Cells for a Xenogeneic Material-Free 3D Neurological Disease Model Neurulation from Pluripotent Cells Using a Human Hydrogel. Curr. Issues Mol. Biol. 2023, 45, 4574-4588. https://doi.org/10.3390/cimb45060290
Valerio LSA, Carrick FR, Bedoya L, Sreerama S, Sugaya K. Neural Differentiation of Induced Pluripotent Stem Cells for a Xenogeneic Material-Free 3D Neurological Disease Model Neurulation from Pluripotent Cells Using a Human Hydrogel. Current Issues in Molecular Biology. 2023; 45(6):4574-4588. https://doi.org/10.3390/cimb45060290
Chicago/Turabian StyleValerio, Luis Sebastian Alexis, Frederick Robert Carrick, Lina Bedoya, Sandeep Sreerama, and Kiminobu Sugaya. 2023. "Neural Differentiation of Induced Pluripotent Stem Cells for a Xenogeneic Material-Free 3D Neurological Disease Model Neurulation from Pluripotent Cells Using a Human Hydrogel" Current Issues in Molecular Biology 45, no. 6: 4574-4588. https://doi.org/10.3390/cimb45060290
APA StyleValerio, L. S. A., Carrick, F. R., Bedoya, L., Sreerama, S., & Sugaya, K. (2023). Neural Differentiation of Induced Pluripotent Stem Cells for a Xenogeneic Material-Free 3D Neurological Disease Model Neurulation from Pluripotent Cells Using a Human Hydrogel. Current Issues in Molecular Biology, 45(6), 4574-4588. https://doi.org/10.3390/cimb45060290







