EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Tissue Collection for Gene Cloning and Expression Analysis
2.3. Embryo and Larvae Collection
2.4. Tissue Collection during Shell Biomineralization
2.5. Tissue Samples from Starved Pacific Abalones
2.6. Tissue Samples from Heat-Stressed Pacific Abalones
2.7. Total RNA Extraction and First-Strand cDNA Synthesis
2.8. Cloning of SMP5 mRNA Sequence in Pacific Abalone
2.8.1. Cloning of Partial Sequence
2.8.2. Cloning of RACE (5′- and 3′) Sequence
2.9. Sequence Analysis of Cloned H. discus Hannai SMP5 (Hdh-SMP5)
2.10. Phylogenetic Analysis
2.11. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.12. Statistical Analysis
3. Results
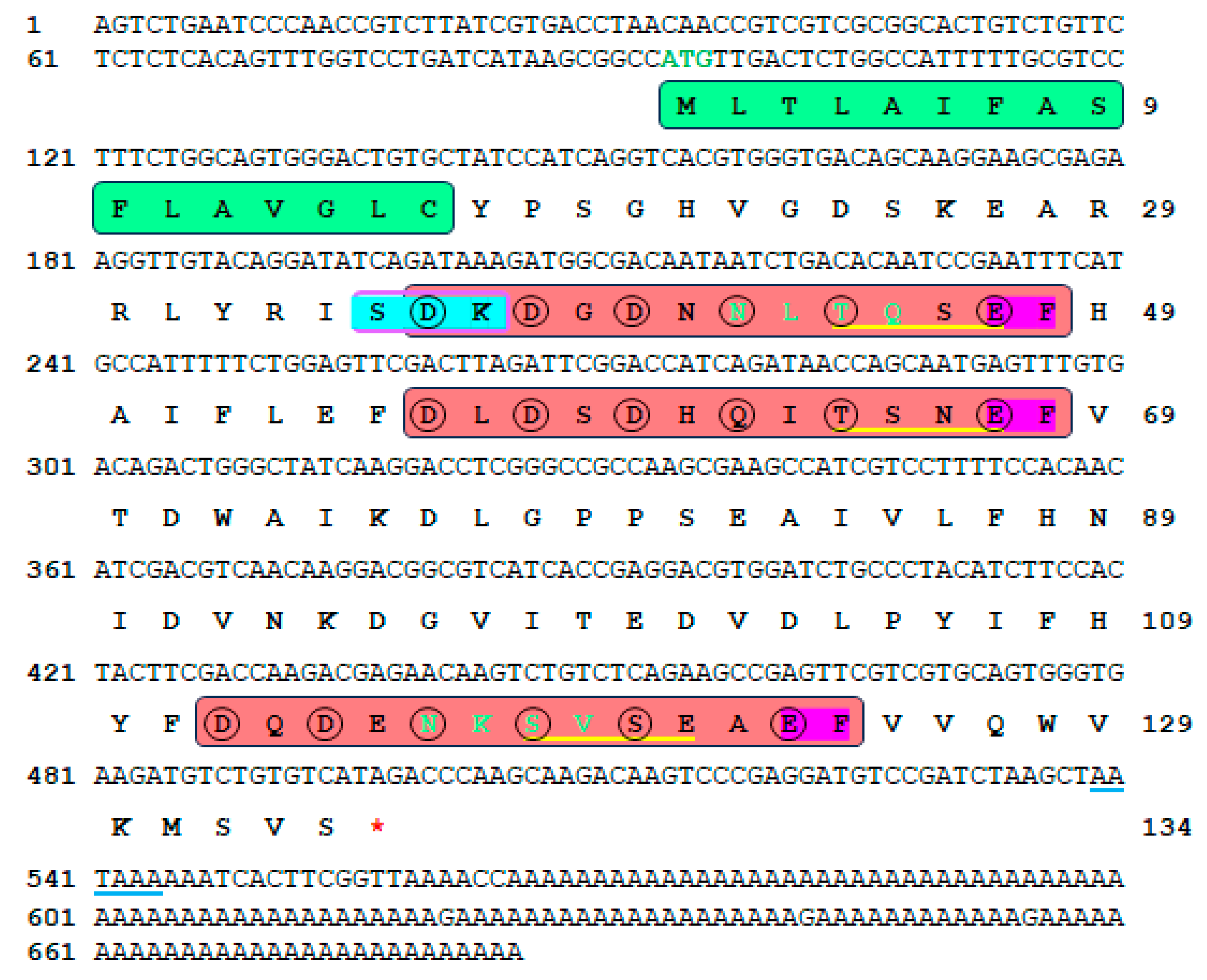
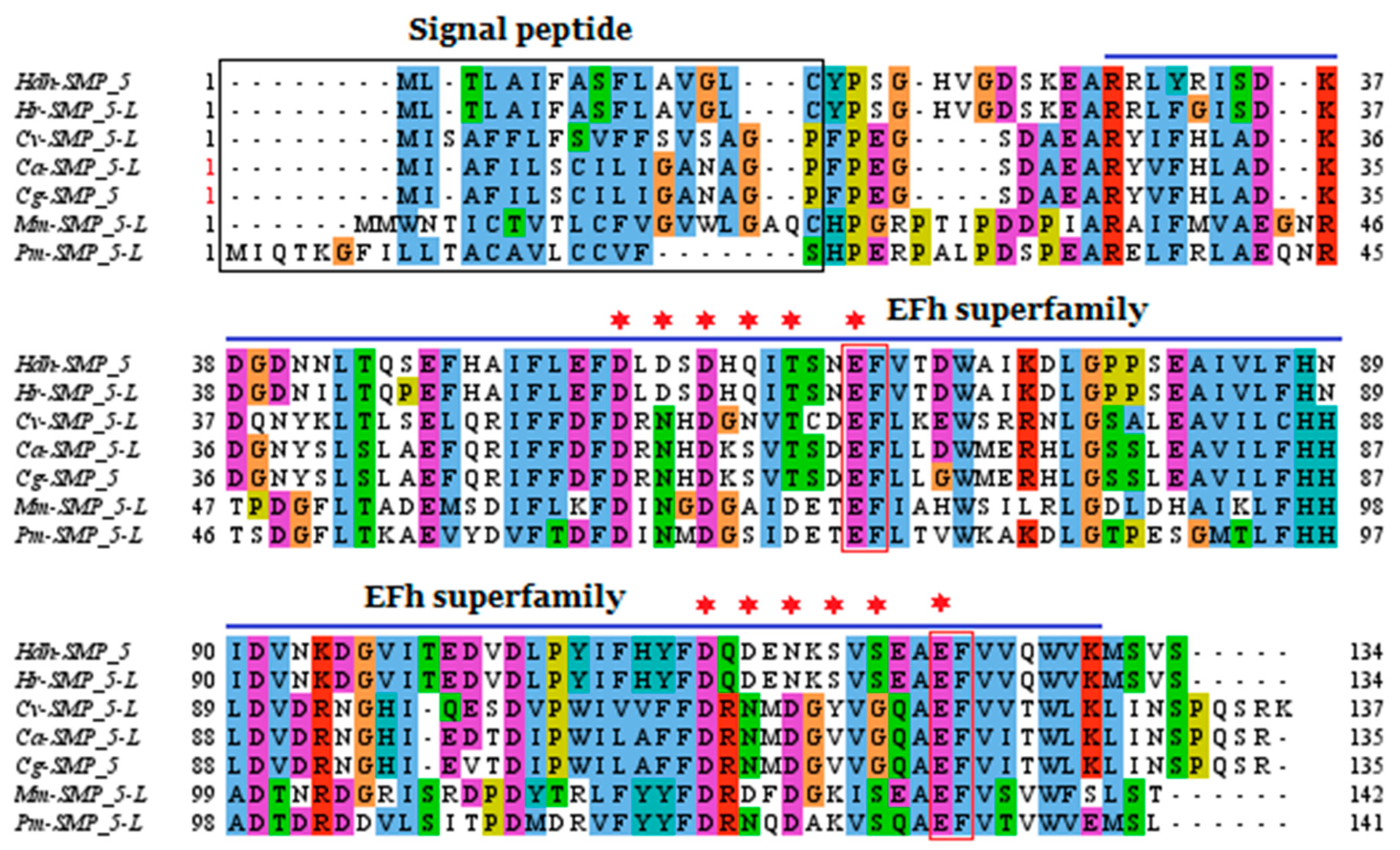
3.1. Hdh-SMP5 Sequence Analysis
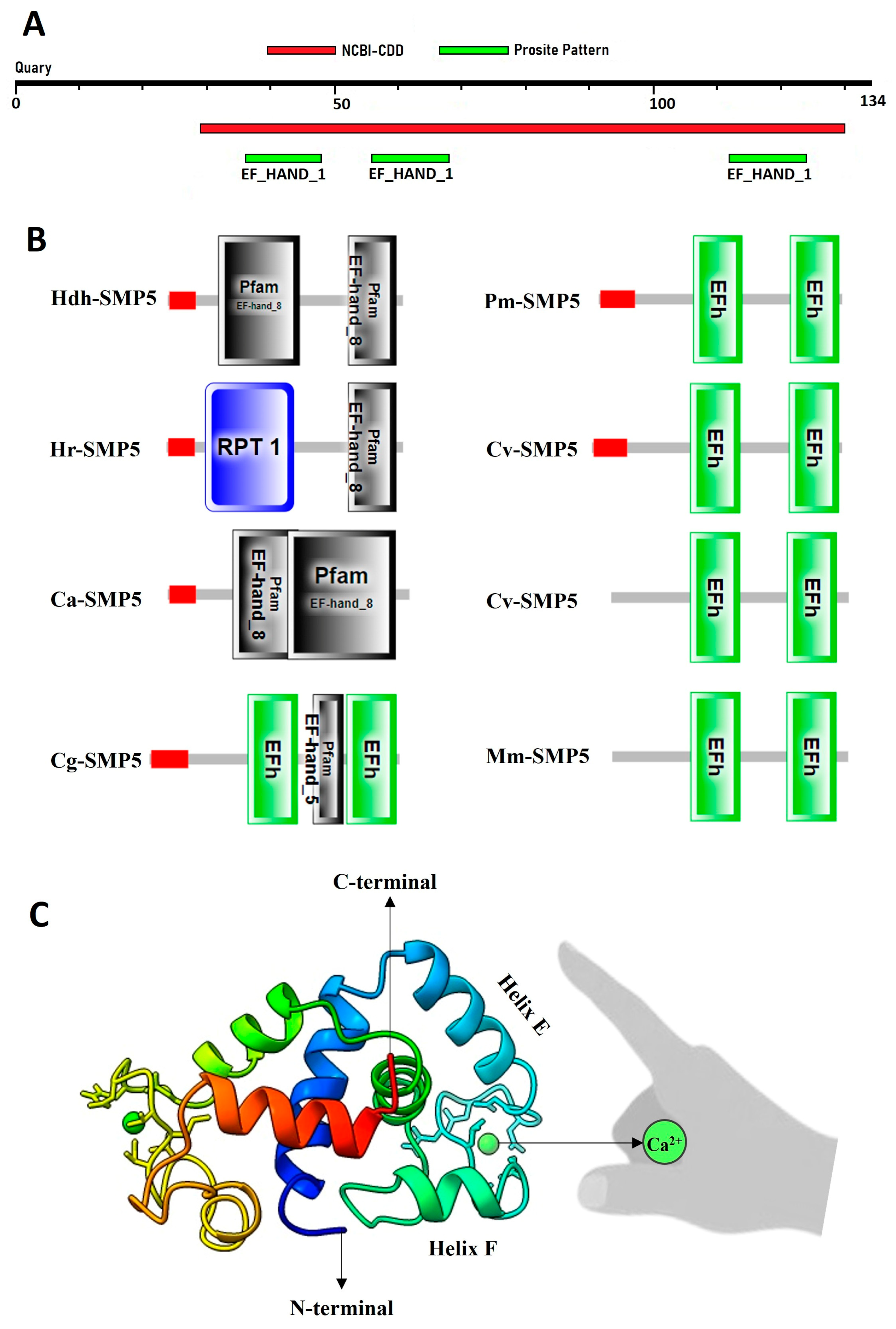
3.2. Structure of Hdh-SMP5
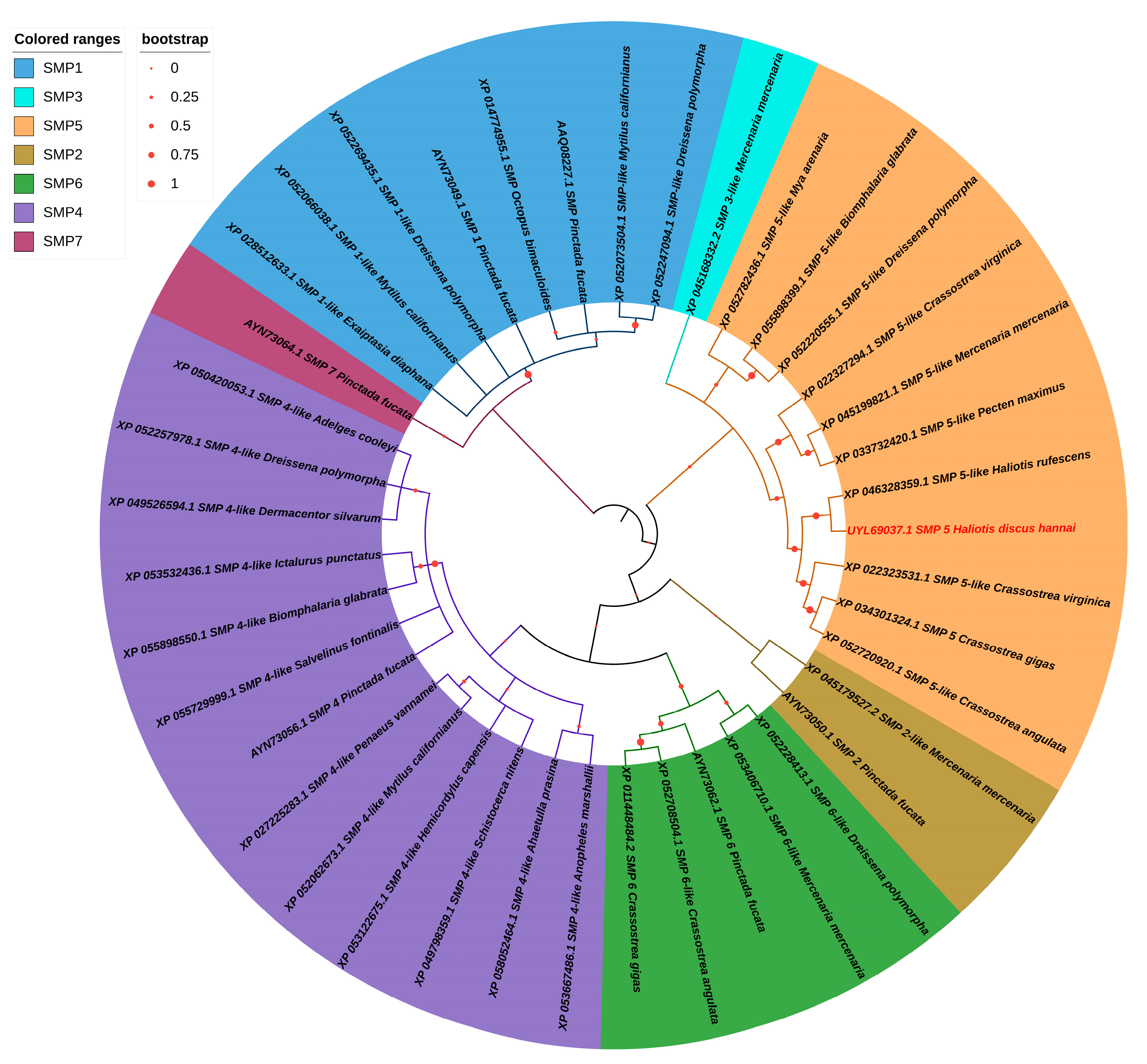
3.3. Phylogenetic Analysis
3.4. Functional Activity Prediction (Gene Ontology)
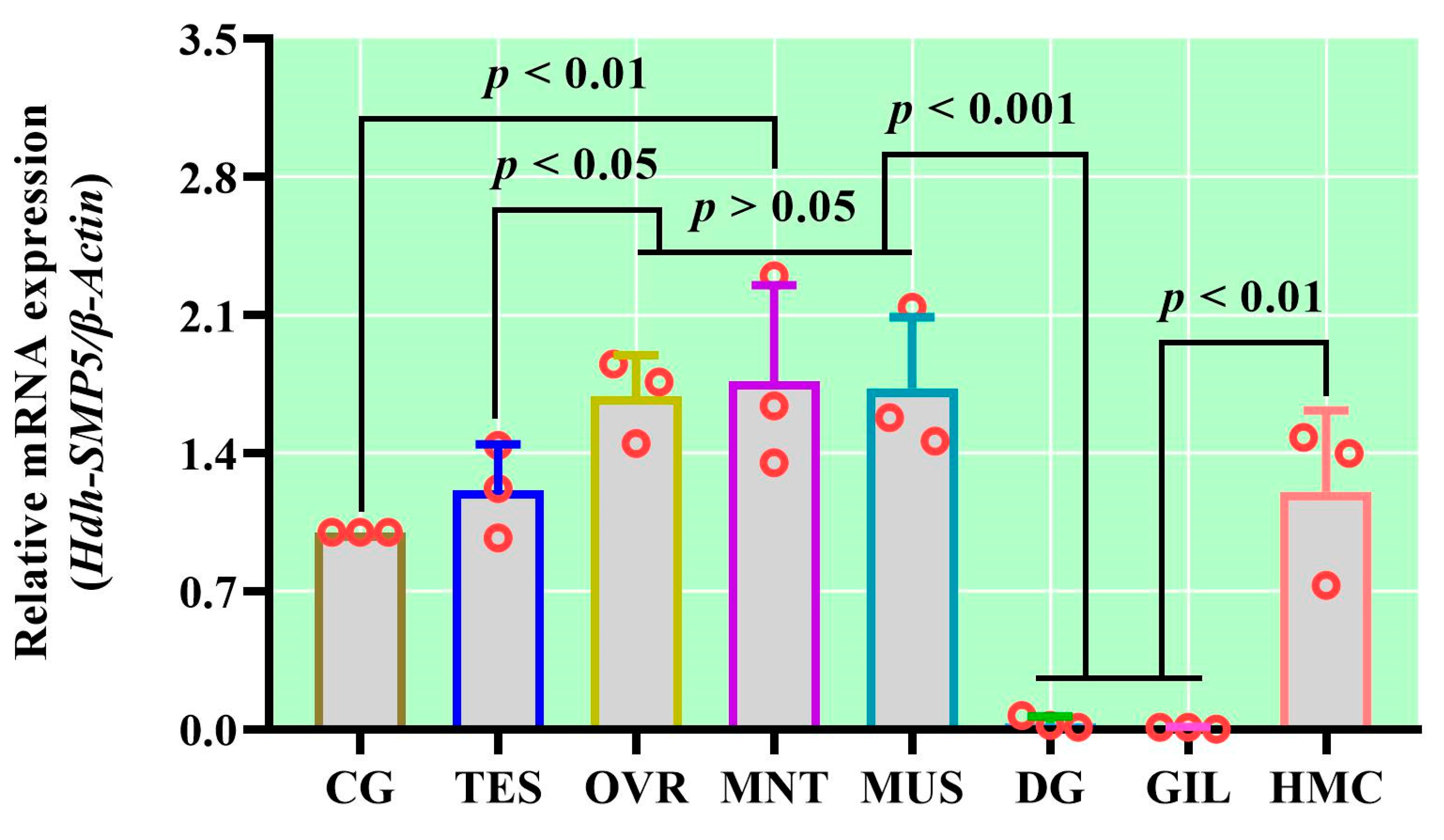
3.5. Tissue-Specific mRNA Expression of Hdh-SMP5
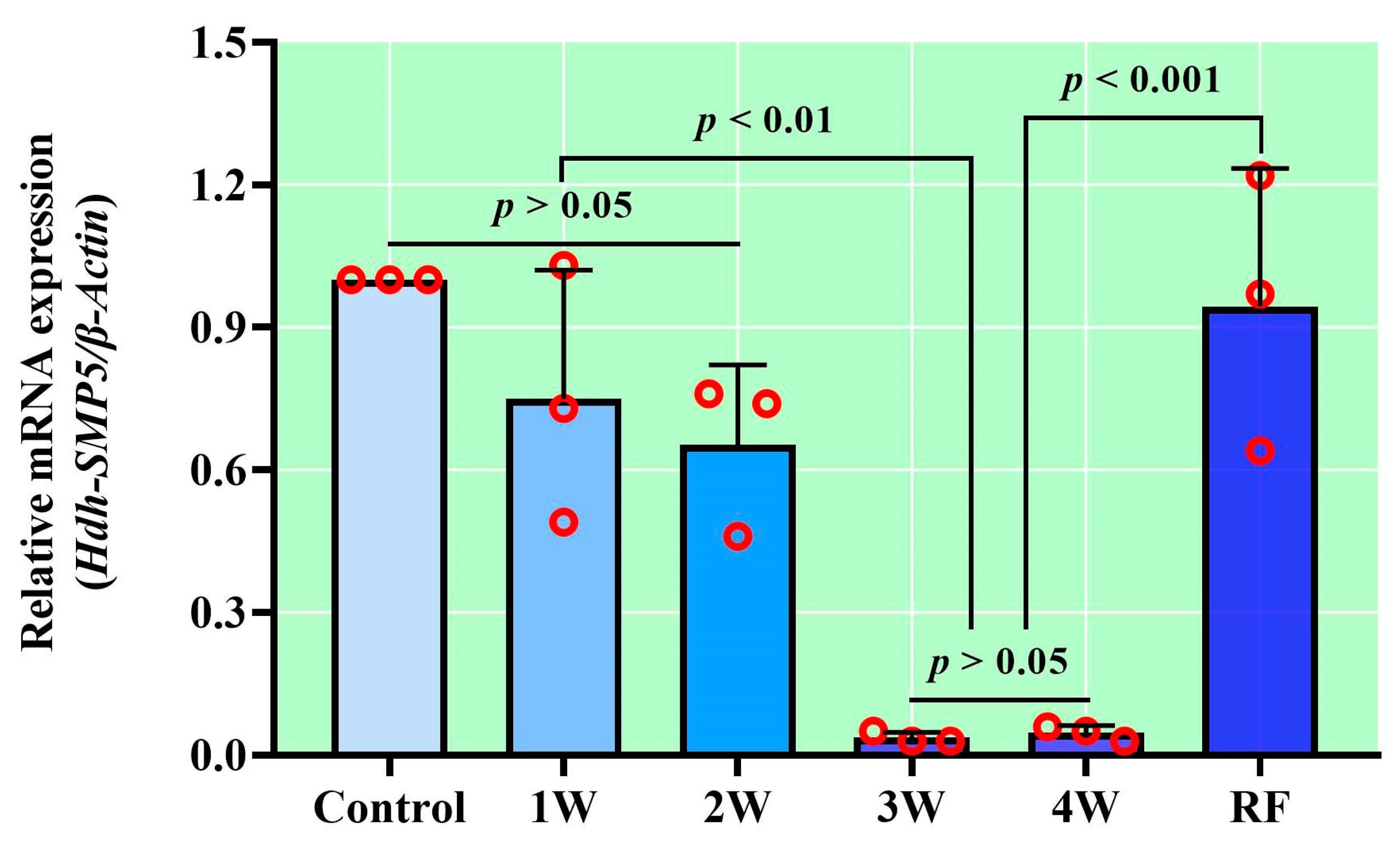
3.6. Hdh-SMP5 Expression in Pacific Abalone during Shell Biomineralization
3.7. Hdh-SMP5 Expression during Starvation
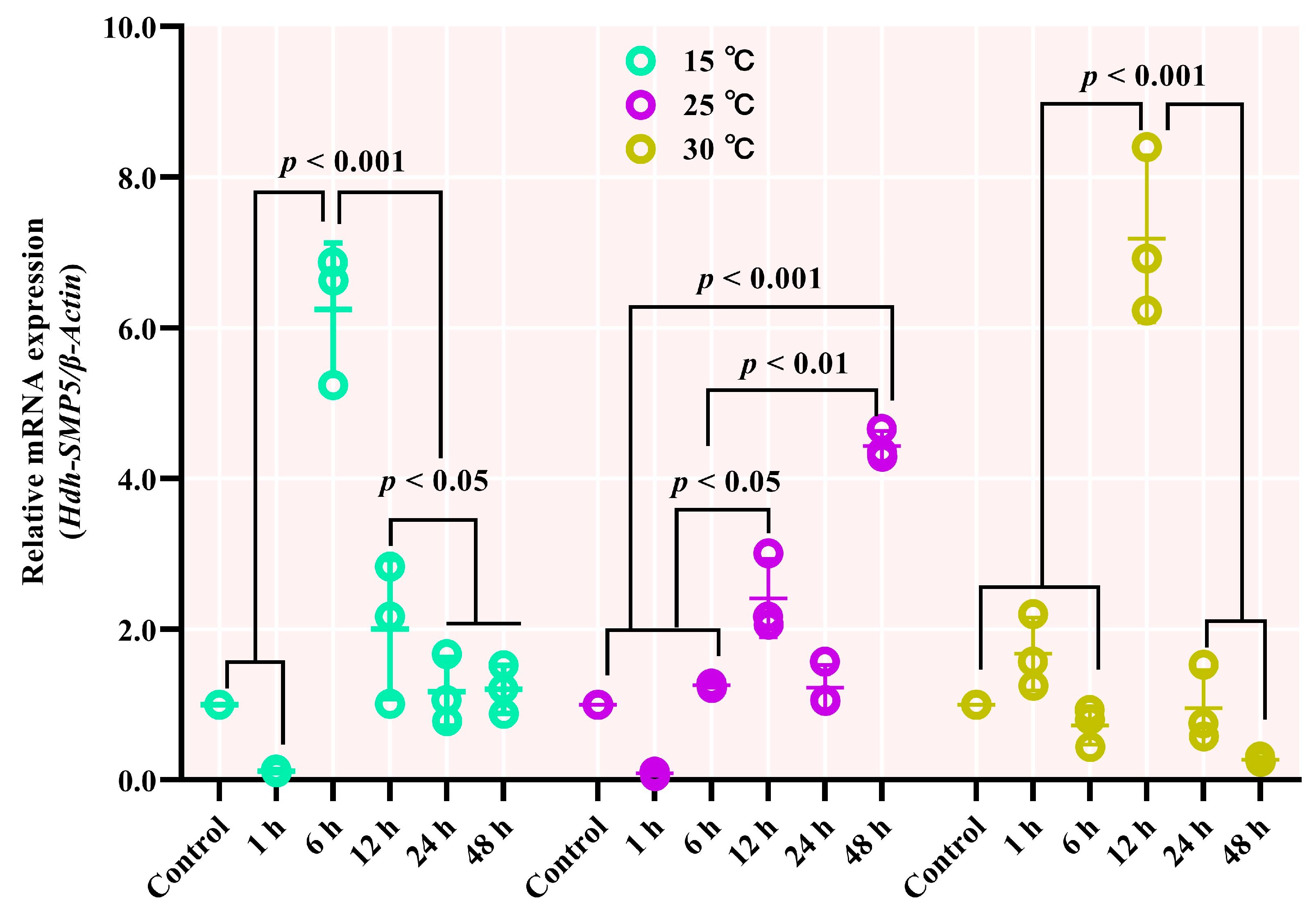
3.8. Hdh-SMP5 mRNA Expression in Heat-Stressed Pacific Abalones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocot, K.M.; Aguilera, F.; McDougall, C.; Jackson, D.J.; Degnan, B.M. Sea shell diversity and rapidly evolving secretomes: Insights into the evolution of biomineralization. Front. Zool. 2016, 13, 23. [Google Scholar] [CrossRef]
- Zhao, R.; Takeuchi, T.; Luo, Y.J.; Ishikawa, A.; Kobayashi, T.; Koyanagi, R.; Villar-Briones, A.; Yamada, L.; Sawada, H.; Iwanaga, S.; et al. Dual gene repertoires for larval and adult shells reveal molecules essential for molluscan shell formation. Mol. Biol. Evol. 2018, 35, 2751–2761. [Google Scholar] [CrossRef]
- Johnson, A.B.; Fogel, N.S.; Lambert, J.D. Growth and morphogenesis of the gastropod shell. Proc. Natl. Acad. Sci. USA 2019, 116, 6878–6883. [Google Scholar] [CrossRef] [PubMed]
- Saruwatari, K.; Matsui, T.; Mukai, H.; Nagasawa, H.; Kogure, T. Nucleation and growth of aragonite crystals at the growth front of nacres in pearl oyster, Pinctada fucata. Biomaterials 2009, 30, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2007, 80, 209–276. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Green, K.; Simpson, F.; Wörheide, G.; Degnan, B.M. A rapidly evolving secretome builds and patterns a sea shell. BMC Biol. 2006, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Wang, L.; Song, L. Recent advances of shell matrix proteins and cellular orchestration in marine molluscan shell biomineralization. Front. Mar. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Jackson, D.; Worheide, G.; Degnan, B. Dynamic expression of ancient and novel molluscan shell genes during ecological transitions. BMC Evol. Biol. 2007, 7, 160. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Liu, C.; Huang, J.; Zheng, G.; Xie, L.; Zhang, R. Hemocytes participate in calcium carbonate crystal formation, transportation and shell regeneration in the pearl oyster Pinctada fucata. Fish Shellfish Immunol. 2016, 51, 263–270. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Falini, G.; Addadi, L.; Weiner, S. Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using cryo-TEM. J. Struct. Biol. 2001, 135, 8–17. [Google Scholar] [CrossRef]
- Liao, Q.; Qin, Y.; Zhou, Y.; Shi, G.; Li, X.; Li, J.; Mo, R.; Zhang, Y.; Yu, Z. Characterization and functional analysis of a chitinase gene: Evidence of ch-chit participates in the regulation of biomineralization in crassostrea hongkongensis. Aquac. Rep. 2021, 21, 100852. [Google Scholar] [CrossRef]
- Rivera-Perez, C.; Ojeda-Ramirez de Areyano, J.J.; Hernandez-Saavedra, N.Y. Purification and functional analysis of the shell matrix protein N66 from the shell of the pearl oyster Pteria sterna. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 235, 19–29. [Google Scholar] [CrossRef]
- Duplat, D.; Puissegur, M.; Bedouet, L.; Rousseau, M.; Boulzaguet, H.; Milet, C.; Sellos, D.; Van Wormhoudt, A.; Lopez, E. Identification of calconectin, a calcium-binding protein specifically expressed by the mantle of Pinctada margaritifera. FEBS Lett. 2006, 580, 2435–2441. [Google Scholar] [CrossRef][Green Version]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chemistry 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Gaume, B.; Denis, F.; Van Wormhoudt, A.; Huchette, S.; Jackson, D.J.; Avignon, S.; Auzoux-Bordenave, S. Characterisation and expression of the biomineralising gene lustrin a during shell formation of the european abalone Haliotis tuberculata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 169, 1–8. [Google Scholar] [CrossRef]
- Mao-Che, L.; Golubic, S.; Le Campion-Alsumard, T.; Payri, C. Developmental aspects of biomineralisation in the Polynesian pearl oyster Pinctada margaritifera var. cumingii. Oceanol. Acta 2001, 24, 37–49. [Google Scholar] [CrossRef]
- Joubert, C.; Piquemal, D.; Marie, B.; Manchon, L.; Pierrat, F.; Zanella-Cléon, I.; Cochennec-Laureau, N.; Gueguen, Y.; Montagnani, C. Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: Focus on biomineralization. BMC Genom. 2010, 11, 613. [Google Scholar] [CrossRef]
- Mann, K.; Siedler, F.; Treccani, L.; Heinemann, F.; Fritz, M. Perlinhibin, a cysteine-, histidine-, and arginine-rich miniprotein from abalone (Haliotis laevigata) nacre, inhibits in vitro calcium carbonate crystallization. Biophys. J. 2007, 93, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Amos, F.F.; Evans, J.S. AP7, a partially disordered pseudo-C-RING protein, is capable of forming stabilized aragonite in vitro. Biochemistry 2009, 48, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Belcher, A.M.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Molecular cloning and characterization of lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufescens. J. Biol. Chem. 1997, 272, 32472–32481. [Google Scholar] [CrossRef] [PubMed]
- de Viçose, G.C.; Viera, M.P.; Bilbao, A.; Izquierdo, M.S. Embryonic and larval development of Haliotis tuberculata coccinea Reeve: An indexed micro-photographic sequence. J. Shellfish Res. 2007, 26, 847–854. [Google Scholar] [CrossRef]
- Jardillier, E.; Rousseau, M.; Gendron-Badou, A.; Fröhlich, F.; Smith, D.C.; Martin, M.; Helléouet, M.-N.; Huchette, S.; Doumenc, D.; Auzoux-Bordenave, S. A morphological and structural study of the larval shell from the abalone Haliotis tuberculata. Mar. Biol. 2008, 154, 735–744. [Google Scholar] [CrossRef]
- Auzoux-Bordenave, S.; Badou, A.; Gaume, B.; Berland, S.; Helléouet, M.N.; Milet, C.; Huchette, S. Ultrastructure, chemistry and mineralogy of the growing shell of the European abalone Haliotis tuberculata. J. Struct. Biol. 2010, 171, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Identification of conserved proteins from diverse shell matrix proteome in Crassostrea gigas: Characterization of genetic bases regulating shell formation. Sci. Rep. 2017, 7, 45754. [Google Scholar] [CrossRef]
- Hanif, M.A.; Hossen, S.; Cho, Y.; Sukhan, Z.P.; Choi, C.Y.; Kho, K.H. Characterization and Expression Analysis of Mollusk-like Growth Factor: A Secreted Protein Involved in Pacific Abalone Embryonic and Larval Development. Biology 2022, 11, 1445. [Google Scholar] [CrossRef]
- Takeuchi, T.; Sarashina, I.; Iijima, M.; Endo, K. In vitro regulation of CaCO3 crystal polymorphism by the highly acidic molluscan shell protein Aspein. FEBS Lett. 2008, 582, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, R. Matrix proteins in the outer shells of molluscs. Mar. Biotechnol. 2006, 8, 572–586. [Google Scholar] [CrossRef]
- Marie, B.; Luquet, G.; Pais de Barros, J.P.; Guichard, N.; Morel, S.; Alcaraz, G.; Bollache, L.; Marin, F. The shell matrix of the freshwater mussel Unio pictorum (Paleoheterodonta, Unionoida). FEBS J. 2007, 274, 2933–2945. [Google Scholar] [CrossRef]
- Kono, M.; Hayashi, N.; Samata, T. Molecular mechanism of the Nacreous layer formation in Pinctada maxima. Biochem. Biophys. Res. Commun. 2000, 269, 213–218. [Google Scholar] [CrossRef]
- Funabara, D.; Ohmori, F.; Kinoshita, S.; Koyama, H.; Mizutani, S.; Ota, A.; Osakabe, Y.; Nagai, K.; Maeyama, K.; Okamoto, K.; et al. Novel genes participating in the formation of prismatic and nacreous layers in the pearl oyster as revealed by their tissue distribution and RNA interference knockdown. PLoS ONE 2014, 9, e84706. [Google Scholar] [CrossRef]
- Hare, P.E. Amino acids in the proteins from aragonite and calcite in the shells of Mytilus californianus. Science 1963, 139, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Marie, B.; Jackson, D.J.; Ramos-Silva, P.; Zanella-Cleon, I.; Guichard, N.; Marin, F. The shell-forming proteome of Lottia gigantea reveals both deep conservations and lineage-specific novelties. FEBS J. 2013, 280, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Gotliv, B.-A.; Addadi, L.; Weiner, S. Mollusk shell acidic proteins: In search of individual functions. Chembiochem 2003, 4, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Liu, S.F.; Ge, Y.J.; Liu, J.; Wang, X.Y.; Xie, L.P.; Zhang, R.Q.; Wang, Z. Identification and characterization of a biomineralization related gene pfmg1 highly expressed in the mantle of Pinctada fucata. Biochemistry 2007, 46, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Shimizu, K.; Isowa, Y.; Takeuchi, T.; Zhao, R.; Kito, K.; Fujie, M.; Satoh, N.; Endo, K. Functional shell matrix proteins tentatively identified by asymmetric snail shell morphology. Sci. Rep. 2020, 10, 9768. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Cui, B.; Li, X.; Lin, Z.; Dong, Y. Characteristics of a novel tyrosinase gene involved in the formation of shell color in hard clam Meretrix meretrix. J. Ocean Univ. China 2020, 19, 183–190. [Google Scholar] [CrossRef]
- Saleuddin, A.S.M.; Petit, H.P. The Mode of Formation and the Structure of the Periostracum. In Mollusca; Saleuddin, A.S.M., Wilbur, K.M., Eds.; Physiology, Part I; Academic Press: New York, NY, USA, 1983; Volume 4, pp. 199–234. [Google Scholar]
- Liu, X.J.; Jin, C.; Li, H.R. Morphological structure of shell and expression patterns of five matrix protein genes during the shell regeneration process in Hyriopsis cumingii. Aquac. Fish. 2018, 3, 225–231. [Google Scholar] [CrossRef]
- Xie, J.; Liang, J.; Sun, J.; Gao, J.; Zhang, S.; Liu, Y.; Xie, L.; Zhang, R. Influence of the extrapallial fluid of Pinctada fucata on the crystallization of calcium carbonate and shell biomineralization. Cryst. Growth Des. 2016, 16, 672–680. [Google Scholar] [CrossRef]
- Matias, D.; Joaquim, S.; Ramos, M.; Sobral, P.; Leitão, A. Biochemical compounds’ dynamics during larval development of the carpet-shell clam Ruditapes decussatus (Linnaeus, 1758): Effects of mono-specific diets and starvation. Helgol. Mar. Res. 2011, 65, 369–379. [Google Scholar] [CrossRef]
- Albentosa, M.; Pérez-Camacho, A.; Fernández-Reiriz, M.J.; Labarta, U. Wheatgerm flour in diets for Manila clam, Ruditapes philippinarum, spat. Aquaculture 2002, 212, 335–345. [Google Scholar] [CrossRef]
- Joubert, C.; Linard, C.; Le Moullac, G.; Soyez, C.; Saulnier, D.; Teaniniuraitemoana, V.; Ky, C.L.; Gueguen, Y. Temperature and food influence shell growth and mantle gene expression of shell matrix proteins in the pearl oyster Pinctada margaritifera. PLoS ONE 2014, 9, e103944. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.C.; He, X. Thermal stability of proteins. Ann. N. Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, X.; Lin, J.; He, M. Seawater acidification and elevated temperature affect gene expression patterns of the pearl oyster Pinctada fucata. PLoS ONE 2012, 7, e33679. [Google Scholar] [CrossRef]
- Pouvreau, S.; Prasil, V. Growth of the black-lip pearl oyster, Pinctada margaritifera, at nine culture sites of French Polynesia: Synthesis of several sampling designs conducted between 1994 and 1999. Aquat. Living Resour. 2001, 14, 155–163. [Google Scholar] [CrossRef]

| Primer Name | Nucleotide Sequences | Purpose |
|---|---|---|
| Oligo dT (OdT) | GGCCACGCGTCGACTAGTACTTTTTTTTTTTTTTTTT | cDNA synthesis |
| Oligo dT adapter (AP) | GGCCACGCGTCGACTAGTAC | |
| SMP5-Fw | GATCATAAGCGGCCATGTTG | Fragment PCR |
| SMP5-Rv | CTTGCTTGGGTCTATGACAC | |
| SMP5-5′ | GATTACGCCAAGCTTCACGTGACCTGATGGATAGCACAGTCC | 5′ RACE PCR |
| SMP5-3′ | GATTACGCCAAGCTTGGACTGTGCTATCCATCAGGTCACGTG | 3′ RACE PCR |
| SMP5-qFw | TGACAGACTGGGCTATCAAG | qRT-PCR |
| SMP5-qRv | CTTGCTTGGGTCTATGACAC | |
| Hdh-β-Actin-Fw | GATAGTGCGAGACATCAAGG | |
| Hdh-β-Actin-Rv | GAGCTCGAAACCTCTCATTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, M.A.; Han, J.D.; Kim, S.C.; Hossen, S.; Kho, K.H. EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai. Curr. Issues Mol. Biol. 2023, 45, 10079-10096. https://doi.org/10.3390/cimb45120629
Hanif MA, Han JD, Kim SC, Hossen S, Kho KH. EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai. Current Issues in Molecular Biology. 2023; 45(12):10079-10096. https://doi.org/10.3390/cimb45120629
Chicago/Turabian StyleHanif, Md Abu, Ji Do Han, Soo Cheol Kim, Shaharior Hossen, and Kang Hee Kho. 2023. "EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai" Current Issues in Molecular Biology 45, no. 12: 10079-10096. https://doi.org/10.3390/cimb45120629
APA StyleHanif, M. A., Han, J. D., Kim, S. C., Hossen, S., & Kho, K. H. (2023). EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai. Current Issues in Molecular Biology, 45(12), 10079-10096. https://doi.org/10.3390/cimb45120629






