Hydroxycitric Acid Alleviated Lung Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Ferroptosis through the Hif-1α Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animal Treatments
2.3. Hematoxylin–Eosin (H&E) Staining
2.4. Lung Injury Score
2.5. Lung Wet/Dry Mass Ratio
2.6. Elisa
2.7. Iron Content Assay
2.8. Cell Culture and Cell Treatment
2.9. Assay of Cell Viability
2.10. Wound Healing Assays
2.11. Transwell Assay
2.12. ROS Assay
2.13. Transmission Electron Microscopy (TEM)
2.14. Western Blot and Real-Time Fluorescence Quantitative PCR
2.15. Flow Cytometry
2.16. Mitochondrial Membrane Potential (MMP) Assay
2.17. Mitochondrial Ferrous Iron (Fe2+) Determination
2.18. Immunofluorescence Staining
2.19. Cell Transfection
2.20. Molecular Docking
2.21. Statistical Analysis
3. Results
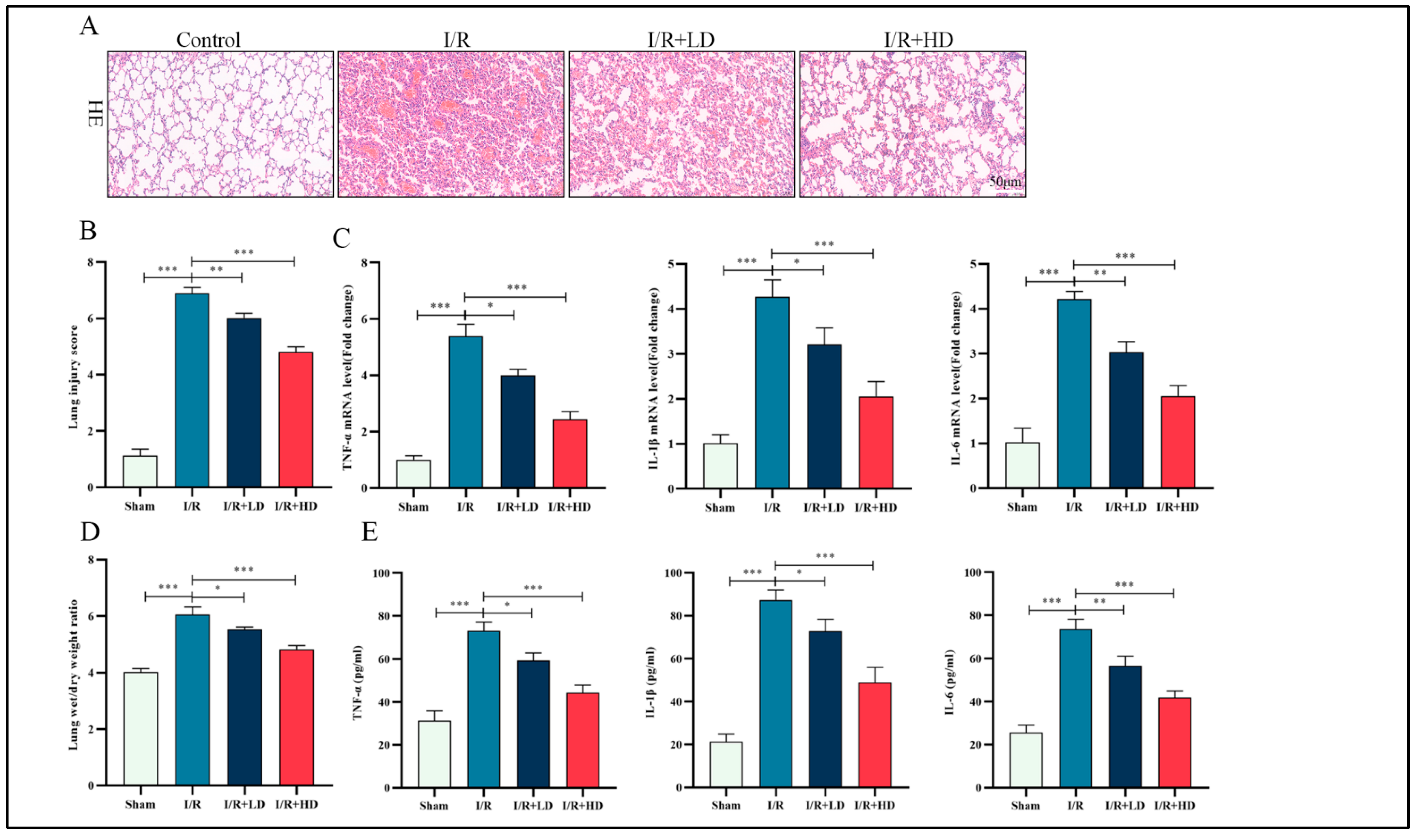
3.1. HCA Pretreatment Attenuates I/R-Induced Lung Injury and Inflammation in Mice
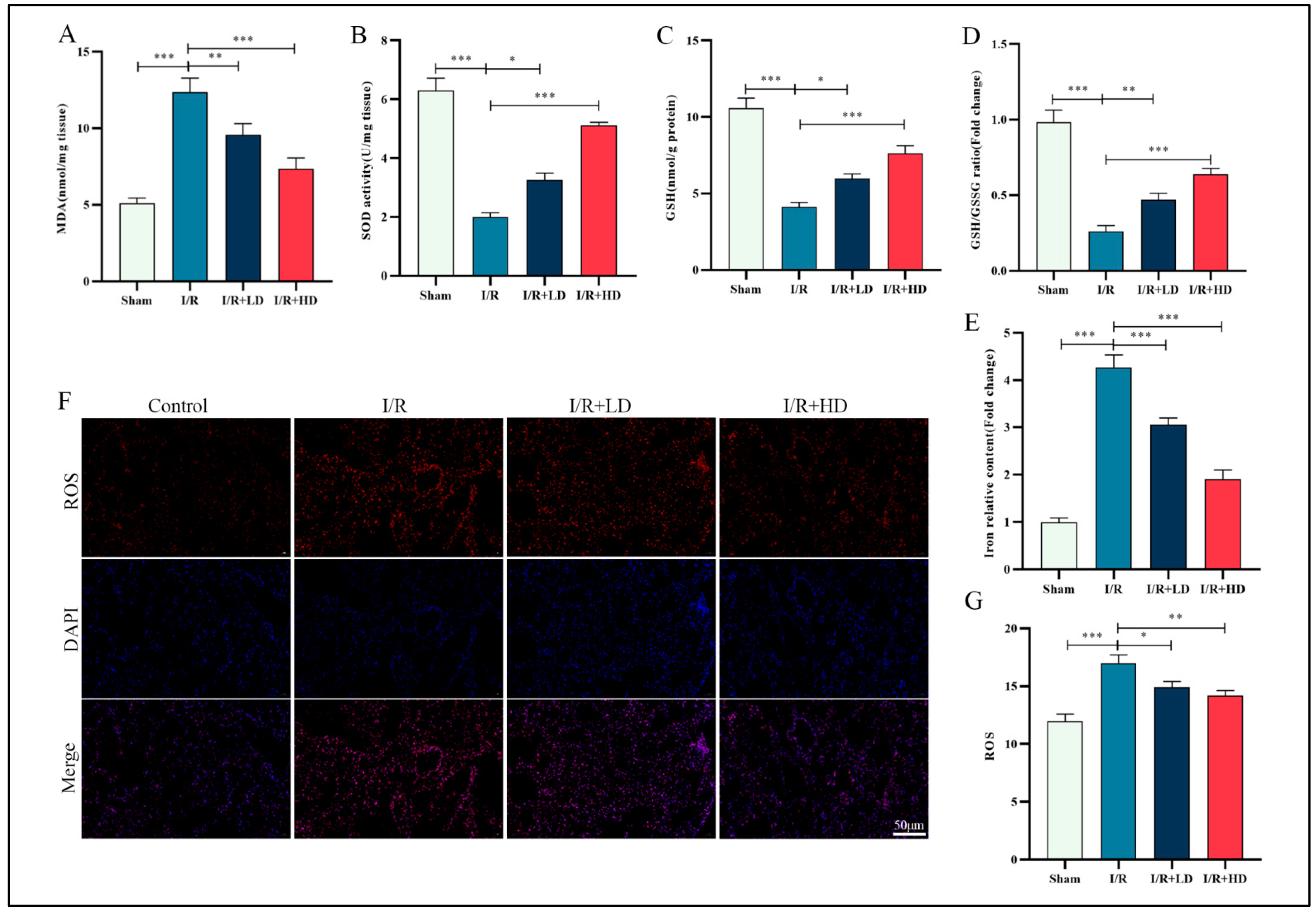
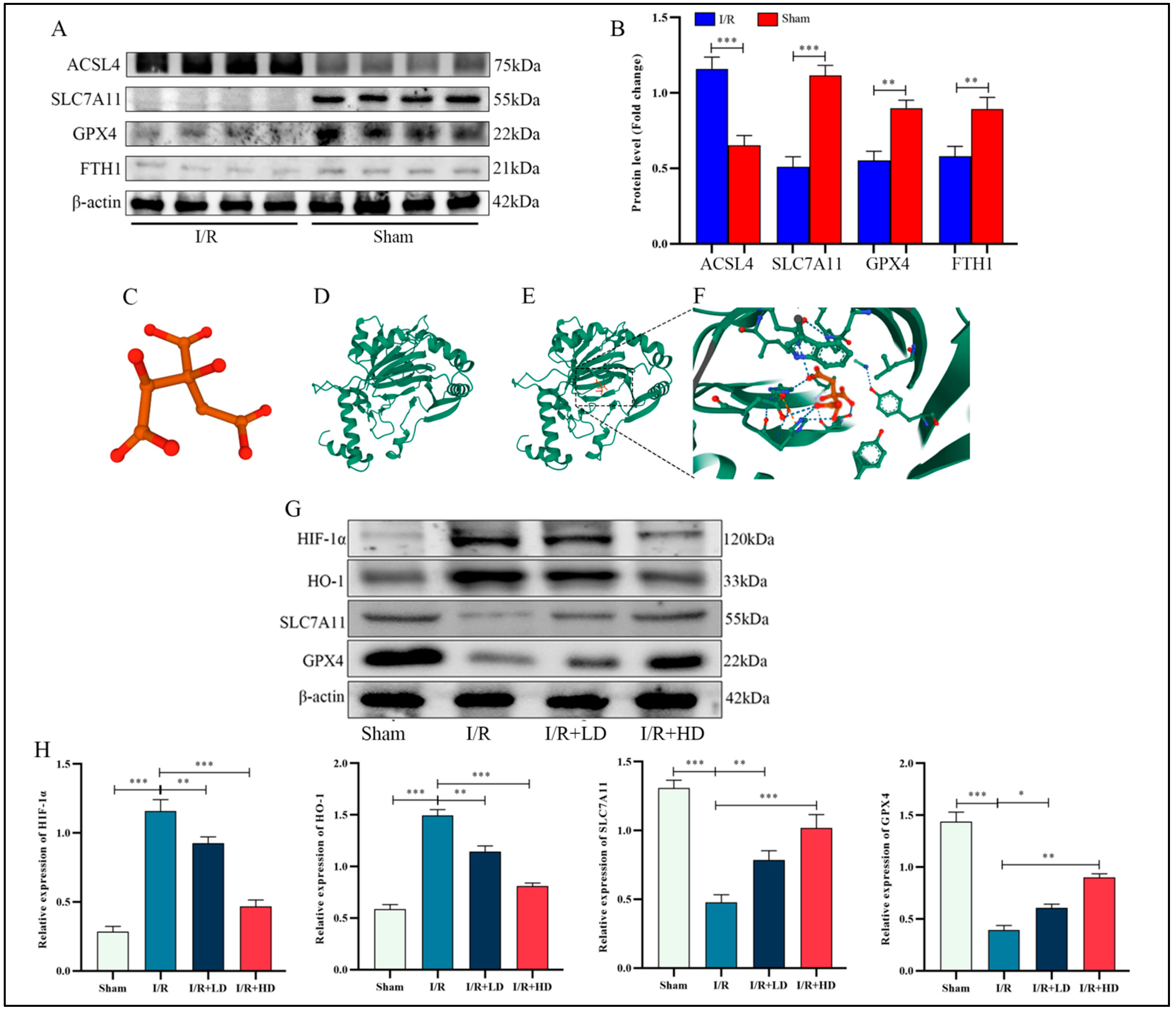
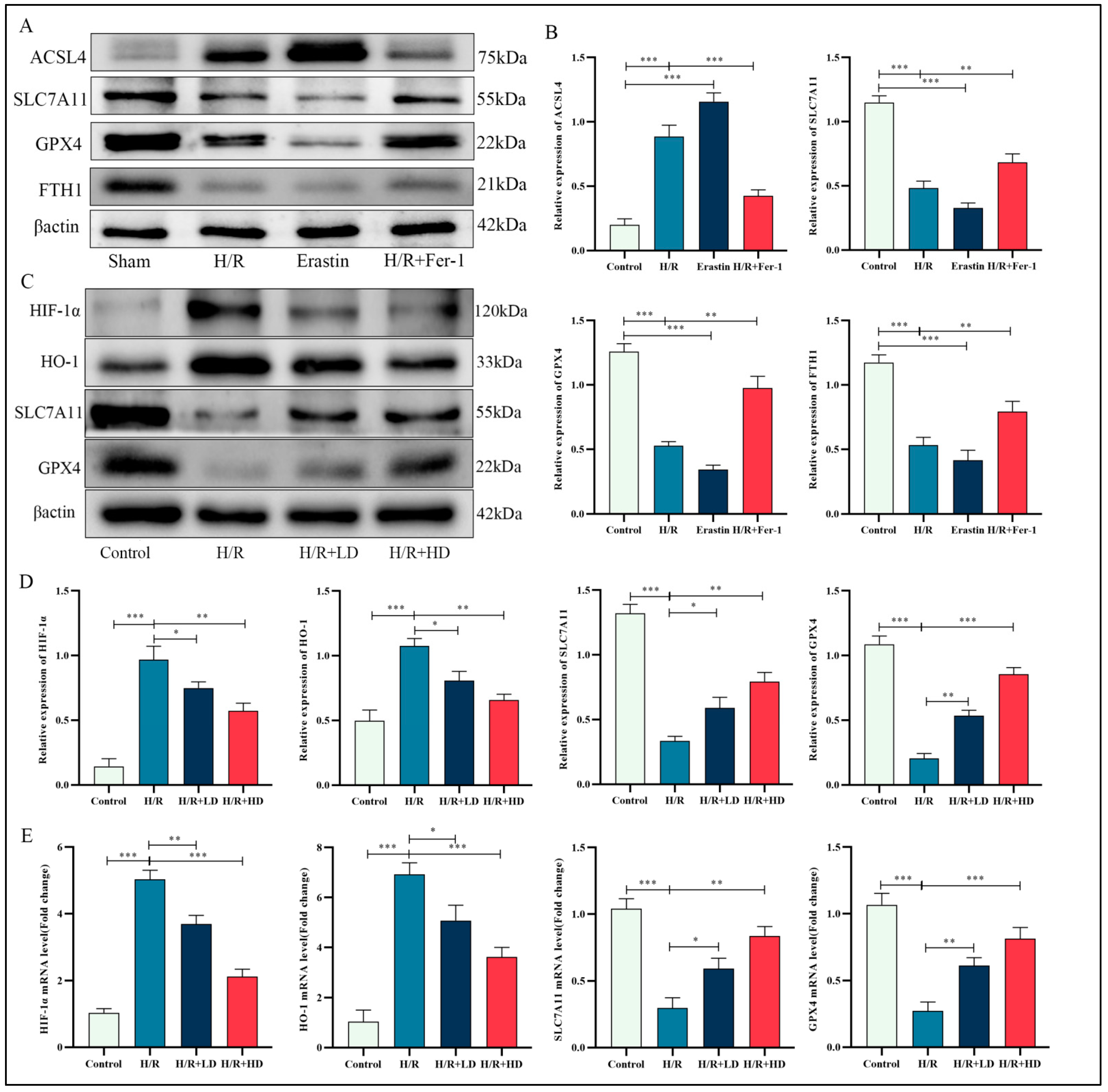
3.2. HCA Demonstrated Inhibitory Effects on Ferroptosis in Mice with LIRI
3.3. The Inhibitory Effect of HCA on Ferroptosis in LIRI Was Mediated through the Suppression of Hif-1α and HO-1
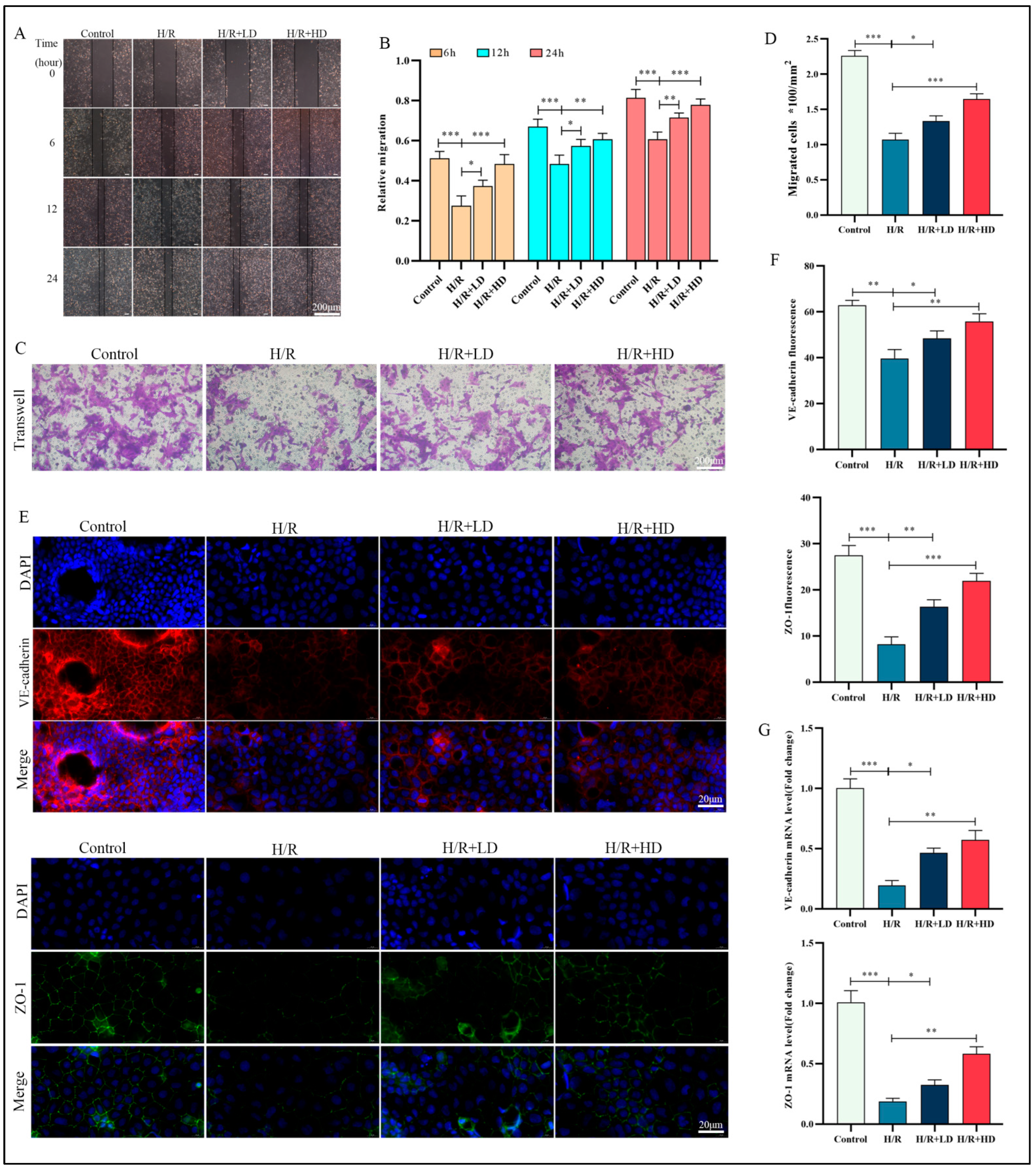
3.4. HCA Enhanced HUVEC Migration Capacity and Ameliorated H/R-Induced Impairment of Endothelial Barrier Function
3.5. Enhanced Protection against H/R-Induced Oxidative Stress in HUVECs Is Observed upon Administration of HCA
3.6. HCA Regulated Proteins Associated with Ferroptosis in HUVECs
3.7. The Effect of HCA on H/R-Induced Ferroptosis in HUVECs Was Abolished by Overexpression of HIF-1α
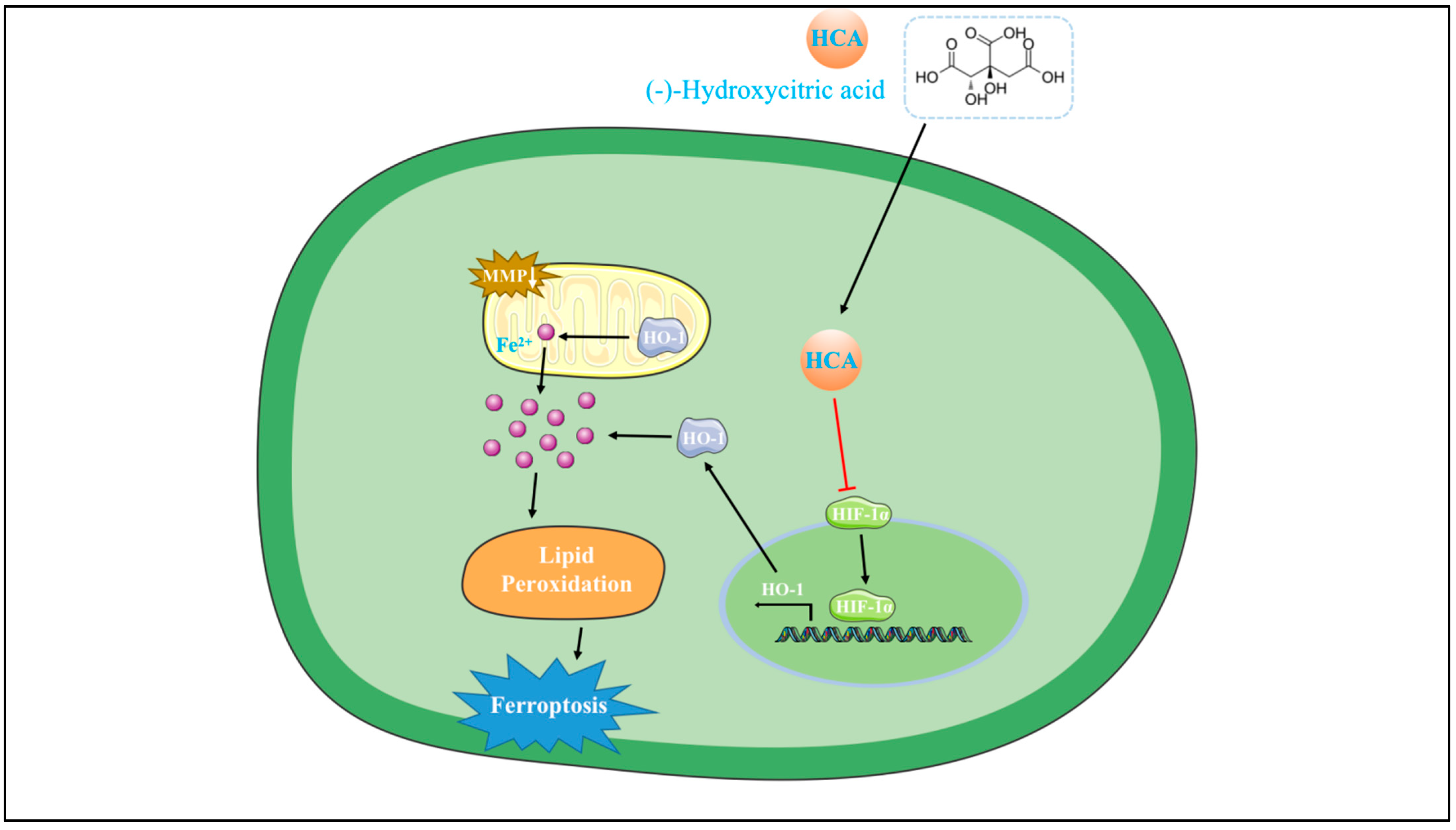
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shepherd, H.M.; Gauthier, J.M.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Innate immunity in lung transplantation. J. Heart Lung Transplant. 2021, 40, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ Transplant. 2016, 21, 246–252. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Ischemia-Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021, 28, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, D.; Ren, M.; Zhang, H.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 2021, 27, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.J.; Luo, X.J.; Tu, H.; Chen, H.; Xiong, X.M.; Li, N.S.; Peng, J. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 401–410. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia cambogia for weight loss. Am. J. Health Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and biochemistry of (−)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Mokhlis, H.A.; Sharaky, M.; Sobhy, M.H.; Hassanein, S.S.; Doghish, A.S.; Salama, S.A.; Mariee, A.D.; Attia, Y.M. Hydroxycitric acid reverses tamoxifen resistance through inhibition of ATP citrate lyase. Pathol. Res. Pract. 2022, 240, 154211. [Google Scholar] [CrossRef]
- Goudarzvand, M.; Afraei, S.; Yaslianifard, S.; Ghiasy, S.; Sadri, G.; Kalvandi, M.; Alinia, T.; Mohebbi, A.; Yazdani, R.; Azarian, S.K.; et al. Hydroxycitric acid ameliorates inflammation and oxidative stress in mouse models of multiple sclerosis. Neural Regen. Res. 2016, 11, 1610–1616. [Google Scholar] [PubMed]
- Liu, X.; Yuan, P.; Sun, X.; Chen, Z. Hydroxycitric Acid Inhibits Renal Calcium Oxalate Deposition by Reducing Oxidative Stress and Inflammation. Curr. Mol. Med. 2020, 20, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Chen, W.R.; Xia, F.; Liu, H.; Meng, X.W.; Zhang, J.; Liu, H.Y.; Xia, Z.Y.; Ji, F.H. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1α signalling. J. Cell. Mol. Med. 2020, 24, 850–861. [Google Scholar] [CrossRef]
- Sharma, A.K.; LaPar, D.J.; Stone, M.L.; Zhao, Y.; Kron, I.L.; Laubach, V.E. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 2255–2267. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Sheikh, M.S.A.; Almaeen, A.; Alduraywish, A.; Alomair, B.M.; Salma, U.; Fei, L.; Yang, T.L. Overexpression of miR-126 Protects Hypoxic-Reoxygenation-Exposed HUVEC Cellular Injury through Regulating LRP6 Expression. Oxid. Med. Cell. Longev. 2022, 2022, 3647744. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Shi, H.; Li, Q.; Zhou, S.; Chen, L. Rhoifolin ameliorates osteoarthritis via the Nrf2/NF-κB axis: In vitro and in vivo experiments. Osteoarthr. Cartil. 2022, 30, 735–745. [Google Scholar] [CrossRef]
- Xia, F.; Chen, H.; Jin, Z.; Fu, Z. Apelin-13 protects the lungs from ischemia-reperfusion injury by attenuating inflammatory and oxidative stress. Hum. Exp. Toxicol. 2021, 40, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. [Google Scholar] [CrossRef] [PubMed]
- Jungraithmayr, W. Novel Strategies for Endothelial Preservation in Lung Transplant Ischemia-Reperfusion Injury. Front. Physiol. 2020, 11, 581420. [Google Scholar] [CrossRef] [PubMed]
- Nisha, V.M.; Priyanka, A.; Anusree, S.S.; Raghu, K.G. (-)-Hydroxycitric acid attenuates endoplasmic reticulum stress-mediated alterations in 3T3-L1 adipocytes by protecting mitochondria and downregulating inflammatory markers. Free Radic. Res. 2014, 48, 1386–1396. [Google Scholar] [CrossRef]
- Maciel, R.A.P.; Cunha, R.S.; Busato, V.; Franco, C.R.C.; Gregório, P.C.; Dolenga, C.J.R.; Nakao, L.S.; Massy, Z.A.; Boullier, A.; Pecoits-Filho, R.; et al. Uremia Impacts VE-Cadherin and ZO-1 Expression in Human Endothelial Cell-to-Cell Junctions. Toxins 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Yao, Y.; Yang, Z.; Ma, H. (-)-Hydroxycitric Acid Suppresses Lipid Droplet Accumulation and Accelerates Energy Metabolism via Activation of the Adiponectin-AMPK Signaling Pathway in Broiler Chickens. J. Agric. Food Chem. 2019, 67, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Tuo, Q.Z.; Lei, P.; Jackman, K.A.; Li, X.L.; Xiong, H.; Li, X.L.; Liuyang, Z.Y.; Roisman, L.; Zhang, S.T.; Ayton, S.; et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 2017, 22, 1520–1530. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Xiao, J.; Shang, J.; Tan, Q.; Ping, F.; Huang, W.; Wu, F.; Zhang, H.; Zhang, X. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, M.; Jiang, L.; Wang, L.; Yang, Y.; Wang, Q.; Qian, X.; Zhao, Y.; Qian, J. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed. Pharmacother. 2022, 154, 113572. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, L.; Tang, P.; Chen, D.; Li, Y.; Li, J.; Bao, C. Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 2021, 47, 52. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Feinman, R.; Deitch, E.A.; Watkins, A.C.; Abungu, B.; Colorado, I.; Kannan, K.B.; Sheth, S.U.; Caputo, F.J.; Lu, Q.; Ramanathan, M.; et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G833–G843. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Gong, X.; Guo, Q.; Zhao, H.; Wang, L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J. Ethnopharmacol. 2019, 228, 70–81. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.-L.; Song, C.-K.; Zou, S.-S.; Pan, S.-Z.; Lai, K.; Li, N.; Geng, Q. Hydroxycitric Acid Alleviated Lung Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Ferroptosis through the Hif-1α Pathway. Curr. Issues Mol. Biol. 2023, 45, 9868-9886. https://doi.org/10.3390/cimb45120616
Lu Z-L, Song C-K, Zou S-S, Pan S-Z, Lai K, Li N, Geng Q. Hydroxycitric Acid Alleviated Lung Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Ferroptosis through the Hif-1α Pathway. Current Issues in Molecular Biology. 2023; 45(12):9868-9886. https://doi.org/10.3390/cimb45120616
Chicago/Turabian StyleLu, Zi-Long, Cong-Kuan Song, Shi-Shi Zou, Shi-Ze Pan, Kai Lai, Ning Li, and Qing Geng. 2023. "Hydroxycitric Acid Alleviated Lung Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Ferroptosis through the Hif-1α Pathway" Current Issues in Molecular Biology 45, no. 12: 9868-9886. https://doi.org/10.3390/cimb45120616
APA StyleLu, Z.-L., Song, C.-K., Zou, S.-S., Pan, S.-Z., Lai, K., Li, N., & Geng, Q. (2023). Hydroxycitric Acid Alleviated Lung Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Ferroptosis through the Hif-1α Pathway. Current Issues in Molecular Biology, 45(12), 9868-9886. https://doi.org/10.3390/cimb45120616






