Amelioration of Motor Performance and Nigrostriatal Dopamine Cell Volume Using a Novel Far-Infrared Ceramic Blanket in an A53T Alpha-Synuclein Transgenic Parkinson’s Disease Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Biosafety, Biosecurity, and Institutional Safety Procedures
2.3. Animals
2.4. Far-Field Infrared Ceramic Blanket
2.5. Rotarod
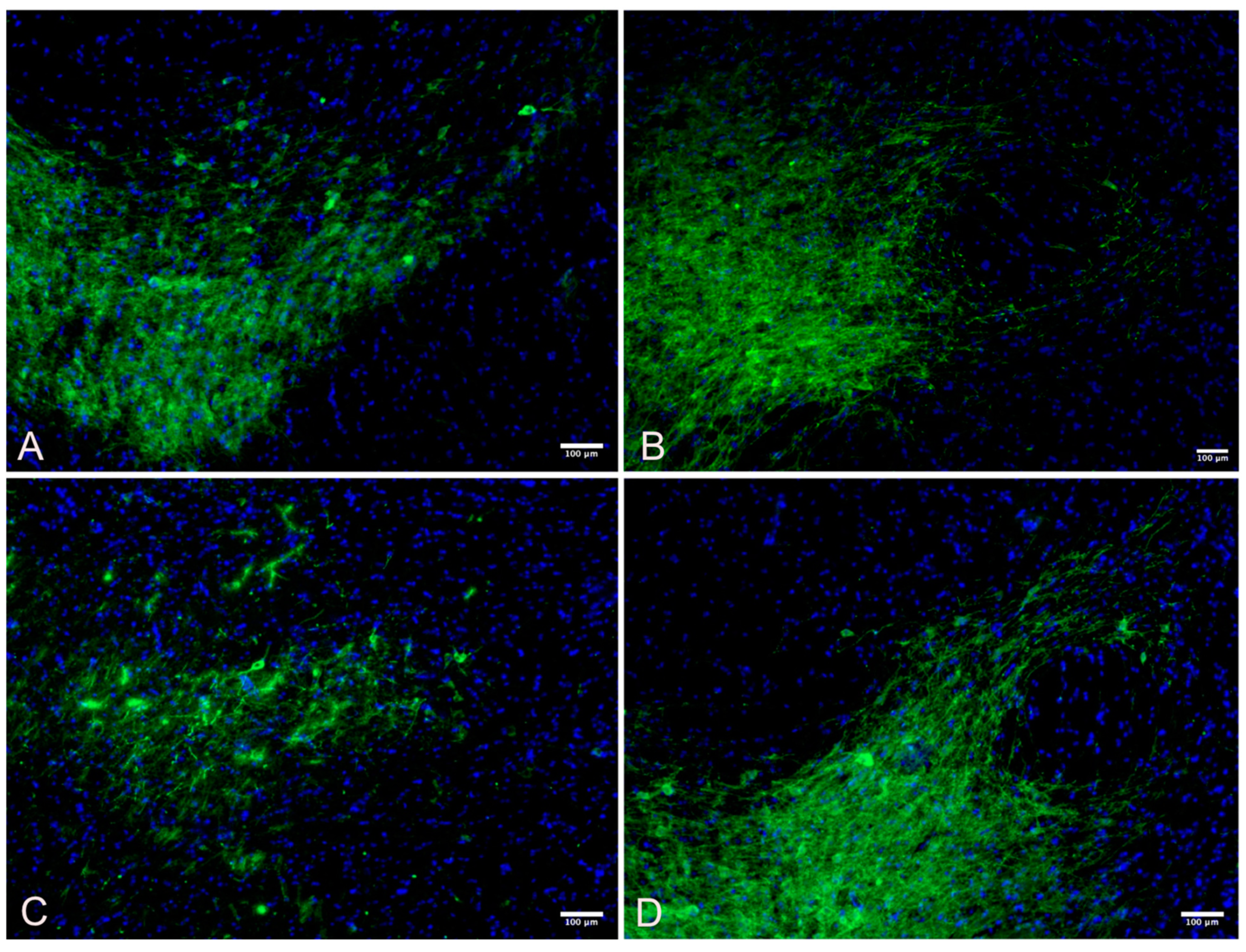
2.6. Immunohistochemistry
2.7. Quantification of Immunofluorescent Imaging
2.8. Statistical Analysis
3. Results
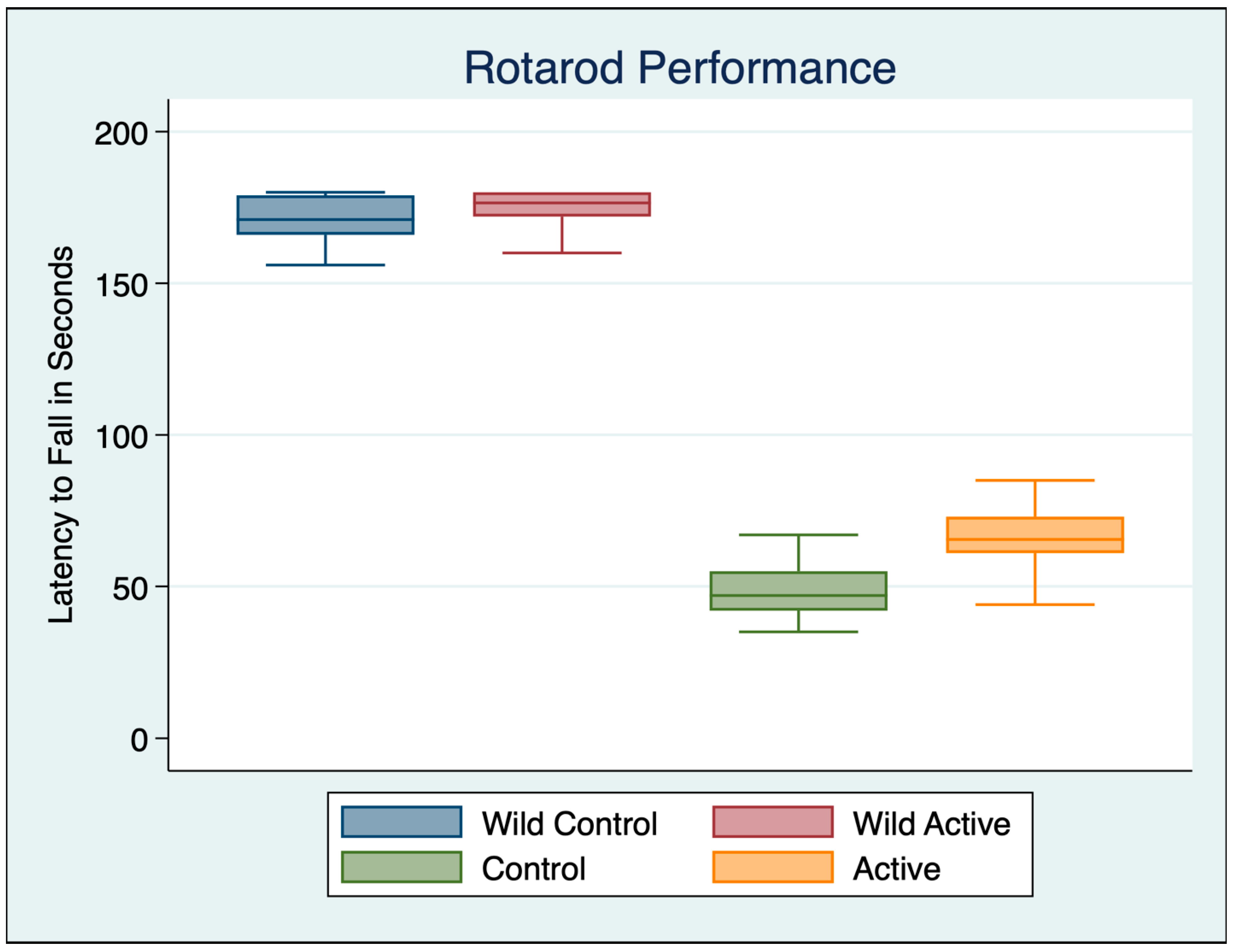
3.1. Rotarod Motor Performance
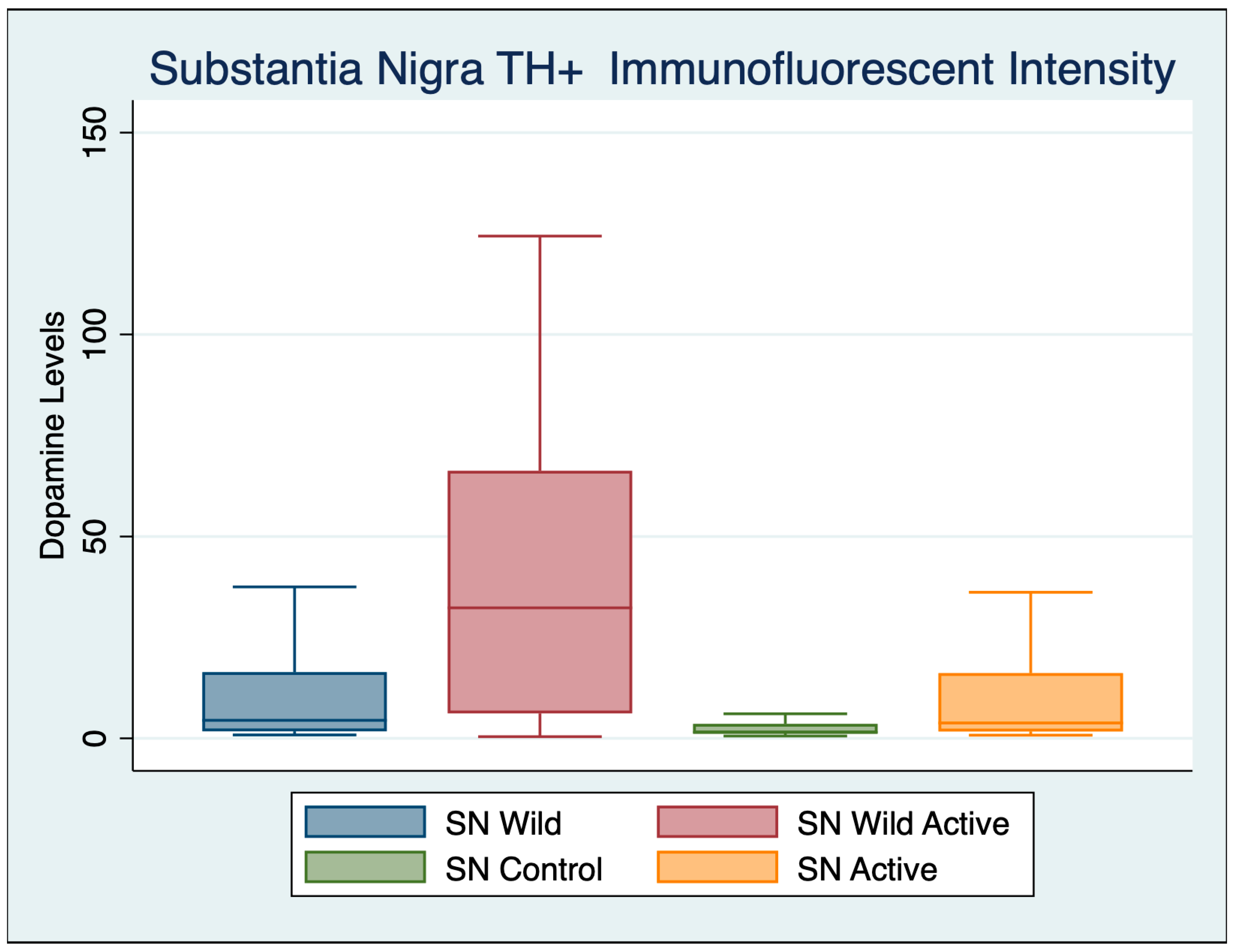
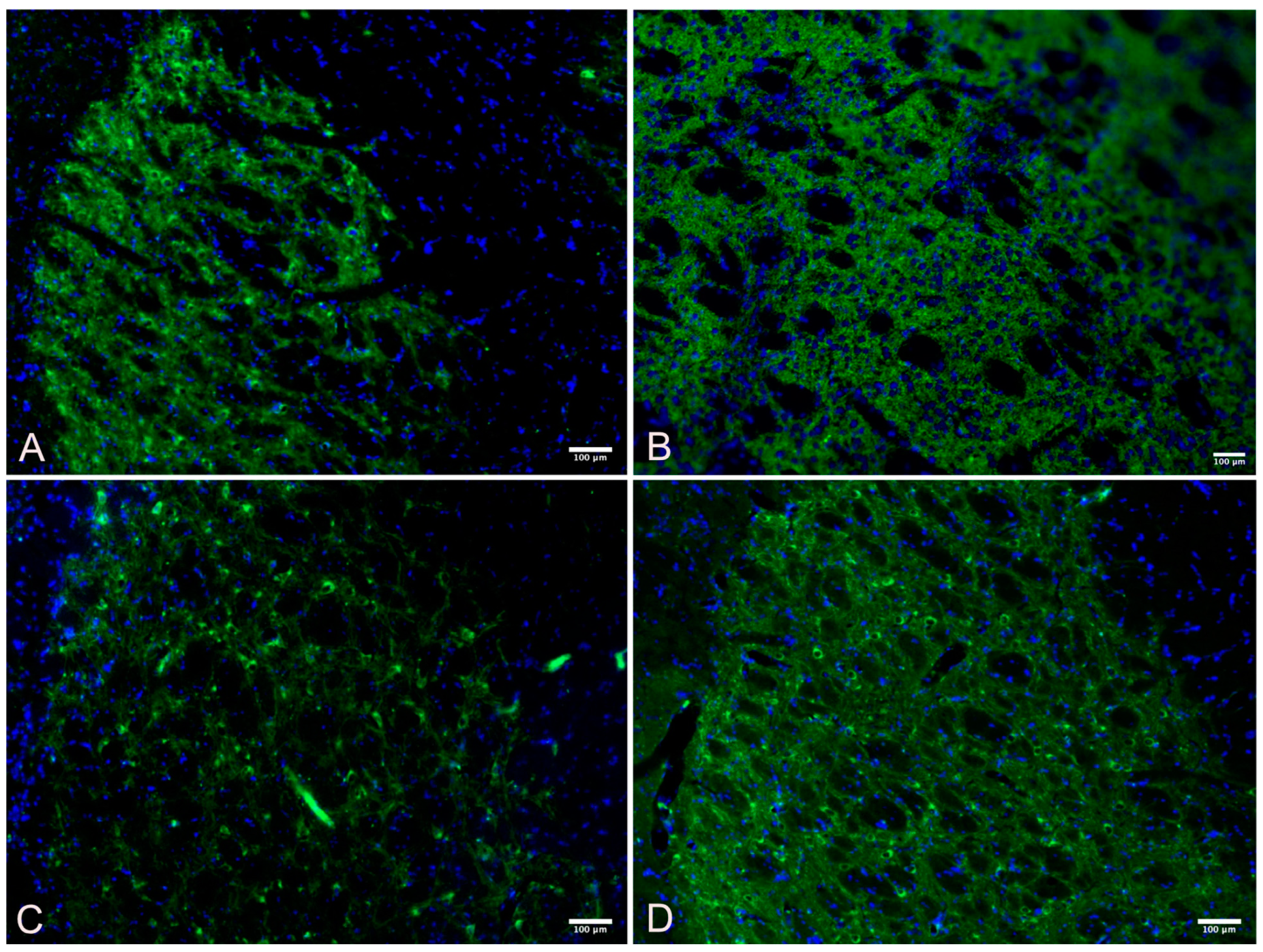
3.2. Substantia Nigra
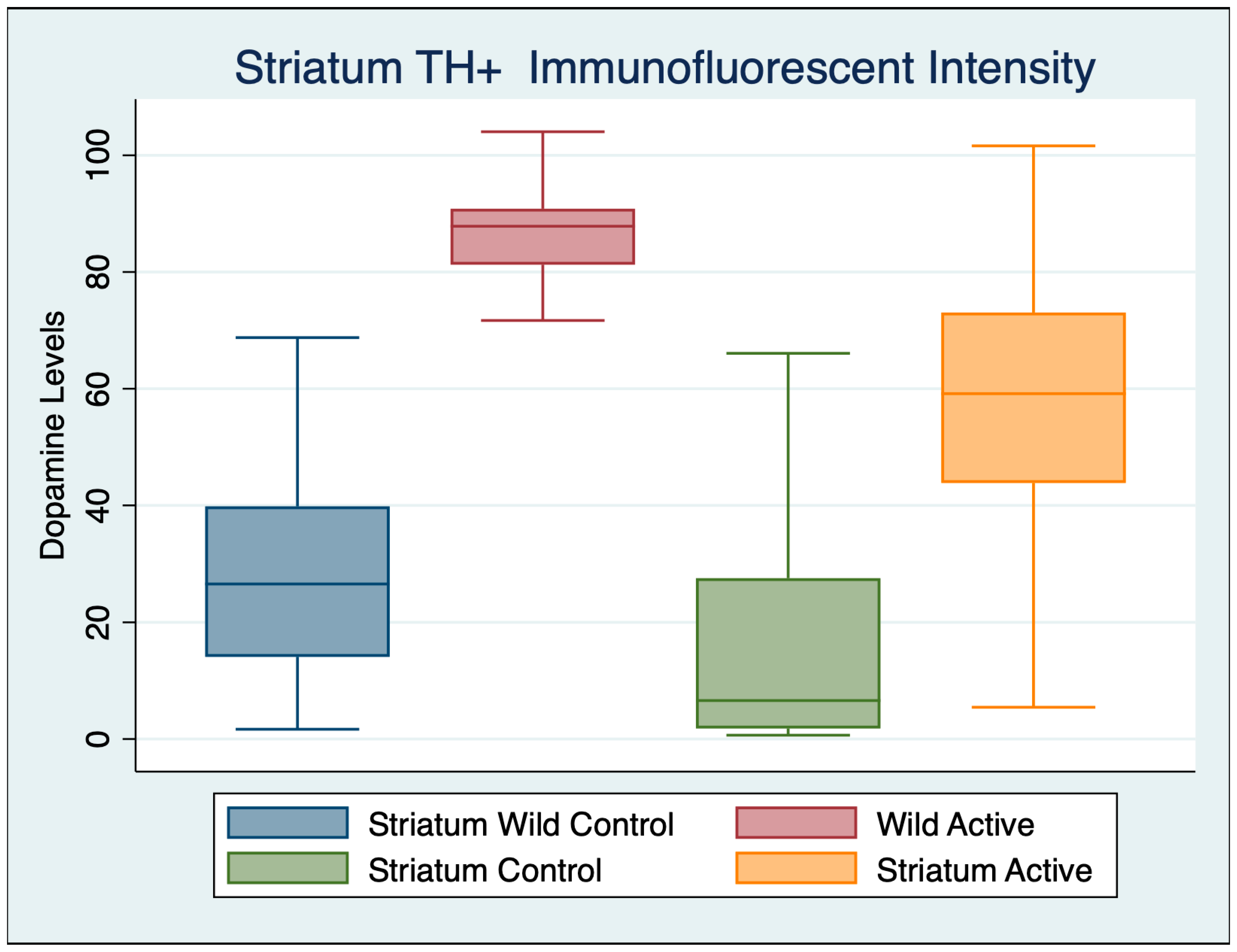
3.3. Striatum
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrick, F.R.; Valerio, L.S.A.; Gonzalez-Vega, M.N.; Engel, D.; Sugaya, K. Accelerated Wound Healing Using a Novel Far-Infrared Ceramic Blanket. Life 2021, 11, 878. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Wey Han, S.A.; Semilan, S.L.A.; Hong, P.K. Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. Int. J. Environ. Res. Public Health 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236; discussion 222. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, L.; Jin, L.; Liu, C.; Liang, Y.; Ren, L.; Liu, Y.; Liu, Y.; Liu, S.; et al. Novel naturally occurring autoantibodies attenuate α-synuclein pathology in a mouse model of Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2023, 49, e12860. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Trojanowski, J.Q. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron 2006, 52, 33–38. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Chen, H.; Lei, H.; Xu, Q. Neuronal activity pattern defects in the striatum in awake mouse model of Parkinson’s disease. Behav. Brain Res. 2018, 341, 135–145. [Google Scholar] [CrossRef]
- Choi, K.; Holly, E.N.; Davatolhagh, M.F.; Beier, K.T.; Fuccillo, M.V. Integrated anatomical and physiological mapping of striatal afferent projections. Eur. J. Neurosci. 2019, 49, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Zhai, S.; Cui, Q.; Simmons, D.V. Rethinking the network determinants of motor disability in Parkinson’s disease. Front. Synaptic Neurosci. 2023, 15, 1186484. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huerta, V.G.; Denton, J.A.; Nakano, Y.; Jaidar, O.; Garcia-Munoz, M.; Arbuthnott, G.W. Striatal bilateral control of skilled forelimb movement. Cell Rep. 2021, 34, 108651. [Google Scholar] [CrossRef]
- Lin, C.P.; Knoop, L.E.J.; Frigerio, I.; Bol, J.; Rozemuller, A.J.M.; Berendse, H.W.; Pouwels, P.J.W.; van de Berg, W.D.J.; Jonkman, L.E. Nigral Pathology Contributes to Microstructural Integrity of Striatal and Frontal Tracts in Parkinson’s Disease. Mov. Disord. 2023, 38, 1655–1667. [Google Scholar] [CrossRef]
- Zhang, Y.; Burock, M.A. Diffusion Tensor Imaging in Parkinson’s Disease and Parkinsonian Syndrome: A Systematic Review. Front. Neurol. 2020, 11, 531993. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Masliah, E. Transgenic models of alpha-synuclein pathology: Past, present, and future. Ann. N. Y. Acad. Sci. 2003, 991, 171–188. [Google Scholar] [CrossRef]
- Ásgrímsdóttir, E.S.; Arenas, E. Midbrain Dopaminergic Neuron Development at the Single Cell Level: In vivo and in Stem Cells. Front. Cell Dev. Biol. 2020, 8, 463. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Peng, Q.; Zhong, S.; Tan, Y.; Zeng, W.; Wang, J.; Cheng, C.; Yang, X.; Wu, Y.; Cao, X.; Xu, Y. The Rodent Models of Dyskinesia and Their Behavioral Assessment. Front. Neurol. 2019, 10, 1016. [Google Scholar] [CrossRef]
- Geibl, F.F.; Henrich, M.T.; Oertel, W.H. Mesencephalic and extramesencephalic dopaminergic systems in Parkinson’s disease. J. Neural Transm. 2019, 126, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Shen, W.; Graves, S.M.; Surmeier, D.J. Dopaminergic modulation of striatal function and Parkinson’s disease. J. Neural Transm. 2019, 126, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Olivas, M.; Wu, L.; Noyce, A. Disruption of Mitochondrial Complex I Induces Progressive Parkinsonism. Mov. Disord. 2022, 37, 478. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656. [Google Scholar] [CrossRef]
- Bridi, J.C.; Hirth, F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Vrancx, C.; Del Vecchio, M.; Swinnen, J.V.; Agostinis, P.; Winderickx, J.; Vangheluwe, P.; Annaert, W. Inter-organellar Communication in Parkinson’s and Alzheimer’s Disease: Looking Beyond Endoplasmic Reticulum-Mitochondria Contact Sites. Front. Neurosci. 2022, 16, 900338. [Google Scholar] [CrossRef]
- Mani, S.; Sevanan, M.; Krishnamoorthy, A.; Sekar, S. A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurol. Sci. 2021, 42, 4459–4469. [Google Scholar] [CrossRef]
- Di Martino, R.; Sisalli, M.J.; Sirabella, R.; Della Notte, S.; Borzacchiello, D.; Feliciello, A.; Annunziato, L.; Scorziello, A. Ncx3-Induced Mitochondrial Dysfunction in Midbrain Leads to Neuroinflammation in Striatum of A53t-α-Synuclein Transgenic Old Mice. Int. J. Mol. Sci. 2021, 22, 8177. [Google Scholar] [CrossRef]
- Milanese, C.; Payán-Gómez, C.; Galvani, M.; Molano González, N.; Tresini, M.; Nait Abdellah, S.; van Roon-Mom, W.M.C.; Figini, S.; Marinus, J.; van Hilten, J.J.; et al. Peripheral mitochondrial function correlates with clinical severity in idiopathic Parkinson’s disease. Mov. Disord. 2019, 34, 1192–1202. [Google Scholar] [CrossRef]
- Walski, T.; Dabrowska, K.; Drohomirecka, A.; Jedruchniewicz, N.; Trochanowska-Pauk, N.; Witkiewicz, W.; Komorowska, M. The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes. Int. J. Radiat. Biol. 2019, 95, 1326–1336. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Quinn, S.M.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 2002, 34, 521–533. [Google Scholar] [CrossRef] [PubMed]
- da Costa Daniele, T.M.; de Bruin, P.F.C.; de Matos, R.S.; de Bruin, G.S.; Maia Chaves, C.J.; de Bruin, V.M.S. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model-A systematic review and meta-analysis. Behav. Brain Res. 2020, 383, 112488. [Google Scholar] [CrossRef] [PubMed]
- Nhu, N.T.; Cheng, Y.J.; Lee, S.D. Effects of Treadmill Exercise on Neural Mitochondrial Functions in Parkinson’s Disease: A Systematic Review of Animal Studies. Biomedicines 2021, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.M.; Hung, S.Y. Current Therapies in Clinical Trials of Parkinson’s Disease: A 2021 Update. Pharmaceuticals 2021, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, L.; Zhang, X. Reprogramming Glial Cells into Functional Neurons for Neuro-regeneration: Challenges and Promise. Neurosci. Bull. 2021, 37, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.L.; Armstrong, S.M. Fine-tuning the circadian system with light treatment for Parkinson’s disease: An in-depth, critical review. Rev. Neurosci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K.; Sibambo, S.R. Trends in the molecular pathogenesis and clinical therapeutics of common neurodegenerative disorders. Int. J. Mol. Sci. 2009, 10, 2510–2557. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tian, J.; Yu, B.; Xu, Y.; Feng, Q. A bone-like nano-hydroxyapatite/collagen loaded injectable scaffold. Biomed. Mater. 2009, 4, 055005. [Google Scholar] [CrossRef]
- Janarthanan, G.; Kim, I.G.; Chung, E.J.; Noh, I. Comparative studies on thin polycaprolactone-tricalcium phosphate composite scaffolds and its interaction with mesenchymal stem cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yu, Y.; Oh, S.Y.; Wang, K.K.; Kim, Y.R.; Jung, S.C.; Kim, H.S.; Jo, I. Far-Infrared Irradiation Inhibits Adipogenic Differentiation and Stimulates Osteogenic Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells: Role of Protein Phosphatase 2B. Cell. Physiol. Biochem. 2019, 52, 240–253. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Xue, J.; Wu, J.; Chang, J.; Wu, C. Defective Black Nano-Titania Thermogels for Cutaneous Tumor-Induced Therapy and Healing. Nano Lett. 2019, 19, 2138–2147. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, Q.; Zhang, Y.; Zhuang, H.; Wang, E.; Xu, Q.; Ma, L.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a Multifunctional Biomaterial Inspired by Ancient Chinese Medicine for Hair Regeneration in Burned Skin. ACS Appl. Mater. Interfaces 2020, 12, 12489–12499. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.T.; Soh, S.H.; Oh, M.W.; Shim, S.; Yoo, H.S. Evaluation of the immunobiological effects of a regenerative far-infrared heating system in pigs. J. Vet. Sci. 2019, 20, e61. [Google Scholar] [CrossRef] [PubMed]
- Iova, O.M.; Marin, G.E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders-An Overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Xiang, B.; Fang, H.; Zhang, B.; Wang, Y.; Zhuo, Y.; Deng, D.; Wang, X. Biomimetic Biomembrane Encapsulation and Targeted Delivery of a Nitric Oxide Release Platform for Therapy of Parkinson’s Disease. ACS Biomater. Sci. Eng. 2023, 9, 2545–2557. [Google Scholar] [CrossRef]
- Wang, L.; Dan, Q.; Xu, B.; Chen, Y.; Zheng, T. Research progress on gas signal molecular therapy for Parkinson’s disease. Open Life Sci. 2023, 18, 20220658. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Yamamoto, T.; Nakamura, K.; Uenishi, T.; Asai, T.; Kita, M.; Kanamura, N. Effects of far infrared radiation by isotropic high-density carbon on the human oral mucosa. Arch. Oral Biol. 2018, 94, 62–68. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Du, F.; Wang, Y.; Wu, Y.; Lim, K.L.; Li, L.; Yang, N.; Yu, C.; Zhang, C. Linking Heat Shock Protein 70 and Parkin in Parkinson’s Disease. Mol. Neurobiol. 2023, 60, 7044–7059. [Google Scholar] [CrossRef] [PubMed]
- Aridon, P.; Geraci, F.; Turturici, G.; D’Amelio, M.; Savettieri, G.; Sconzo, G. Protective role of heat shock proteins in Parkinson’s disease. Neurodegener. Dis. 2011, 8, 155–168. [Google Scholar] [CrossRef]
- Vendredy, L.; Adriaenssens, E.; Timmerman, V. Small heat shock proteins in neurodegenerative diseases. Cell Stress Chaperones 2020, 25, 679–699. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Flint Beal, M. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013, 63, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Foo, A.S.C.; Soong, T.W.; Yeo, T.T.; Lim, K.L. Mitochondrial Dysfunction and Parkinson’s Disease-Near-Infrared Photobiomodulation as a Potential Therapeutic Strategy. Front. Aging Neurosci. 2020, 12, 89. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, H.; Zhang, H.; Han, Y.; Yuan, J.; Wang, T.; Gao, Y.; Li, Z. Ameliorating Mitochondrial Dysfunction of Neurons by Biomimetic Targeting Nanoparticles Mediated Mitochondrial Biogenesis to Boost the Therapy of Parkinson’s Disease. Adv. Sci. 2023, 10, e2300758. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, Y.W.; Lee, D.; Kim, D.; Kim, K.; Kim, T.; Baek, C.; Lee, Y.; Lee, J.; Lee, H.; et al. Far-infrared rays enhance mitochondrial biogenesis and GLUT3 expression under low glucose conditions in rat skeletal muscle cells. Korean J. Physiol. Pharmacol. 2021, 25, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, Y.W.; Kim, J.H.; Yang, M.; Bae, H.; Lim, I.; Bang, H.; Go, K.C.; Yang, G.W.; Rho, Y.H.; et al. Improvement Characteristics of Bio-active Materials Coated Fabric on Rat Muscular Mitochondria. Korean J. Physiol. Pharmacol. 2015, 19, 283–289. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Q.-T.; Oristian, D.S.; Lu, C.P.; Zheng, Q.; Wang, H.-W.; Fuchs, E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell 2011, 144, 341–352. [Google Scholar] [CrossRef]
- Watanabe, T.; Noritake, J.; Kaibuchi, K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005, 15, 76–83. [Google Scholar] [CrossRef]
- Kodama, A.; Karakesisoglou, I.; Wong, E.; Vaezi, A.; Fuchs, E. ACF7: An essential integrator of microtubule dynamics. Cell 2003, 115, 343–354. [Google Scholar] [CrossRef]
- Facchin, F.; Canaider, S.; Tassinari, R.; Zannini, C.; Bianconi, E.; Taglioli, V.; Olivi, E.; Cavallini, C.; Tausel, M.; Ventura, C. Physical energies to the rescue of damaged tissues. World J. Stem Cells 2019, 11, 297. [Google Scholar] [CrossRef]
| Variable | Obs | Mean | Std. Dev. | Min | Max |
|---|---|---|---|---|---|
| Wild Control | 30 | 169.800 | 10.480 | 137 | 180 |
| Wild Active | 30 | 173.933 | 6.817 | 160 | 180 |
| Control | 30 | 48.967 | 9.891 | 35 | 78 |
| Active | 30 | 66.067 | 9.336 | 44 | 85 |
| ANOVA Multiple Comparisons F (3, 116) = 1551 p < 0.001 | Mean Diff | 95% CI | p | Cohen’s d |
|---|---|---|---|---|
| Wild Control vs. Wild Active | −4.133 | −10.54 to 2.269 | 0.5145 | 0.4676 |
| Wild Control vs. Control | 120.8 | 114.4 to 127.2 | <0.001 | 11.8588 |
| Wild Control vs. Active | 103.7 | 97.33 to 110.1 | <0.001 | 10.4523 |
| Wild Active vs. Control | 125 | 118.6 to 131.4 | <0.001 | 14.7119 |
| Wild Active vs. Active | 107.9 | 101.5 to 114.3 | <0.001 | 13.1955 |
| Control vs. Active | −17.1 | −23.5 to −10.7 | <0.001 | 1.7780 |
| Variable | Obs | Mean | Std. Dev. | Min | Max |
|---|---|---|---|---|---|
| Wild Control | 102 | 10.903 | 14.428 | 0.848 | 94.010 |
| Wild Active | 65 | 40.136 | 36.569 | 0.410 | 124.367 |
| Control | 103 | 4.103 | 7.176 | 0.572 | 56.719 |
| Active | 97 | 13.587 | 22.989 | 0.778 | 106.737 |
| ANOVA Multiple Comparisons F (3, 446) = 50.07 p < 0.001 | Mean Diff | 95% CI | p | Cohen’s d |
|---|---|---|---|---|
| Wild Control vs. Wild Active | −29.67 | −37.71 to −21.63 | <0.001 | 1.1500 |
| Wild Control vs. Control | 6.764 | 0.2375 to 13.29 | 0.038 | 0.5976 |
| Wild Control vs. Active | −3.811 | −10.5 to 2.874 | 0.790 | 0.1406 |
| Wild Active vs. Control | 36.43 | 28.41 to 44.46 | <0.001 | 0.3545 |
| Wild Active vs. Active | 25.86 | 17.71 to 34.01 | <0.001 | 0.9095 |
| Control vs. Active | −10.57 | −17.24 to −3.914 | <0.001 | 0.3428 |
| Variable | Obs | Mean | Std. Dev. | Min | Max |
|---|---|---|---|---|---|
| Wild Control | 92 | 27.672 | 17.706 | 1.673 | 85.779 |
| Wild Active | 62 | 86.381 | 7.731 | 51.204 | 104.041 |
| Control | 77 | 19.955 | 25.800 | 0.650 | 101.263 |
| Active | 48 | 56.996 | 22.921 | 5.439 | 101.619 |
| ANOVA Multiple Comparisons F (3, 271) = 203.1 | Mean Diff | 95% CI | p | Cohen’s d |
|---|---|---|---|---|
| Wild Control vs. Wild Active | −59.83 | −68.21 to −51.45 | <0.001 | 4.035203 |
| Wild Control vs. Control | 14.43 | 6.192 to 22.68 | <0.001 | 0.354508 |
| Wild Control vs. Active | −22.39 | −30.37 to −14.41 | <0.001 | 1.493189 |
| Wild Active vs. Control | 74.26 | 65.71 to 82.81 | <0.001 | 0.354508 |
| Wild Active vs. Active | 37.44 | 29.14 to 45.74 | <0.001 | 1.81406 |
| Control vs. Active | −36.82 | −44.98 to −28.66 | <0.001 | 1.497221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrick, F.R.; Hernandez, L.S.A.V.; Sugaya, K. Amelioration of Motor Performance and Nigrostriatal Dopamine Cell Volume Using a Novel Far-Infrared Ceramic Blanket in an A53T Alpha-Synuclein Transgenic Parkinson’s Disease Mouse Model. Curr. Issues Mol. Biol. 2023, 45, 9823-9837. https://doi.org/10.3390/cimb45120613
Carrick FR, Hernandez LSAV, Sugaya K. Amelioration of Motor Performance and Nigrostriatal Dopamine Cell Volume Using a Novel Far-Infrared Ceramic Blanket in an A53T Alpha-Synuclein Transgenic Parkinson’s Disease Mouse Model. Current Issues in Molecular Biology. 2023; 45(12):9823-9837. https://doi.org/10.3390/cimb45120613
Chicago/Turabian StyleCarrick, Frederick Robert, Luis Sebastian Alexis Valerio Hernandez, and Kiminobu Sugaya. 2023. "Amelioration of Motor Performance and Nigrostriatal Dopamine Cell Volume Using a Novel Far-Infrared Ceramic Blanket in an A53T Alpha-Synuclein Transgenic Parkinson’s Disease Mouse Model" Current Issues in Molecular Biology 45, no. 12: 9823-9837. https://doi.org/10.3390/cimb45120613
APA StyleCarrick, F. R., Hernandez, L. S. A. V., & Sugaya, K. (2023). Amelioration of Motor Performance and Nigrostriatal Dopamine Cell Volume Using a Novel Far-Infrared Ceramic Blanket in an A53T Alpha-Synuclein Transgenic Parkinson’s Disease Mouse Model. Current Issues in Molecular Biology, 45(12), 9823-9837. https://doi.org/10.3390/cimb45120613








