Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy
Abstract
:1. Introduction
2. Principles of Antimicrobial PDT
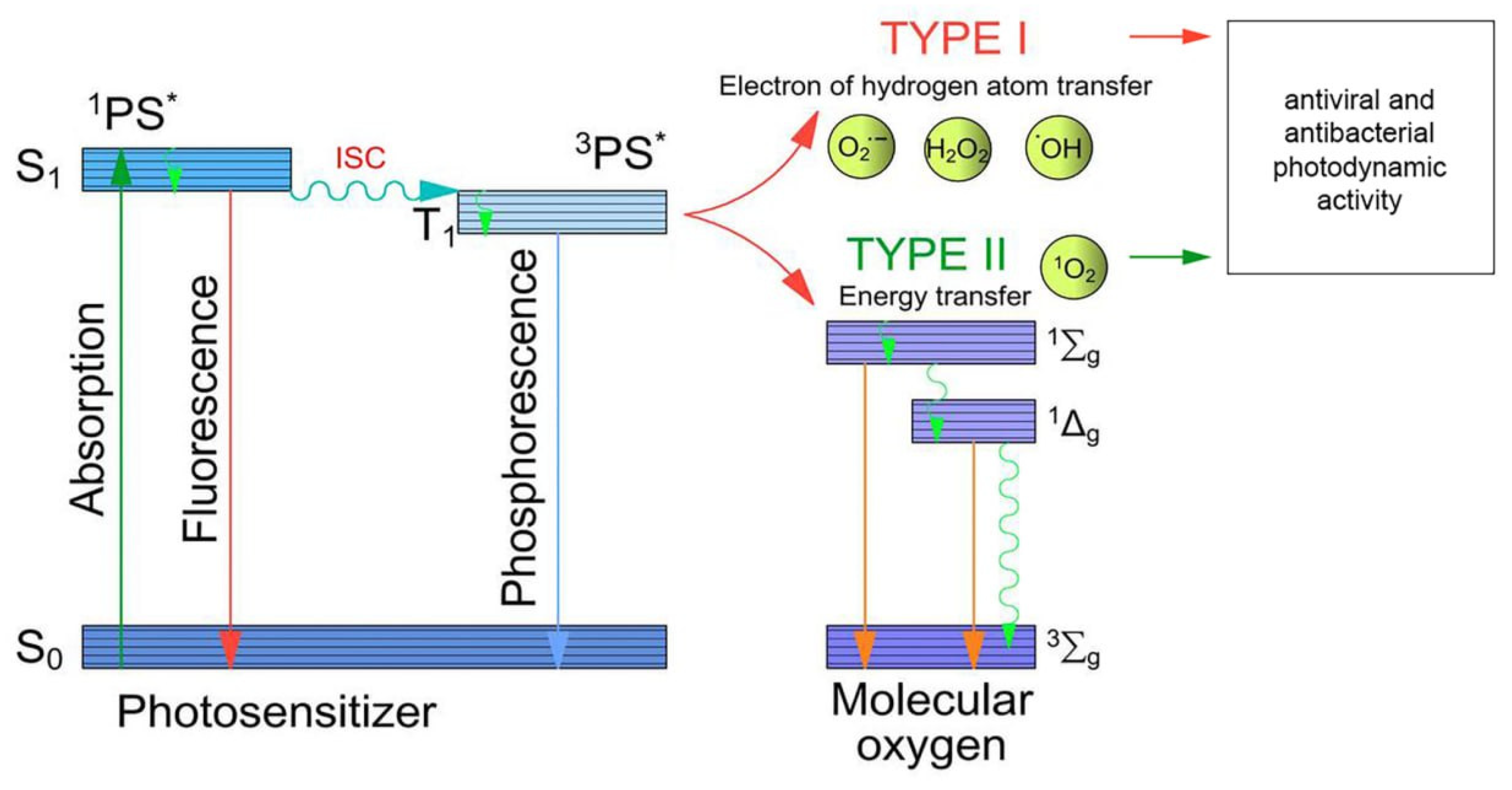
2.1. Mechanisms of Photodynamic Action
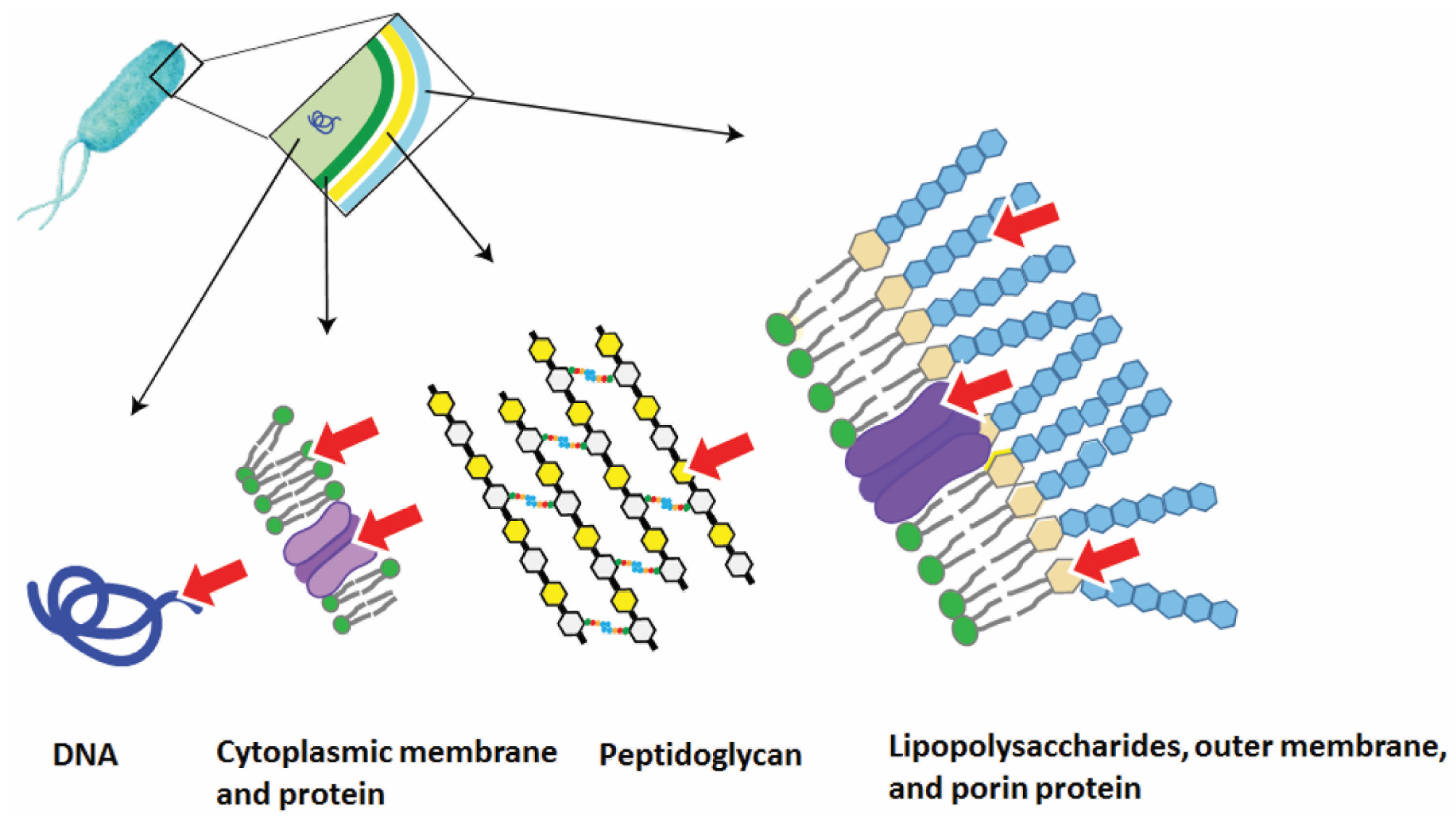
2.2. The Mechanisms of Photodynamic Antibacterial Action
- The PS accumulates in the intercellular space, generates reactive oxygen species, which can subsequently diffuse into the target cells, causing their oxidative damage;
- The PS binds to the structural elements of the bacterial cell due to hydrophobic or Coulombic interactions and transfers its energy to the intracellular biomolecules. This leads to the formation of ROS and the destruction of pathogenic tissues;
- The PS independently penetrates the cell and binds to the intracellular targets, for example, polyanionic macromolecules, such as DNA [55].
2.3. The Mechanisms of Photodynamic Antiviral Action
- The accumulation stage is similar to that of bacteria;
- When affecting viral targets, ROS (generated via type I or type II mechanisms) can have a damaging effect on the viral nucleic acids, capsid proteins, as well as on the receptor proteins and lipoprotein envelope lipids for large viruses [56];
- The negatively charged phospholipids of the virus envelope may play a decisive role in electrostatic interactions with the PS.
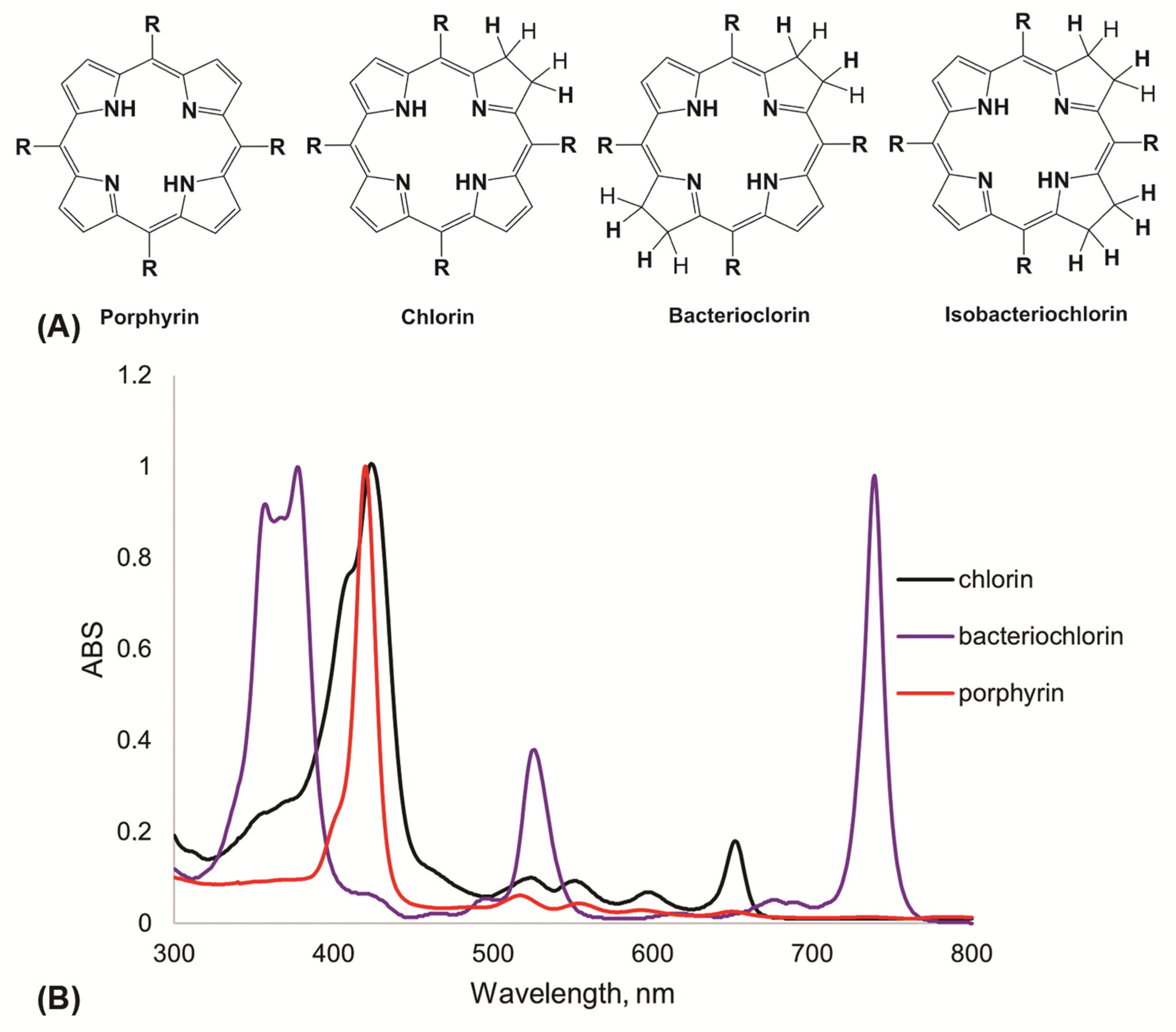
2.4. Photosensitizers for APDT
2.5. Antibacterial and Antiviral Activity of Cationic Porphyrins
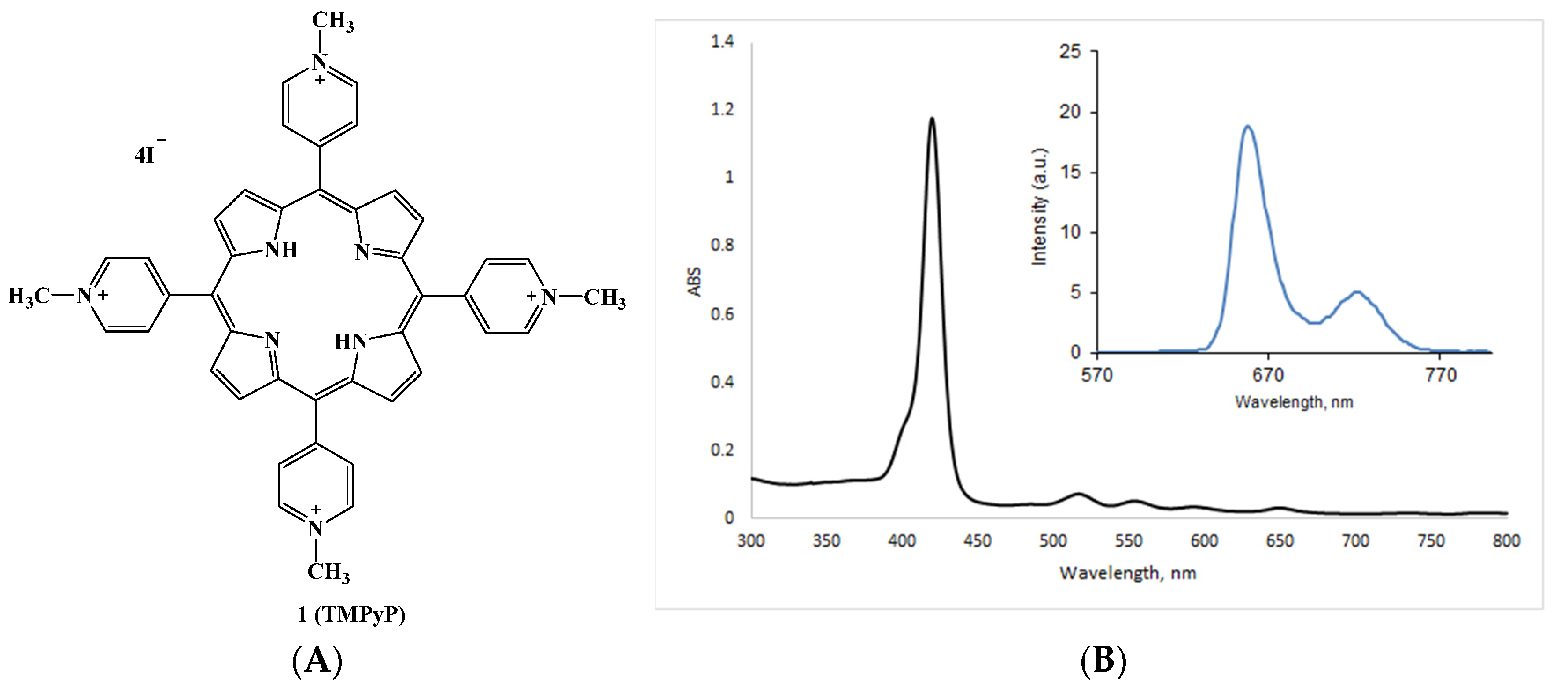
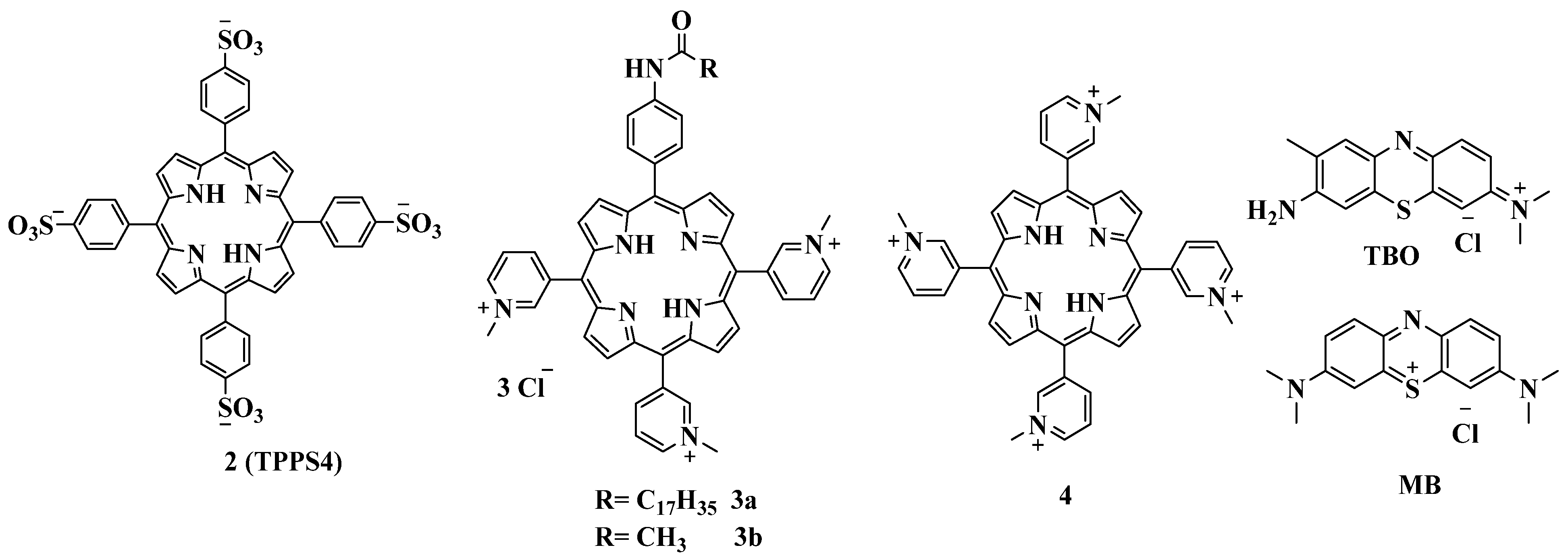
2.5.1. Cationic Porphyrins Based on Tetrapyridylporphyrin (TMPyP) and Its Derivatives
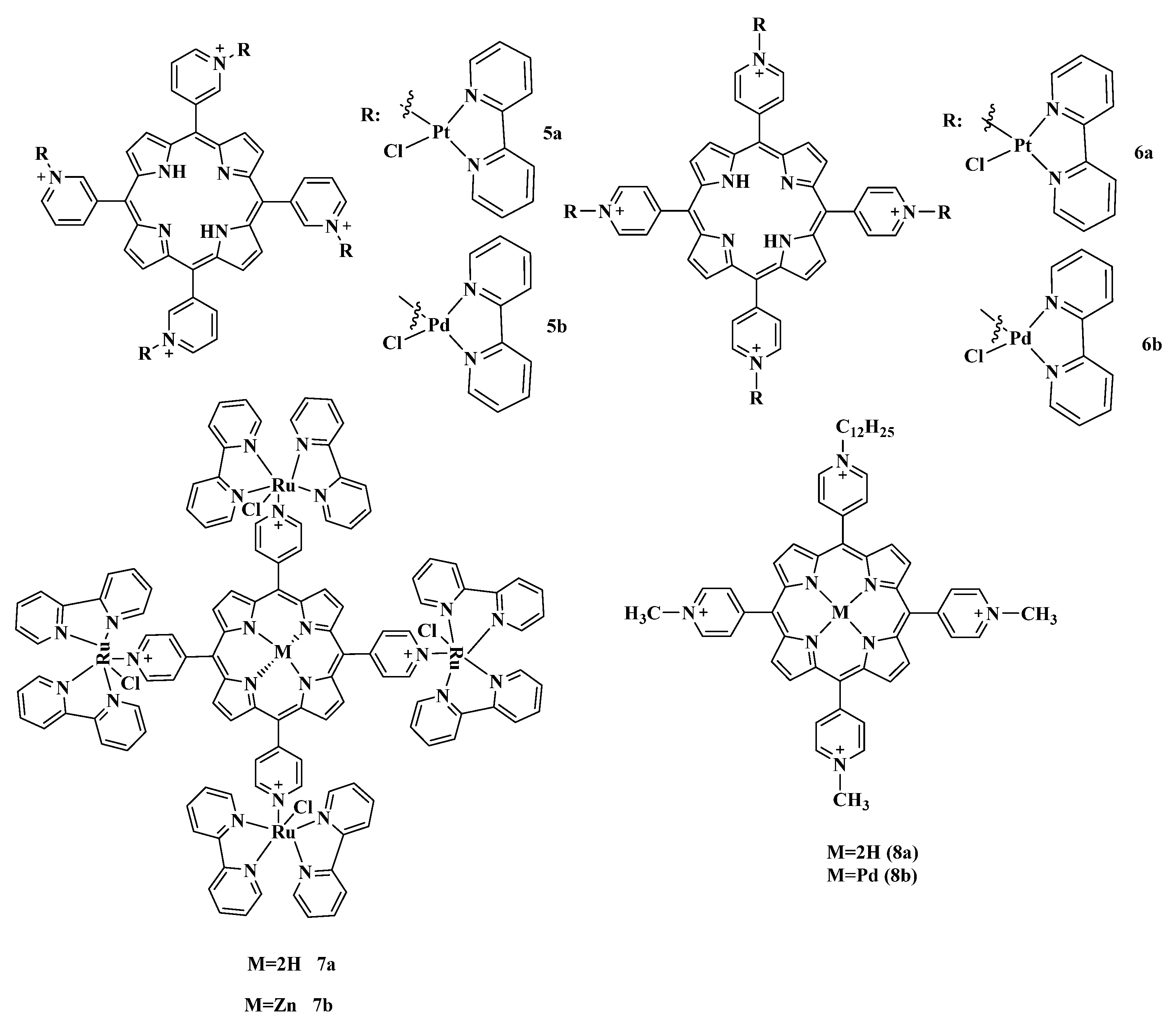
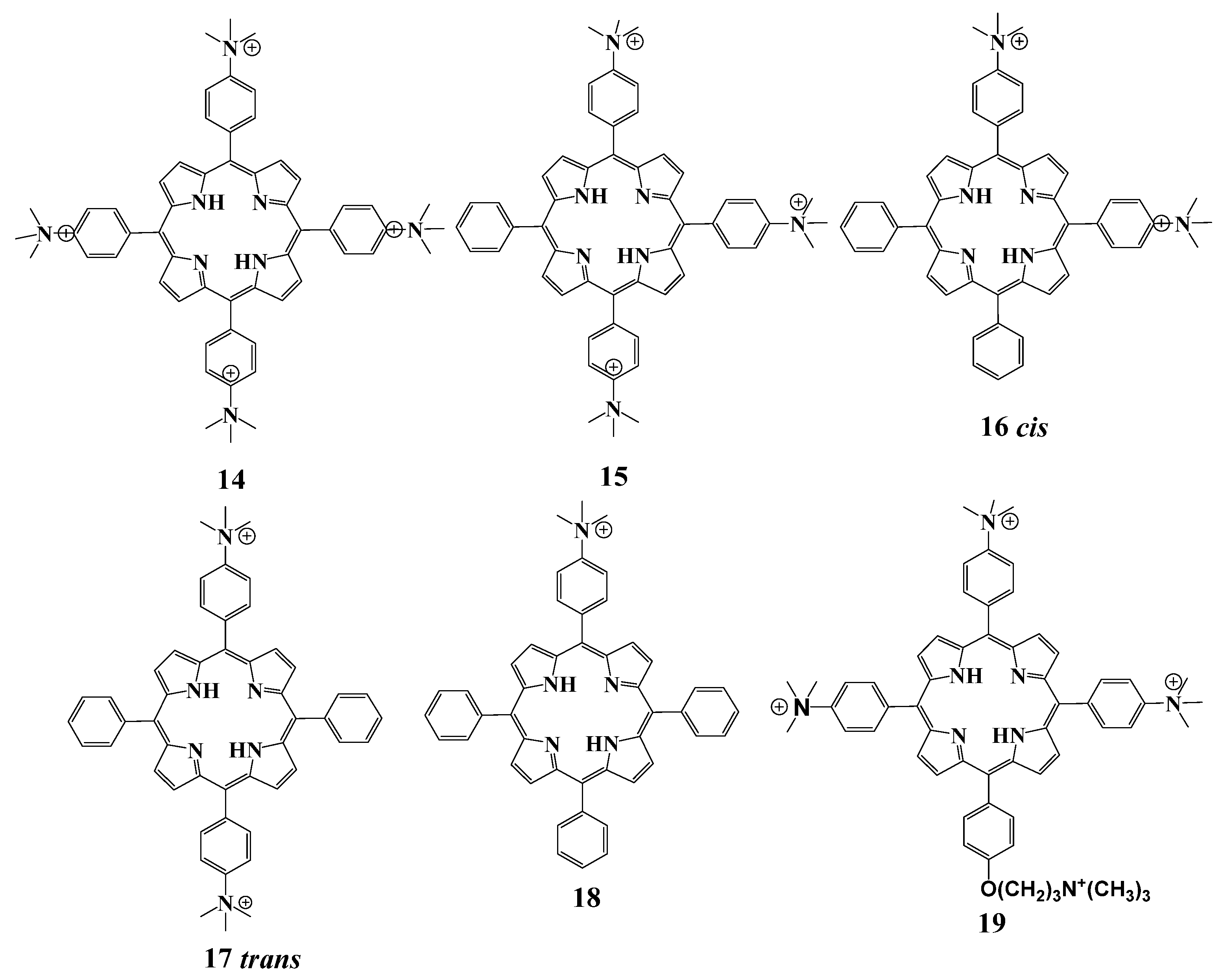
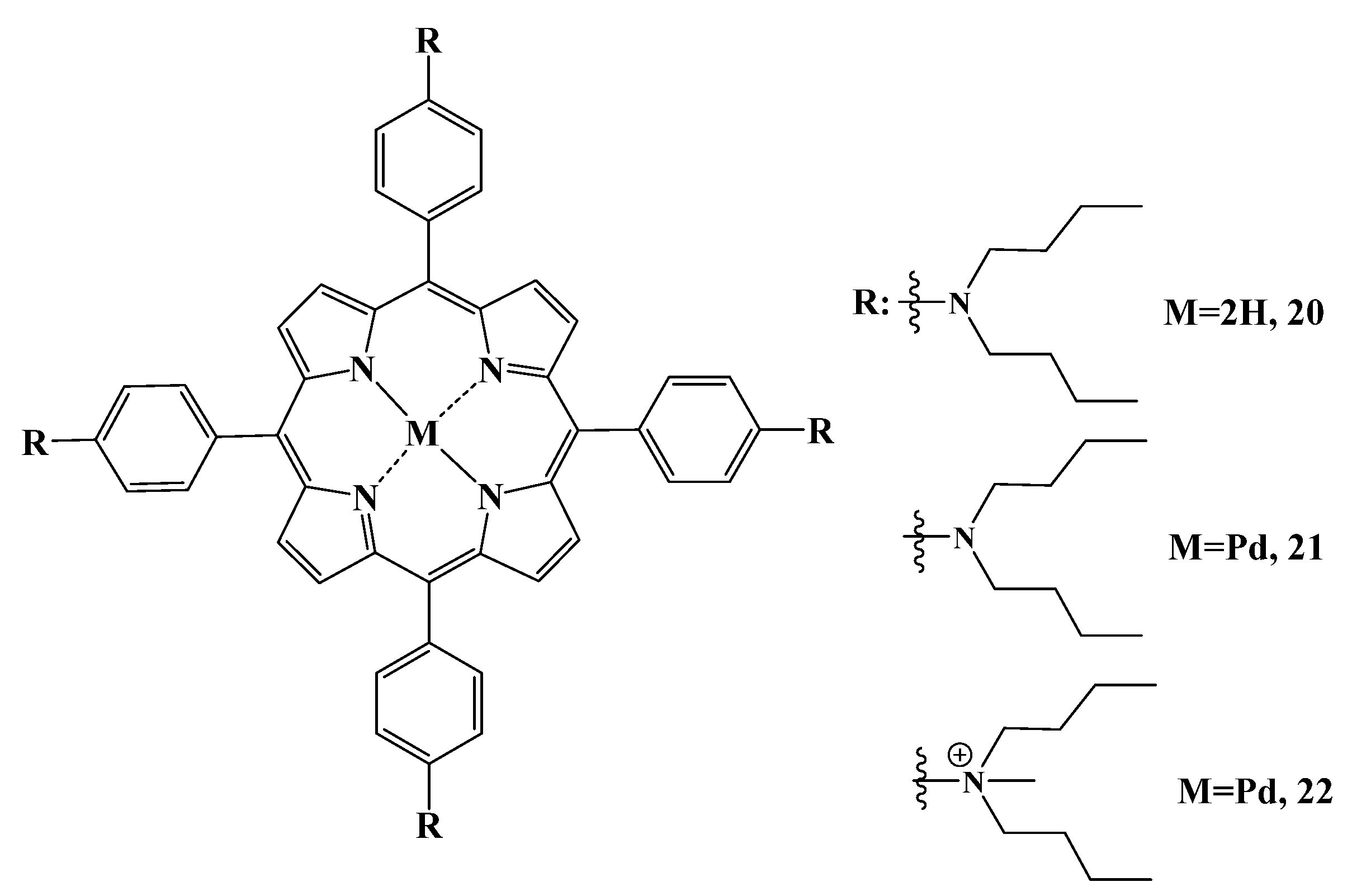
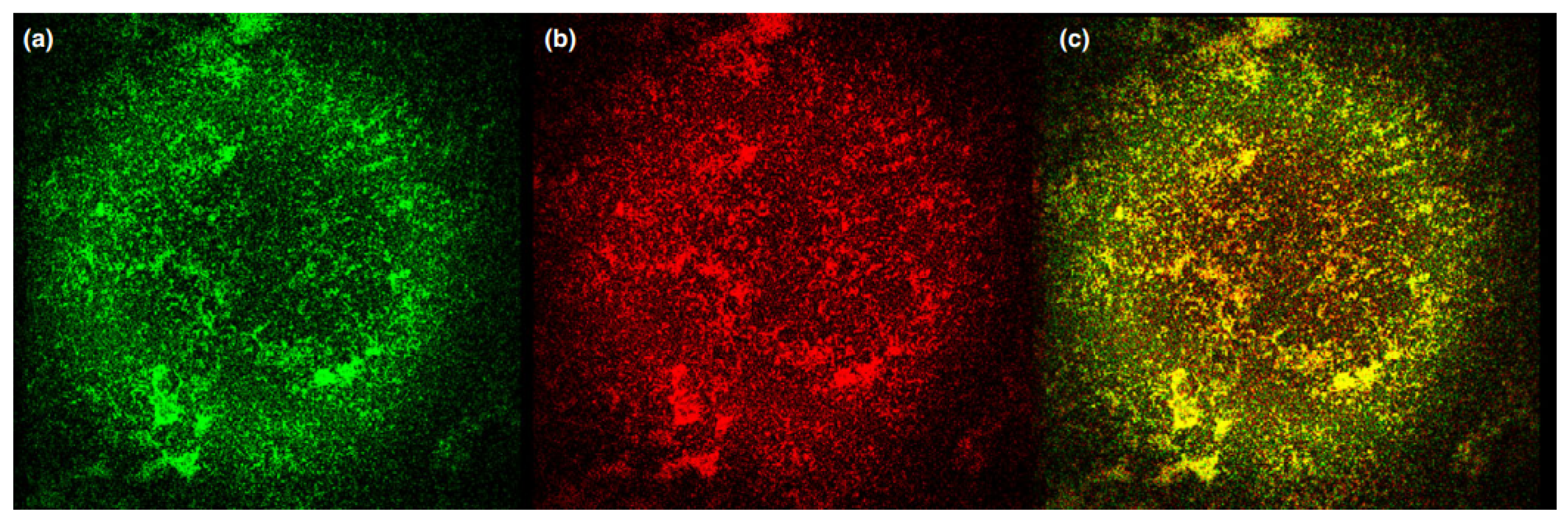
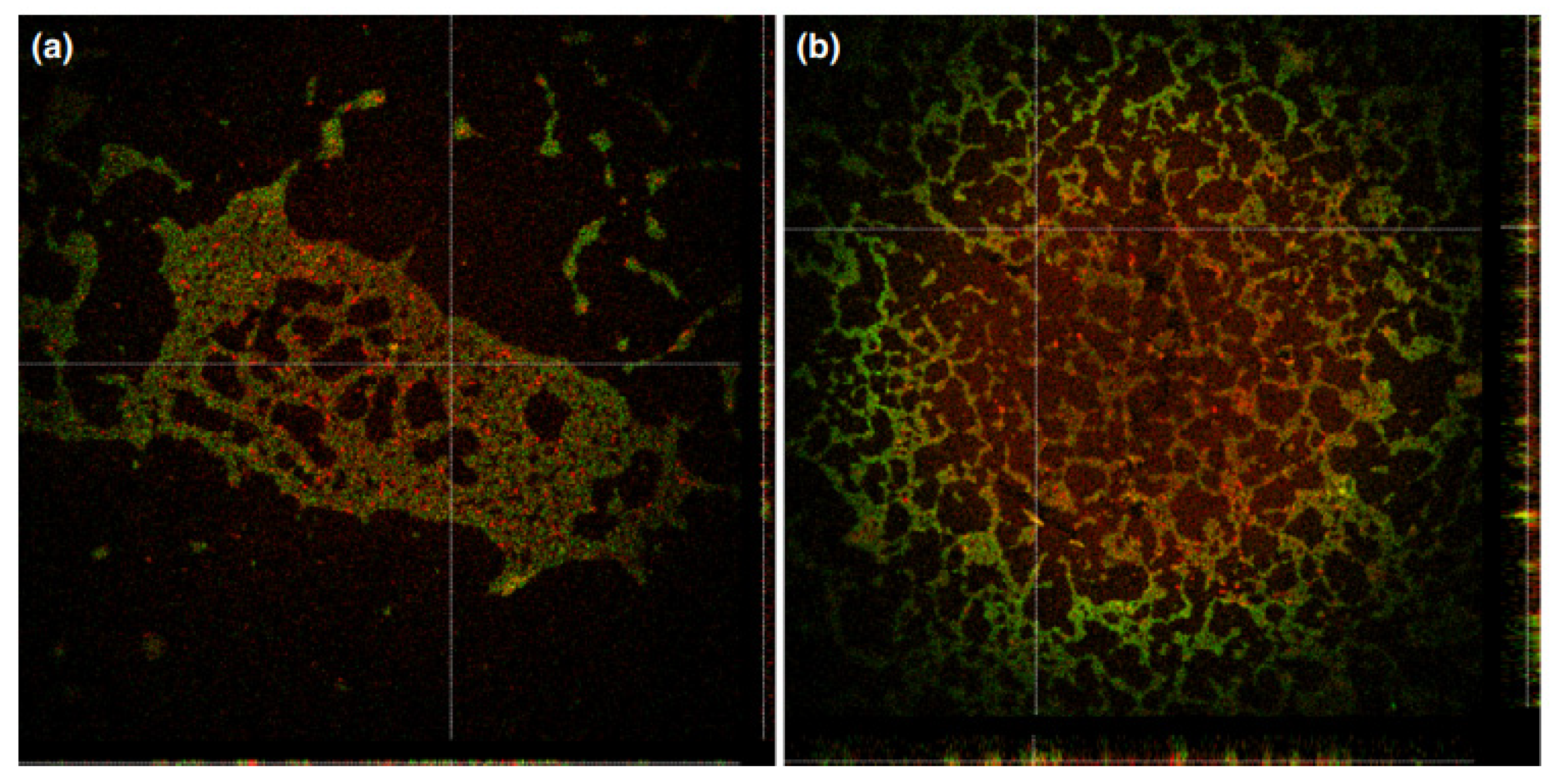
2.5.2. Cationic Ammonium Derivatives
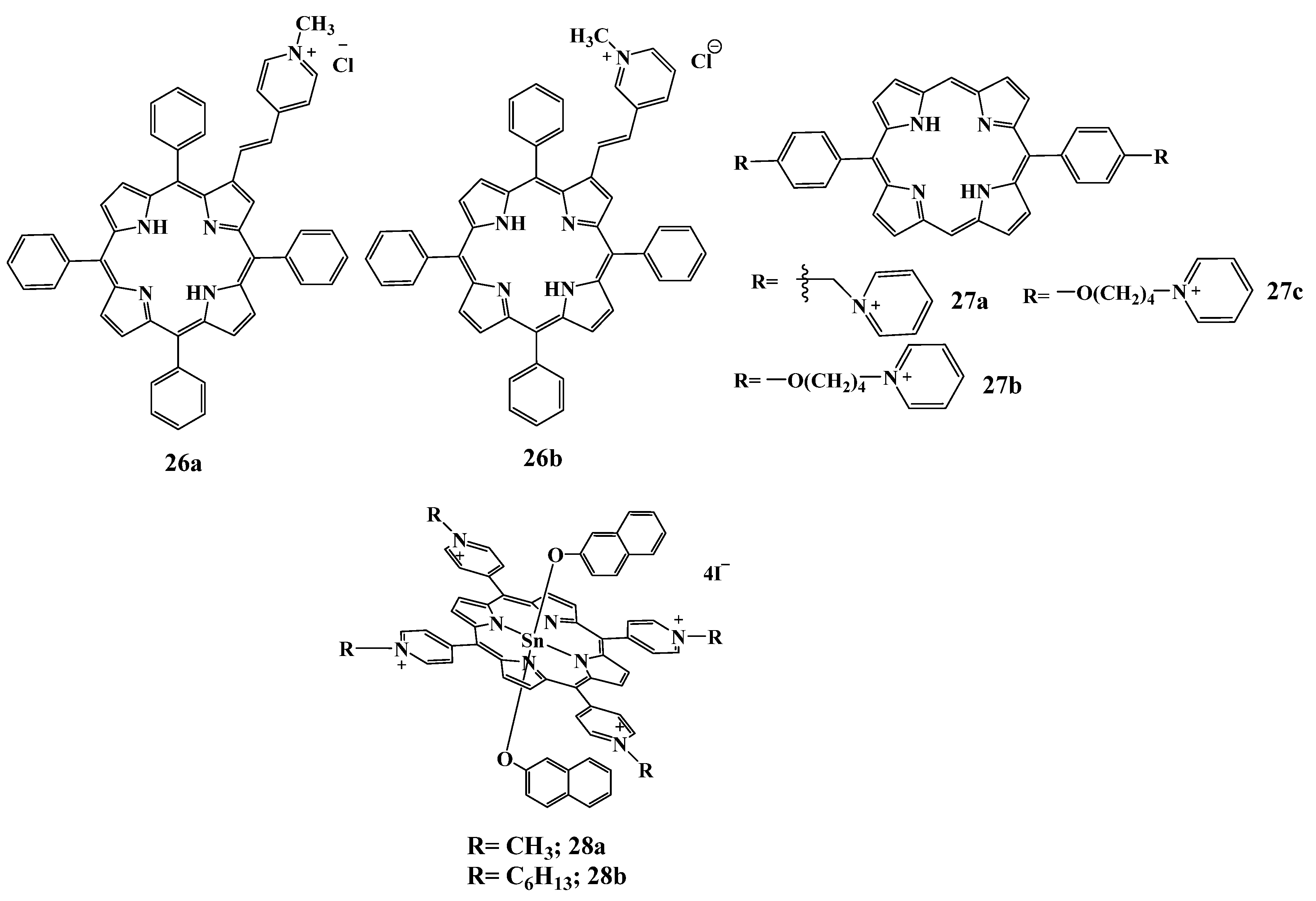
2.5.3. Cationic Imidazolium Derivatives
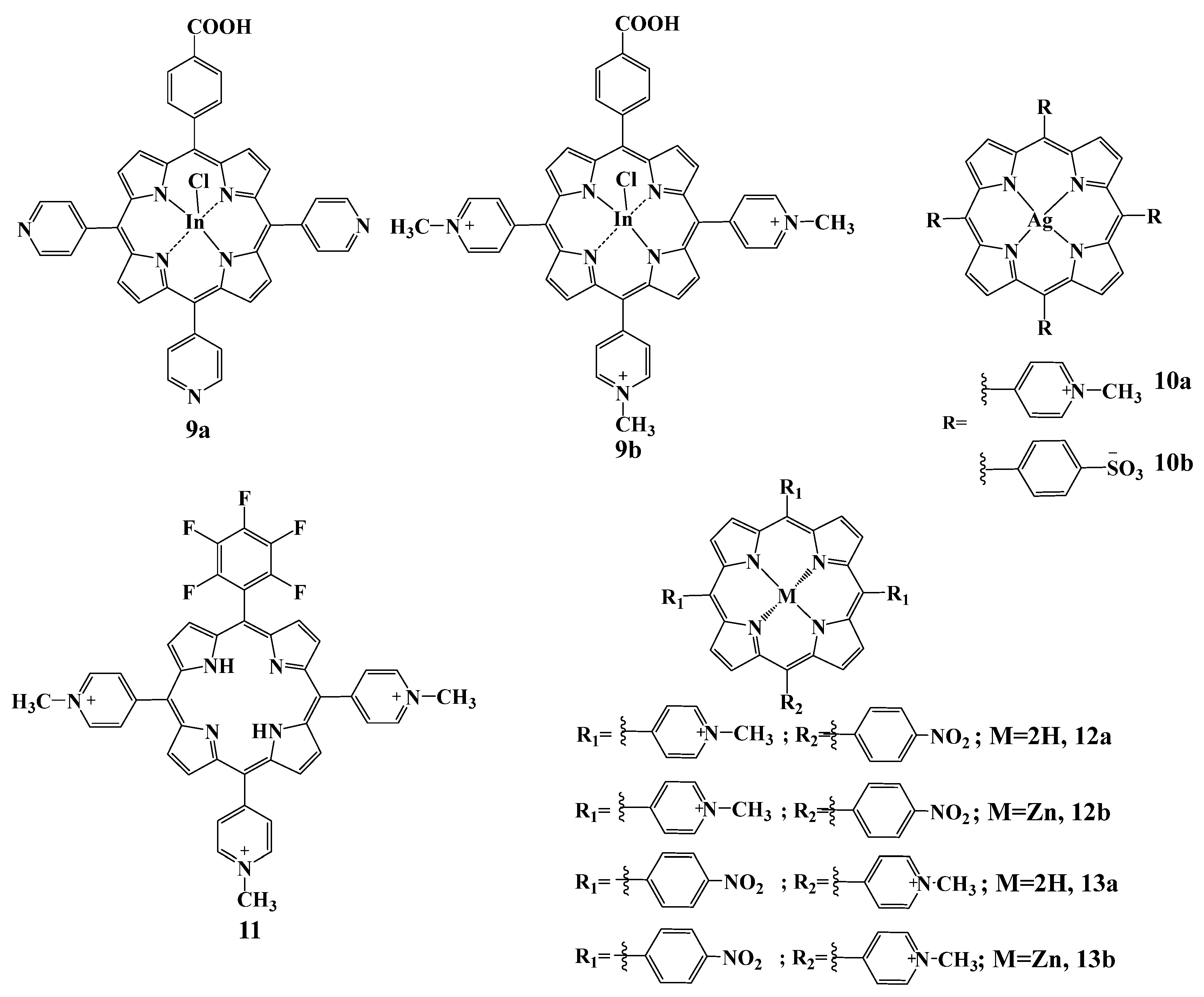
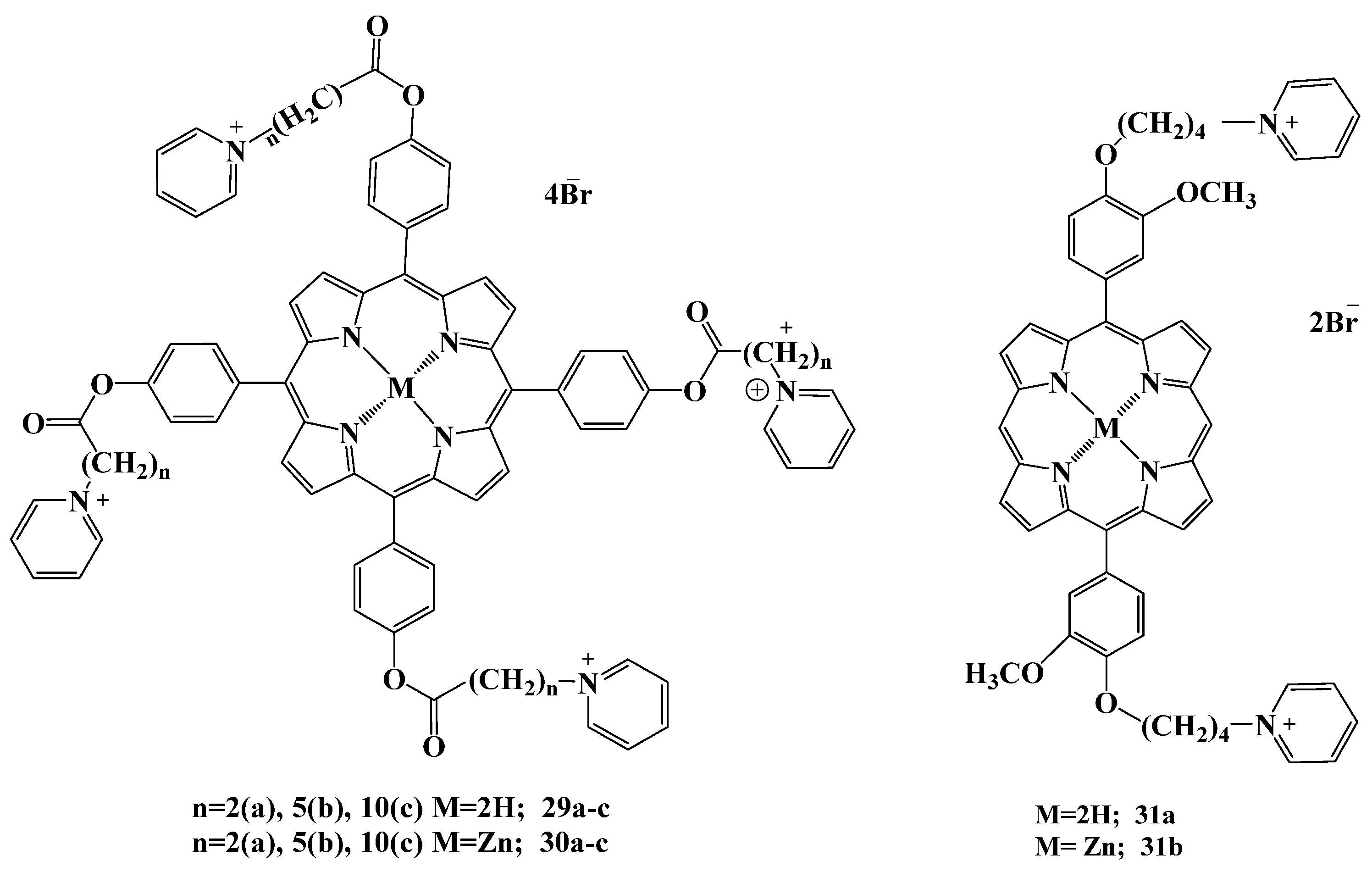
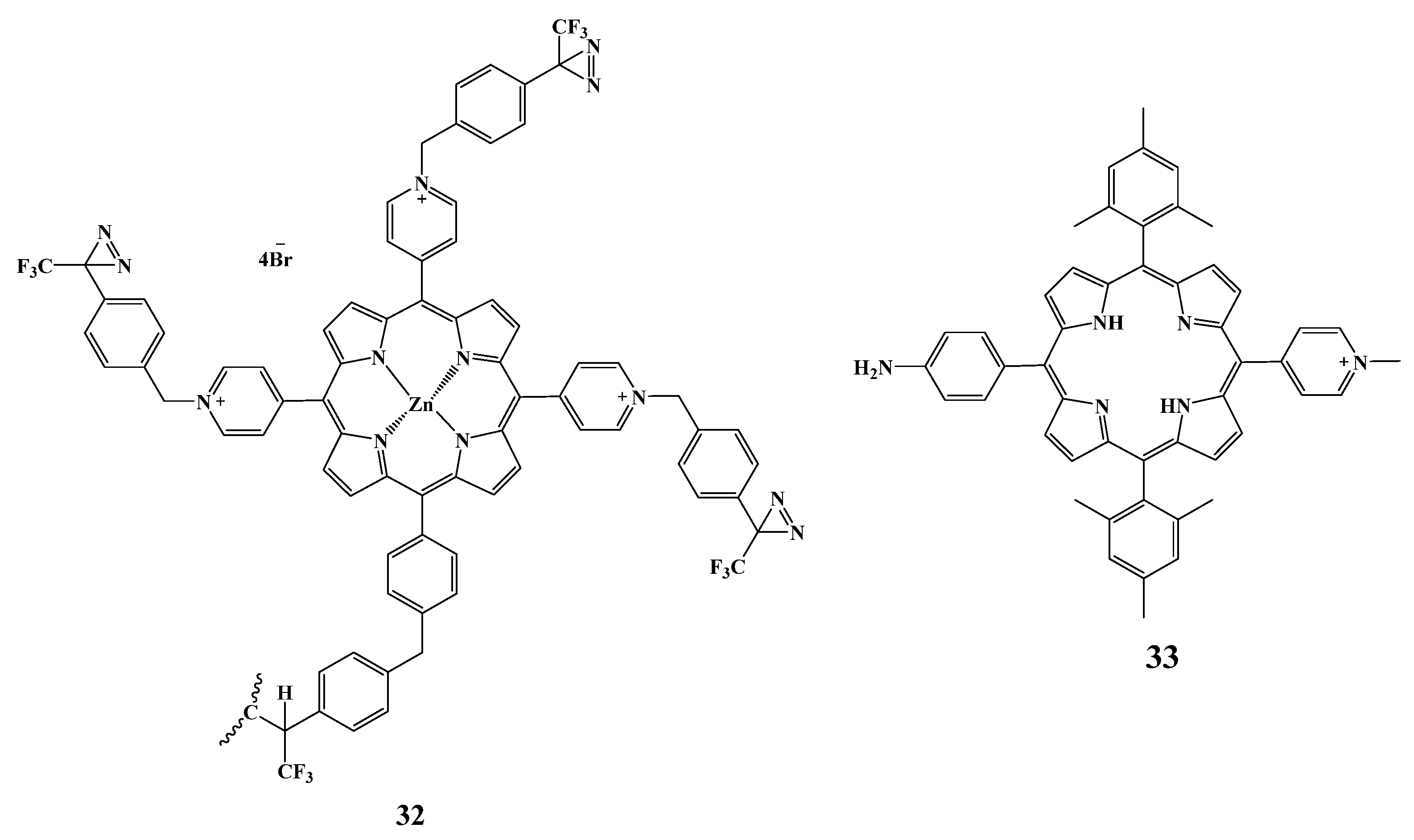
2.5.4. Other Cationic Derivatives
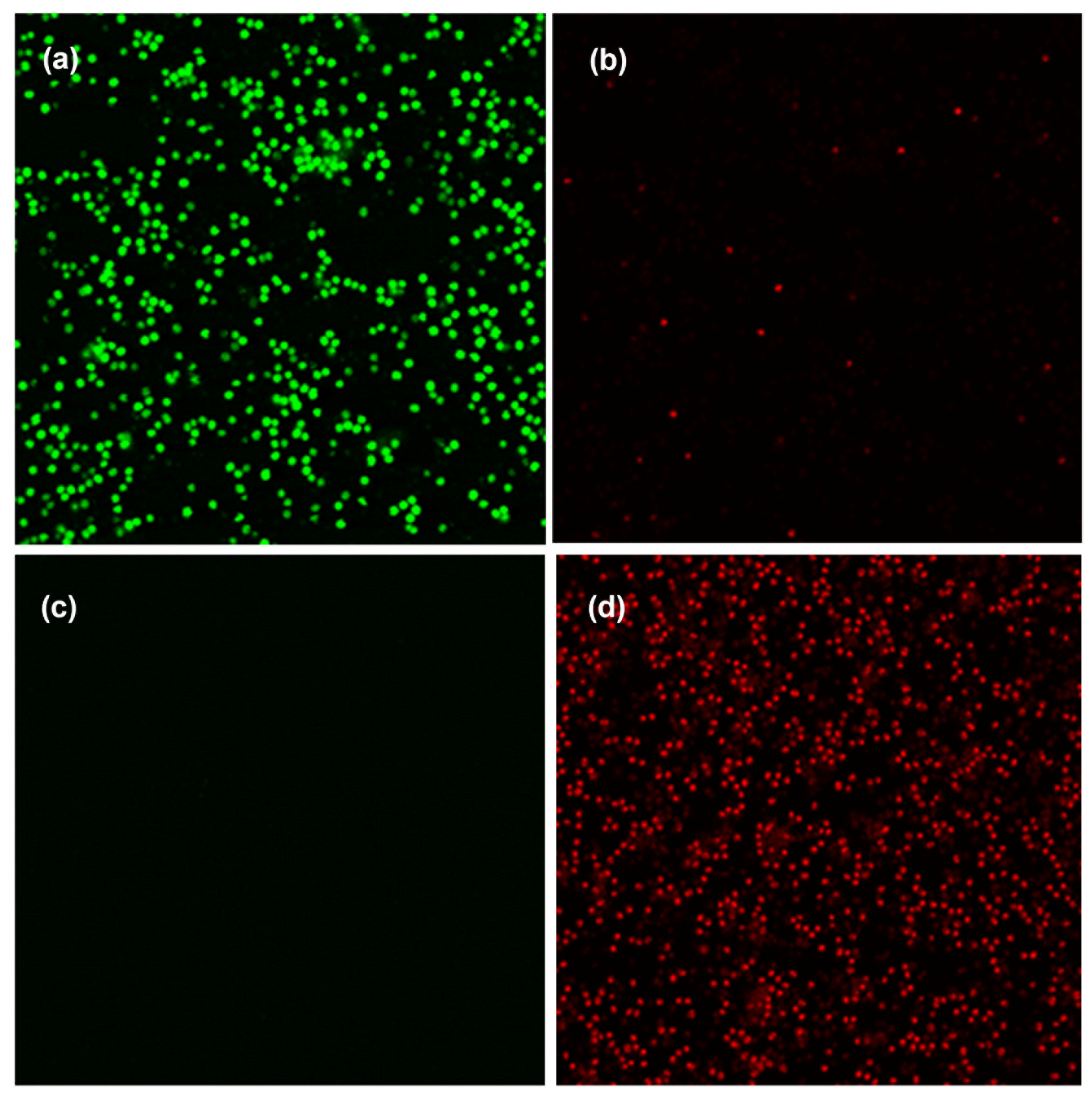
2.6. Approaches to Improve Antibacterial Effect of PS
2.6.1. Development of Antimicrobial Surfaces
2.6.2. Preparation of Conjugates with Antibiotics
2.6.3. Preparation of Conjugates with Nanoparticles
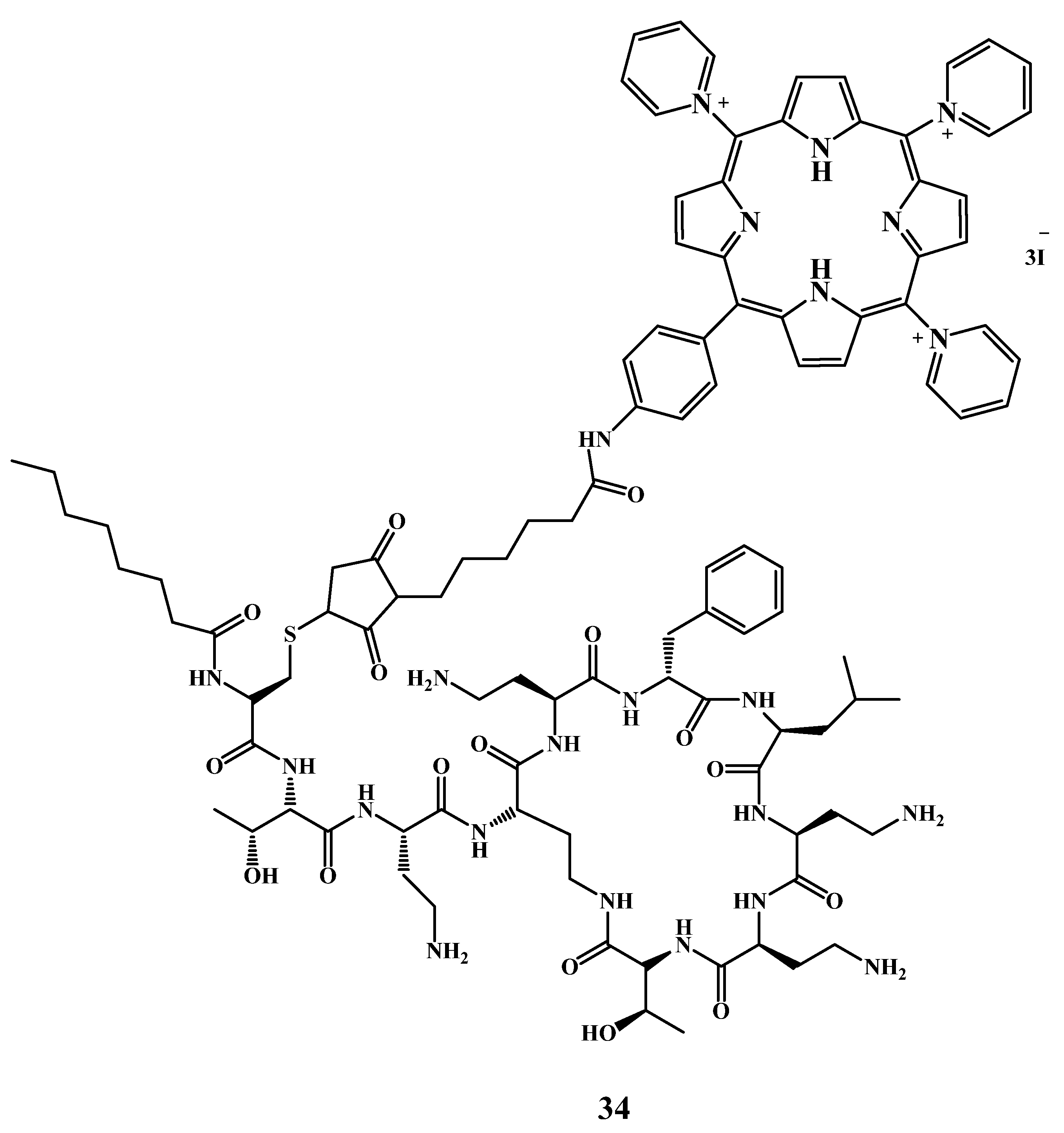
2.6.4. Preparation of Conjugates with Antimicrobial Peptides
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lai, Z.; Shan, A. Advances of Antimicrobial Peptide-Based Biomaterials for the Treatment of Bacterial Infections. Adv. Sci. 2023, 10, 2206602. [Google Scholar] [CrossRef] [PubMed]
- 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO.
- Strathdee, S.A.; Davies, S.C.; Marcelin, J.R. Confronting Antimicrobial Resistance beyond the COVID-19 Pandemic and the 2020 US Election. Lancet 2020, 396, 1050–1053. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human Viruses: Discovery and Emergence. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Hernandez, R.; Jácome, R.; López Vidal, Y.; Ponce de León, S. Are RNA Viruses Candidate Agents for the Next Global Pandemic? A Review. ILAR J. 2017, 58, 343–358. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Y.; Fan, D.; Huang, S.; Ma, Y.; Ding, J.; Luo, Z.; Chen, F.; Zeng, W. Shining Light on Multidrug-Resistant Bacterial Infections: Rational Design of Multifunctional Organosilver-Based AIEgen Probes as Light-Activated Theranostics for Combating Biofilms and Liver Abscesses. Adv. Funct. Mater. 2023, 33, 2304974. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in Bacterial Resistance Mechanisms and the Potential Ways to Combat Them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef]
- Feng, Y.; Coradi Tonon, C.; Ashraf, S.; Hasan, T. Photodynamic and Antibiotic Therapy in Combination against Bacterial Infections: Efficacy, Determinants, Mechanisms, and Future Perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef]
- Mohan Raj, J.R.; Ajakkala, P.B.; Krishna Kumar, B.; Deekshit, V.K.; Rai, P. Phage Therapy: A Promising Approach to Counter Antimicrobial Drug Resistance. In Biotechnology in Healthcare; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 195–204. [Google Scholar]
- Sun, Y.; Liu, Y.; Zhang, B.; Shi, S.; Zhang, T.; Zhao, D.; Tian, T.; Li, Q.; Lin, Y. Erythromycin Loaded by Tetrahedral Framework Nucleic Acids Are More Antimicrobial Sensitive against Escherichia coli (E. coli). Bioact. Mater. 2021, 6, 2281–2290. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, X.; Tan, Y.; Wu, J.; Cai, Y.; Zhou, J.; Wang, X. A Systematical Review on Antimicrobial Peptides and Their Food Applications. Biomater. Adv. 2023, 155, 213684. [Google Scholar] [CrossRef]
- Alghamdi, S. The Role of Vaccines in Combating Antimicrobial Resistance (AMR) Bacteria. Saudi J. Biol. Sci. 2021, 28, 7505–7510. [Google Scholar] [CrossRef]
- Nazarov, P.A. Alternatives to Antibiotics: Phage Lytic Enzymes and Phage Therapy. Bull. Russ. State Med. Univ. 2018, 1, 5–15. [Google Scholar] [CrossRef]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to Antibiotics—A Pipeline Portfolio Review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Inusa, I.; Sanghvi, G.; Mandaliya, V.B.; Bishoyi, A.K. The Current Status of Phage Therapy and Its Advancement towards Establishing Standard Antimicrobials for Combating Multi Drug-Resistant Bacterial Pathogens. Microb. Pathog. 2023, 181, 106199. [Google Scholar] [CrossRef]
- Mironov, A.F.; Ostroverkhov, P.V.; Tikhonov, S.I.; Pogorilyy, V.A.; Kirin, N.S.; Chudakova, O.O.; Tsygankov, A.A.; Grin, M.A. Amino Acid Derivatives of Natural Chlorins as a Platform for the Creation of Targeted Photosensitizers in Oncology. Fine Chem. Technol. 2021, 15, 16–33. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.R.; Jeong, H.; Lee, J.; Wu, X.; Li, H.; Yoon, J. Photodynamic and Photothermal Therapies for Bacterial Infection Treatment. Smart Mol. 2023, 1, e20220010. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Yan, E.; Kwek, G.; Qing, N.S.; Lingesh, S.; Xing, B. Antimicrobial Photodynamic Therapy for the Remote Eradication of Bacteria. Chempluschem 2023, 88, e202300009. [Google Scholar] [CrossRef]
- Koifman, O.I.; Ageeva, T.A.; Kuzmina, N.S.; Otvagin, V.F.; Nyuchev, A.V.; Fedorov, A.Y.; Belykh, D.V.; Lebedeva, N.S.; Yurina, E.S.; Syrbu, S.A.; et al. Synthesis Strategy of Tetrapyrrolic Photosensitizers for Their Practical Application in Photodynamic Therapy. Macroheterocycles 2022, 15, 207–304. [Google Scholar] [CrossRef]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of Photodynamic Therapy in Wound Healing: A Systematic Review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef]
- Varela Kellesarian, S.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Yunker, M.; Varela Kellesarian, T.; Romanos, G.E.; Javed, F. Efficacy of Antimicrobial Photodynamic Therapy in the Disinfection of Acrylic Denture Surfaces: A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 17, 103–110. [Google Scholar] [CrossRef]
- Lago, A.D.N.; Furtado, G.S.; Ferreira, O.C.; Diniz, R.S.; Gonçalves, L.M. Resolution of Herpes Simplex in the Nose Wing Region Using Photodynamic Therapy and Photobiomodulation. Photodiagnosis Photodyn. Ther. 2018, 23, 237–239. [Google Scholar] [CrossRef]
- Liang, F.; Han, P.; Chen, R.; Lin, P.; Luo, M.; Cai, Q.; Huang, X. Topical 5-Aminolevulinic Acid Photodynamic Therapy for Laryngeal Papillomatosistosis Treatment. Photodiagnosis Photodyn. Ther. 2019, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Belousova, I.M.; Kislyakov, I.M.; Muraviova, T.D.; Starodubtsev, A.M.; Kris’ko, T.K.; Selivanov, E.A.; Sivakova, N.P.; Golovanova, I.S.; Volkova, S.D.; Shtro, A.A.; et al. Photodynamic Inactivation of Enveloped Virus in Protein Plasma Preparations by Solid-Phase Fullerene-Based Photosensitizer. Photodiagnosis Photodyn. Ther. 2014, 11, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Schlenke, P. Pathogen Inactivation Technologies for Cellular Blood Components: An Update. Transfus. Med. Hemotherapy 2014, 41, 309–325. [Google Scholar] [CrossRef]
- Lozano, M.; Cid, J.; Müller, T.H. Plasma Treated with Methylene Blue and Light: Clinical Efficacy and Safety Profile. Transfus. Med. Rev. 2013, 27, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.L.; Scholle, F.; Sadeghifar, H.; Francis, A.J.; Boltersdorf, J.; Weare, W.W.; Argyropoulos, D.S.; Maggard, P.A.; Ghiladi, R.A. Synthesis, Characterization, and Antimicrobial Efficacy of Photomicrobicidal Cellulose Paper. Biomacromolecules 2015, 16, 2482–2492. [Google Scholar] [CrossRef]
- Stanley, S.L.; Scholle, F.; Zhu, J.; Lu, Y.; Zhang, X.; Situ, X.; Ghiladi, R.A. Photosensitizer-Embedded Polyacrylonitrile Nanofibers as Antimicrobial Non-Woven Textile. Nanomaterials 2016, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.A.; McCoy, C.P.; Gorman, S.P.; Jones, D.S. Photosensitisers—The Progression from Photodynamic Therapy to Anti-Infective Surfaces. Expert. Opin. Drug Deliv. 2015, 12, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Brovko, L. Antibacterial Photosensitization-Based Treatment for Food Safety. Food Eng. Rev. 2013, 5, 185–199. [Google Scholar] [CrossRef]
- Ghate, V.S.; Zhou, W.; Yuk, H. Perspectives and Trends in the Application of Photodynamic Inactivation for Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Aznar, R.; Sánchez, G. Curcumin-Mediated Photodynamic Inactivation of Norovirus Surrogates. Food Env. Virol. 2016, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic Diarylporphyrins: In Vitro Versatile Anticancer and Antibacterial Photosensitizers. J. Photochem. Photobiol. B 2019, 197, 111548. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Karcz, D.; Boroń, B.; Matwijczuk, A.; Furso, J.; Staroń, J.; Ratuszna, A.; Fiedor, L. Lessons from Chlorophylls: Modifications of Porphyrinoids towards Optimized Solar Energy Conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef]
- Bortnevskaya, Y.S.; Shiryaev, N.A.; Zakharov, N.S.; Kitoroage, O.O.; Gradova, M.A.; Karpechenko, N.Y.; Novikov, A.S.; Nikolskaya, E.D.; Mollaeva, M.R.; Yabbarov, N.G.; et al. Synthesis and Biological Properties of EGFR-Targeted Photosensitizer Based on Cationic Porphyrin. Pharmaceutics 2023, 15, 1284. [Google Scholar] [CrossRef]
- Dandash, F.; Leger, D.Y.; Diab-Assaf, M.; Sol, V.; Liagre, B. Porphyrin/Chlorin Derivatives as Promising Molecules for Therapy of Colorectal Cancer. Molecules 2021, 26, 7268. [Google Scholar] [CrossRef]
- Lazewski, D.; Kucinska, M.; Potapskiy, E.; Kuzminska, J.; Tezyk, A.; Popenda, L.; Jurga, S.; Teubert, A.; Gdaniec, Z.; Kujawski, J.; et al. Novel Short PEG Chain-Substituted Porphyrins: Synthesis, Photochemistry, and In Vitro Photodynamic Activity against Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Liu, Y.-C.; Sun, H.; Guo, D.-S. Type I Photodynamic Therapy by Organic–Inorganic Hybrid Materials: From Strategies to Applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Involvement of Type I and Type II Mechanisms on the Photoinactivation of Non-Enveloped DNA and RNA Bacteriophages. J. Photochem. Photobiol. B 2013, 120, 10–16. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II Mechanisms of Antimicrobial Photodynamic Therapy: An in Vitro Study on Gram-Negative and Gram-Positive Bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.; Tomé, J.P. Photodynamic Inactivation of Bacteria: Finding the Effective Targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Nucleic Acid Changes during Photodynamic Inactivation of Bacteria by Cationic Porphyrins. Bioorganic Med. Chem. 2013, 21, 4311–4318. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet Oxygen: There Is Still Something New under the Sun, and It Is Better than Ever. Photochem. Photobiol. Sci. 2010, 9, 1543–1560. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The Photodegradation of Porphyrins in Cells Can Be Used to Estimate the Lifetime of Singlet Oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Pereira, M.A.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Influence of External Bacterial Structures on the Efficiency of Photodynamic Inactivation by a Cationic Porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef]
- Awad, M.M.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Important Cellular Targets for Antimicrobial Photodynamic Therapy. Appl. Microbiol. Biotechnol. 2016, 100, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [CrossRef] [PubMed]
- González-Delgado, J.A.; Kennedy, P.J.; Ferreira, M.; Tomé, J.P.C.; Sarmento, B. Use of Photosensitizers in Semisolid Formulations for Microbial Photodynamic Inactivation. J. Med. Chem. 2016, 59, 4428–4442. [Google Scholar] [CrossRef] [PubMed]
- Mariewskaya, K.A.; Tyurin, A.P.; Chistov, A.A.; Korshun, V.A.; Alferova, V.A.; Ustinov, A.V. Photosensitizing Antivirals. Molecules 2021, 26, 3971. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-Mrugalska, P.; Mielcarek, J.; Goslinski, T.; Balzarini, J. Photosensitizers Mediated Photodynamic Inactivation Against Virus Particles. Mini-Rev. Med. Chem. 2015, 15, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Neris, R.L.S.; Figueiredo, C.M.; Higa, L.M.; Araujo, D.F.; Carvalho, C.A.M.; Verçoza, B.R.F.; Silva, M.O.L.; Carneiro, F.A.; Tanuri, A.; Gomes, A.M.O.; et al. Co-Protoporphyrin IX and Sn-Protoporphyrin IX Inactivate Zika, Chikungunya and Other Arboviruses by Targeting the Viral Envelope. Sci. Rep. 2018, 8, 9805. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Douaisi, M.P.; Mondal, D.; Kane, R.S. Light-Activated Nanotubeporphyrin Conjugates as Effective Antiviral Agents. Nanotechnology 2012, 23, 105101. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Heldwein, E.E.; Krummenacher, C. Entry of Herpesviruses into Mammalian Cells. Cell. Mol. Life Sci. 2008, 65, 1653–1668. [Google Scholar] [CrossRef]
- Zurzolo, C.; Polistina, C.; Saini, M.; Gentile, R.; Aloj, L.; Migliaccio, G.; Bonatti, S.; Nitsch, L. Opposite Polarity of Virus Budding and of Viral Envelope Glycoprotein Distribution in Epithelial Cells Derived from Different Tissues. J. Cell Biol. 1992, 117, 551–564. [Google Scholar] [CrossRef]
- Collander, R.; Lindholm, M.; Haug, C.M.; Stene, J.; Sörensen, N.A. The Partition of Organic Compounds between Higher Alcohols and Water. Acta Chem. Scand. 1951, 5, 774–780. [Google Scholar] [CrossRef]
- Mariewskaya, K.A.; Gvozdev, D.A.; Chistov, A.A.; Straková, P.; Huvarová, I.; Svoboda, P.; Kotouček, J.; Ivanov, N.M.; Krasilnikov, M.S.; Zhitlov, M.Y.; et al. Membrane-Targeting Perylenylethynylphenols Inactivate Medically Important Coronaviruses via the Singlet Oxygen Photogeneration Mechanism. Molecules 2023, 28, 6278. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Millioni, R.; Puricelli, L.; Tessari, P.; Arrigoni, G.; Franchin, C.; Segalla, A.; Teardo, E.; Reddi, E. Molecular Targets of Antimicrobial Photodynamic Therapy Identified by a Proteomic Approach. J. Proteom. 2012, 77, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gan, C.R.R.; Gao, J.; Loh, X.J. A Perspective on the Trends and Challenges Facing Porphyrin-Based Anti-Microbial Materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Kustov, A.V.; Belykh, D.V.; Smirnova, N.L.; Venediktov, E.A.; Kudayarova, T.V.; Kruchin, S.O.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and Investigation of Water-Soluble Chlorophyll Pigments for Antimicrobial Photodynamic Therapy. Dye. Pigment 2018, 149, 553–559. [Google Scholar] [CrossRef]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-Based Cationic Amphiphilic Photosensitisers as Potential Anticancer, Antimicrobial and Immunosuppressive Agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation beyond the Medical Scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic Inactivation of Escherichia Coli with Cationic Meso-Tetraarylporphyrins—The Charge Number and Charge Distribution Effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Garcia-Sampedro, A.; Tabero, A.; Mahamed, I.; Acedo, P. Multimodal Use of the Porphyrin TMPyP: From Cancer Therapy to Antimicrobial Applications. In Porphyrin Science by Women; World Scientific Publishing Co.: Singapore, 2020; Volume 3, pp. 11–27. ISBN 9789811223556. [Google Scholar]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid Photosensitizers Mediated Photodynamic Inactivation against Bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Cenklová, V. Photodynamic Therapy with TMPyP—Porphyrine Induces Mitotic Catastrophe and Microtubule Disorganization in HeLa and G361 Cells, a Comprehensive View of the Action of the Photosensitizer. J. Photochem. Photobiol. B 2017, 173, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Zorzet, S.; Zacchigna, M.; Della Pietra, E.; Cogoi, S.; Xodo, L.E. Anticancer Activity of Cationic Porphyrins in Melanoma Tumour-Bearing Mice and Mechanistic in Vitro Studies. Mol. Cancer 2014, 13, 75. [Google Scholar] [CrossRef]
- Pratviel, G. Porphyrins in Complex with DNA: Modes of Interaction and Oxidation Reactions. Coord. Chem. Rev. 2016, 308, 460–477. [Google Scholar] [CrossRef]
- Sannikova, N.E.; Timofeev, I.O.; Chubarov, A.S.; Lebedeva, N.S.; Semeikin, A.S.; Kirilyuk, I.A.; Tsentalovich, Y.P.; Fedin, M.V.; Bagryanskaya, E.G.; Krumkacheva, O.A. Application of EPR to Porphyrin-Protein Agents for Photodynamic Therapy. J. Photochem. Photobiol. B 2020, 211, 112008. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.M.; Miotto, R.; Baptista, M.S. Photodynamic Efficiency of Cationic Meso-Porphyrins at Lipid Bilayers: Insights from Molecular Dynamics Simulations. J. Phys. Chem. B 2012, 116, 14618–14627. [Google Scholar] [CrossRef]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villaneuva, A.; Jori, G. Meso-Substituted Cationic Porphyrins as Efficient Photosensitizers of Gram-Positive and Gram-Negative Bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial Activity of Tetraaryl-Porphyrin Photosensitizers: An in Vitro Study on Gram Negative and Gram Positive Bacteria. J. Photochem. Photobiol. B 2006, 85, 28–38. [Google Scholar] [CrossRef]
- Valduga, G.; Breda, B.; Giacometti, G.M.; Jori, G.; Reddi, E. Photosensitization of Wild and Mutant Strains Of Escherichia Coli by meso-Tetra (N-Methyl-4-Pyridyl)Porphine. Biochem. Biophys. Res. Commun. 1999, 256, 84–88. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Cassidy, C.M.; Elborn, J.S.; Tunney, M.M. Delivery of Photosensitisers and Light through Mucus: Investigations into the Potential Use of Photodynamic Therapy for Treatment of Pseudomonas Aeruginosa Cystic Fibrosis Pulmonary Infection. J. Control. Release 2007, 117, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Guterres, K.B.; Rossi, G.G.; Menezes, L.B.; Anraku de Campos, M.M.; Iglesias, B.A. Preliminary Evaluation of the Positively and Negatively Charge Effects of Tetra-Substituted Porphyrins on Photoinactivation of Rapidly Growing Mycobacteria. Tuberculosis 2019, 117, 45–51. [Google Scholar] [CrossRef]
- Lesar, A.; Mušković, M.; Begić, G.; Lončarić, M.; Tomić Linšak, D.; Malatesti, N.; Gobin, I. Cationic Porphyrins as Effective Agents in Photodynamic Inactivation of Opportunistic Plumbing Pathogen Legionella Pneumophila. Int. J. Mol. Sci. 2020, 21, 5367. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.; Zeng, W.; Chen, T.; Liao, W.; Qian, J.; Lin, J.; Zhou, C.; Tian, X.; Cao, J.; et al. Mechanisms of Heteroresistance and Resistance to Imipenem in Pseudomonas aeruginosa. Infect. Drug Resist. 2020, 13, 1419–1428. [Google Scholar] [CrossRef]
- Vital-Lopez, F.G.; Reifman, J.; Wallqvist, A. Biofilm Formation Mechanisms of Pseudomonas Aeruginosa Predicted via Genome-Scale Kinetic Models of Bacterial Metabolism. PLoS Comput. Biol. 2015, 11, e1004452. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Urquhart, C.G.; da Rosa Pinheiro, T.; da Silva, J.L.G.; Leal, D.B.R.; Burgo, T.A.L.; Iglesias, B.A.; Santos, R.C.V. Antimicrobial Activity of Water-Soluble Tetra-Cationic Porphyrins on Pseudomonas Aeruginosa. Photodiagnosis Photodyn. Ther. 2023, 42, 103266. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms that Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals 2021, 14, 603. [Google Scholar] [CrossRef] [PubMed]
- Bryden, F.; Boyle, R.W. Metalloporphyrins for Medical Imaging Applications. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 2016; pp. 141–221. [Google Scholar]
- Imran, M.; Ramzan, M.; Qureshi, A.; Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Sáfar, G.A.M.; Gontijo, R.N.; Fantini, C.; Martins, D.C.S.; Idemori, Y.M.; Pinheiro, M.V.B.; Krambrock, K. Enhanced Oxygen Singlet Production by Hybrid System of Porphyrin and Enriched (6,5) Single-Walled Carbon Nanotubes for Photodynamic Therapy. J. Phys. Chem. C 2015, 119, 4344–4350. [Google Scholar] [CrossRef]
- da Silveira, C.H.; Vieceli, V.; Clerici, D.J.; Santos, R.C.V.; Iglesias, B.A. Investigation of Isomeric Tetra-Cationic Porphyrin Activity with Peripheral [Pd(Bpy)Cl]+ Units by Antimicrobial Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2020, 31, 101920. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.C.; Acunha, T.V.; Santurio, J.M.; Denardi, L.B.; Iglesias, B.A. Investigation of Powerful Fungicidal Activity of Tetra-Cationic Platinum(II) and Palladium(II) Porphyrins by Antimicrobial Photodynamic Therapy Assays. Photodiagnosis Photodyn. Ther. 2021, 36, 102550. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.G.; Guterres, K.B.; Moreira, K.S.; Burgo, T.A.L.; de Campos, M.M.A.; Iglesias, B.A. Photo-Damage Promoted by Tetra-Cationic Palladium(II) Porphyrins in Rapidly Growing Mycobacteria. Photodiagnosis Photodyn. Ther. 2021, 36, 102514. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Özkan, M.; Khaligh, A.; Tuncel, D. Water-Dispersible Glycosylated Poly (2,5′-Thienylene)Porphyrin-Based Nanoparticles for Antibacterial Photodynamic Therapy. Photochem. Photobiol. Sci. 2019, 18, 1147–1155. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Yao, Y.; Yuan, H.; Wang, S.; Wang, Z.; Zhang, X. Supramolecular Photosensitizers with Enhanced Antibacterial Efficiency. Angew. Chem. Int. Ed. 2013, 52, 8285–8289. [Google Scholar] [CrossRef] [PubMed]
- Clerici, D.J.; Hahn da Silveira, C.; Iglesias, B.A.; Christ Vianna Santos, R. The First Evidence of Antibiofilm Action of Proteus Mirabilis with Tetra-Cationic Porphyrins Containing Cisplatin by Antimicrobial Photodynamic Therapy. Microb. Pathog. 2023, 174, 105859. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel Metals and Metal Complexes as Platforms for Cancer Therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef]
- Yuan, M.; Chua, S.L.; Liu, Y.; Drautz-Moses, D.I.; Yam, J.K.H.; Aung, T.T.; Beuerman, R.W.; Salido, M.M.S.; Schuster, S.C.; Tan, C.-H.; et al. Repurposing the Anticancer Drug Cisplatin with the Aim of Developing Novel Pseudomonas Aeruginosa Infection Control Agents. Beilstein J. Org. Chem. 2018, 14, 3059–3069. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Bezzerra, F.C.; Teles, A.V.; Menezes, L.B.; Alves, K.M.; Alonso, L.; Alonso, A.; Andrade, M.A.; Borissevitch, I.E.; Souza, G.R.L.; et al. Photoinactivation of Salmonella enterica (Serovar Typhimurium) by Tetra-Cationic Porphyrins Containing Peripheral [Ru(Bpy)2Cl]+ Units. J. Photochem. Photobiol. A Chem. 2020, 391, 112375. [Google Scholar] [CrossRef]
- Xuan, W.; Huang, L.; Wang, Y.; Hu, X.; Szewczyk, G.; Huang, Y.Y.; El-Hussein, A.; Bommer, J.C.; Nelson, M.L.; Sarna, T.; et al. Amphiphilic Tetracationic Porphyrins Are Exceptionally Active Antimicrobial Photosensitizers: In Vitro and in Vivo Studies with the Free-Base and Pd-Chelate. J. Biophotonics 2019, 12, e201800318. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Iamamoto, Y.; Baptista, M.S. Mechanism and Efficiency of Cell Death of Type II Photosensitizers: Effect of Zinc Chelation. Photochem. Photobiol. 2012, 88, 774–781. [Google Scholar] [CrossRef]
- Gyulkhandanyan, A.G.; Paronyan, M.H.; Gyulkhandanyan, A.G.; Ghazaryan, K.R.; Parkhats, M.V.; Dzhagarov, B.M.; Korchenova, M.V.; Lazareva, E.N.; Tuchina, E.S.; Gyulkhandanyan, G.V.; et al. Meso-Substituted Cationic 3- and 4-N-Pyridylporphyrins and Their Zn(II) Derivatives for Antibacterial Photodynamic Therapy. J. Innov. Opt. Health Sci. 2022, 15, 2142007. [Google Scholar] [CrossRef]
- Makola, L.C.; Managa, M.; Nyokong, T. Enhancement of Photodynamic Antimicrobialtherapy through the Use of Cationic Indium Porphyrin Conjugated to Ag/CuFe2O4 Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 30, 101736. [Google Scholar] [CrossRef]
- Ha, A.C.; Nguyen, T.; Nguyen, P.A.; Nguyen, V.M. Antibacterial Activity of Green Fabricated Silver-Doped Titanates. Fine Chem. Technol. 2022, 17, 335–345. [Google Scholar] [CrossRef]
- Hossein Mohammadi, A.; Sobhani-Nasab, A.; Nejati, M.; Hadi, S.; Behjati, M.; Mirzaii-Dizgah, I.; Moradi Hasan-Abad, A.; Karami, M. Preparation and Characterization of CuO, Ag2O and ZnO Nanoparticles and Investigation of Their Antibacterial and Anticancer Properties on HCT-116 and C26 Cells. Inorg. Chem. Commun. 2023, 149, 110404. [Google Scholar] [CrossRef]
- Guterres, K.B.; Rossi, G.G.; de Campos, M.M.A.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A. Nanomolar Effective Report of Tetra-Cationic Silver(II) Porphyrins against Non-Tuberculous Mycobacteria in Antimicrobial Photodynamic Approaches. Photodiagnosis Photodyn. Ther. 2022, 38, 102770. [Google Scholar] [CrossRef] [PubMed]
- Majiya, H.; Adeyemi, O.O.; Stonehouse, N.J.; Millner, P. Photodynamic Inactivation of Bacteriophage MS2: The A-Protein Is the Target of Virus Inactivation. J. Photochem. Photobiol. B 2018, 178, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.; Nelson, K.L. Sunlight-Mediated Inactivation of MS2 Coliphage via Exogenous Singlet Oxygen Produced by Sensitizers in Natural Waters. Env. Sci. Technol. 2007, 41, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Evaluation of Resistance Development and Viability Recovery by a Non-Enveloped Virus after Repeated Cycles of APDT. Antivir. Res. 2011, 91, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Chayavichitsilp, P.; Buckwalter, J.V.; Krakowski, A.C.; Friedlander, S.F. Herpes Simplex. Pediatr. Rev. 2009, 30, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.V.; Oliveira, T.M.A.; Bezerra, F.C.; Alonso, L.; Alonso, A.; Borissevitch, I.E.; Gonçalves, P.J.; Souza, G.R.L. Photodynamic Inactivation of Bovine Herpesvirus Type 1 (BoHV-1) by Porphyrins. J. Gen. Virol. 2018, 99, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Timilsina, U.; Mazumder, Z.H.; Mukherjee, A.; Ghimire, D.; Markandey, M.; Upadhyaya, K.; Sharma, D.; Mishra, N.; Jha, T.; et al. Dual Activity of Amphiphilic Zn(II) Nitroporphyrin Derivatives as HIV-1 Entry Inhibitors and in Cancer Photodynamic Therapy. Eur. J. Med. Chem. 2019, 174, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.N.; Scarbrough, B.; Saleh, R.; Hovey, J.; Ari, F.; Goyal, S.; Chi, R.J.; Troutman, J.M.; Vivero-Escoto, J.L. Influence of Cationic Meso-Substituted Porphyrins on the Antimicrobial Photodynamic Efficacy and Cell Membrane Interaction in Escherichia coli. Int. J. Mol. Sci. 2019, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Caminos, D.A.; Durantini, E.N. Photodynamic Inactivation of Escherichia coli Immobilized on Agar Surfaces by a Tricationic Porphyrin. Bioorganic Med. Chem. 2006, 14, 4253–4259. [Google Scholar] [CrossRef]
- Sen, P.; Soy, R.; Mgidlana, S.; Mack, J.; Nyokong, T. Light-Driven Antimicrobial Therapy of Palladium Porphyrins and Their Chitosan Immobilization Derivatives and Their Photophysical-Chemical Properties. Dye Pigment 2022, 203, 110313. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical Formulations and Wound Healing Applications of Chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and Biological Activity of Vicinal Diaryl-Substituted 1H-Imidazoles. Tetrahedron 2007, 63, 4571–4624. [Google Scholar] [CrossRef]
- Moreira, X.; Santos, P.; Faustino, M.A.F.; Raposo, M.M.M.; Costa, S.P.G.; Moura, N.M.M.; Gomes, A.T.P.C.; Almeida, A.; Neves, M.G.P.M.S. An Insight into the Synthesis of Cationic Porphyrin-Imidazole Derivatives and Their Photodynamic Inactivation Efficiency against Escherichia coli. Dye Pigment 2020, 178, 108330. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on APDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef]
- Le Guern, F.; Ouk, T.-S.; Yerzhan, I.; Nurlykyz, Y.; Arnoux, P.; Frochot, C.; Leroy-Lhez, S.; Sol, V. Photophysical and Bactericidal Properties of Pyridinium and Imidazolium Porphyrins for Photodynamic Antimicrobial Chemotherapy. Molecules 2021, 26, 1122. [Google Scholar] [CrossRef]
- Prasanth, C.S.; Karunakaran, S.C.; Paul, A.K.; Kussovski, V.; Mantareva, V.; Ramaiah, D.; Selvaraj, L.; Angelov, I.; Avramov, L.; Nandakumar, K.; et al. Antimicrobial Photodynamic Efficiency of Novel Cationic Porphyrins towards Periodontal Gram-Positive and Gram-Negative Pathogenic Bacteria. Photochem. Photobiol. 2014, 90, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Parish, J.H.; Brown, S.B. Mechanism of Uptake of a Cationic Water-Soluble Pyridinium Zinc Phthalocyanine across the Outer Membrane of Escherichia coli. Antimicrob. Agents Chemother. 2000, 44, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.P.; Giuntini, F.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Silva, A.M.S.; Santana-Marques, M.G.; Ferrer-Correia, A.J.; Cavaleiro, J.A.S.; et al. Synthesis of Cationic β-Vinyl Substituted Meso-Tetraphenylporphyrins and Their in Vitro Activity against Herpes Simplex Virus Type 1. Bioorganic Med. Chem. Lett. 2005, 15, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Garzotto, F.; Caruso, E. Photoinactivation of Pseudomonas Aeruginosa Biofilm by Dicationic Diaryl-Porphyrin. Int. J. Mol. Sci. 2021, 22, 6808. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Soy, R.C.; Mack, J.; Nyokong, T. Non-Aggregated Lipophilic Water-Soluble Tin Porphyrins as Photosensitizers for Photodynamic Therapy and Photodynamic Antimicrobial Chemotherapy. New J. Chem. 2020, 44, 11006–11012. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Savelyeva, I.O.; Ignatova, A.A.; Gradova, M.A.; Gradov, O.V.; Lobanov, A.V.; Feofanov, A.V.; Mironov, A.F.; Bragina, N.A. Synthesis and Photodynamic Antimicrobial Activity of Amphiphilic Meso-Arylporphyrins with Pyridyl Moieties. Dye Pigment 2020, 181, 108561. [Google Scholar] [CrossRef]
- Savelyeva, I.O.; Bortnevskaya, Y.S.; Usanev, A.Y.; Bragina, N.A.; Mironov, A.F.; Ignatova, A.A.; Feofanov, A.V.; Zhdanova, K.A. Photoinactivation of S. Aureus by Cationic Meso-Arylporphyrin and Its Zn(II) Complex. Macroheterocycles 2021, 14, 140–146. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Savel’eva, I.O.; Usanev, A.Y.; Usachev, M.N.; Shmigol, T.A.; Gradova, M.A.; Bragina, N.A. Synthesis of Trans-Substituted Cationic Zinc Porphynates and Study of Their Photodynamic Antimicrobial Activity. Russ. J. Inorg. Chem. 2022, 67, 1756–1762. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Savelyeva, I.O.; Ezhov, A.V.; Zhdanov, A.P.; Zhizhin, K.Y.; Mironov, A.F.; Bragina, N.A.; Babayants, A.A.; Frolova, I.S.; Filippova, N.I.; et al. Novel Cationic Meso-Arylporphyrins and Their Antiviral Activity against HSV-1. Pharmaceuticals 2021, 14, 242. [Google Scholar] [CrossRef]
- Magaela, N.B.; Makola, L.C.; Managa, M.; Nyokong, T. Photodynamic Activity of Novel Cationic Porphyrins Conjugated to Graphene Quantum Dots against Staphylococcus aureus. J. Porphyr. Phthalocyanines 2022, 26, 392–402. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-Based Nanovehicles: Recent Advances in Anticancer Therapeutic Applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef] [PubMed]
- Shatila, F.; Tieman, G.M.O.; Musolino, S.F.; Wulff, J.E.; Buckley, H.L. Antimicrobial Photodynamic Inactivation of Planktonic and Biofilm Cells by Covalently Immobilized Porphyrin on Polyethylene Terephthalate Surface. Int. Biodeterior. Biodegrad. 2023, 178, 105567. [Google Scholar] [CrossRef]
- Ringot, C.; Sol, V.; Barrière, M.; Saad, N.; Bressollier, P.; Granet, R.; Couleaud, P.; Frochot, C.; Krausz, P. Triazinyl Porphyrin-Based Photoactive Cotton Fabrics: Preparation, Characterization, and Antibacterial Activity. Biomacromolecules 2011, 12, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Yu, J.; Zhu, X.; Wu, Y.; Liu, J.; Zhang, Y. Photodynamic-Based Combinatorial Cancer Therapy Strategies: Tuning the Properties of Nanoplatform According to Oncotherapy Needs. Coord. Chem. Rev. 2022, 461, 214495. [Google Scholar] [CrossRef]
- González-Delgado, J.A.; Castro, P.M.; Machado, A.; Araújo, F.; Rodrigues, F.; Korsak, B.; Ferreira, M.; Tomé, J.P.C.; Sarmento, B. Hydrogels Containing Porphyrin-Loaded Nanoparticles for Topical Photodynamic Applications. Int. J. Pharm. 2016, 510, 221–231. [Google Scholar] [CrossRef]
- Zagami, R.; Franco, D.; Pipkin, J.D.; Antle, V.; De Plano, L.; Patanè, S.; Guglielmino, S.; Monsù Scolaro, L.; Mazzaglia, A. Sulfobutylether-β-Cyclodextrin/5,10,15,20-Tetrakis(1-Methylpyridinium-4-Yl)Porphine Nanoassemblies with Sustained Antimicrobial Phototherapeutic Action. Int. J. Pharm. 2020, 585, 119487. [Google Scholar] [CrossRef]
- Brogden, K.A.; Ackermann, M.; McCray, P.B.; Tack, B.F. Antimicrobial Peptides in Animals and Their Role in Host Defences. Int. J. Antimicrob. Agents 2003, 22, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.P.; Chen, J.Y. Conjugation of Antimicrobial Peptides to Enhance Therapeutic Efficacy. Eur. J. Med. Chem. 2023, 259, 115680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qi, G.-B.; Wang, H. A Purpurin-Peptide Derivative for Selective Killing of Gram-Positive Bacteria via Insertion into Cell Membrane. J. Mater. Chem. B 2016, 4, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.D.P.; Uliana, M.P.; De Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic Therapy Enhanced by the Peptide Aurein. Sci. Rep. 2018, 8, 412. [Google Scholar] [CrossRef]
- Dosselli, R.; Gobbo, M.; Bolognini, E.; Campestrini, S.; Reddi, E. Porphyrin−Apidaecin Conjugate as a New Broad Spectrum Antibacterial Agent. ACS Med. Chem. Lett. 2010, 1, 35–38. [Google Scholar] [CrossRef]
- Dosselli, R.; Tampieri, C.; Ruiz-González, R.; De Munari, S.; Ragàs, X.; Sánchez-García, D.; Agut, M.; Nonell, S.; Reddi, E.; Gobbo, M. Synthesis, Characterization, and Photoinduced Antibacterial Activity of Porphyrin-Type Photosensitizers Conjugated to the Antimicrobial Peptide Apidaecin 1b. J. Med. Chem. 2013, 56, 1052–1063. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savelyeva, I.O.; Zhdanova, K.A.; Gradova, M.A.; Gradov, O.V.; Bragina, N.A. Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy. Curr. Issues Mol. Biol. 2023, 45, 9793-9822. https://doi.org/10.3390/cimb45120612
Savelyeva IO, Zhdanova KA, Gradova MA, Gradov OV, Bragina NA. Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy. Current Issues in Molecular Biology. 2023; 45(12):9793-9822. https://doi.org/10.3390/cimb45120612
Chicago/Turabian StyleSavelyeva, Inga O., Kseniya A. Zhdanova, Margarita A. Gradova, Oleg V. Gradov, and Natal’ya A. Bragina. 2023. "Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy" Current Issues in Molecular Biology 45, no. 12: 9793-9822. https://doi.org/10.3390/cimb45120612
APA StyleSavelyeva, I. O., Zhdanova, K. A., Gradova, M. A., Gradov, O. V., & Bragina, N. A. (2023). Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy. Current Issues in Molecular Biology, 45(12), 9793-9822. https://doi.org/10.3390/cimb45120612


_Kim.png)



