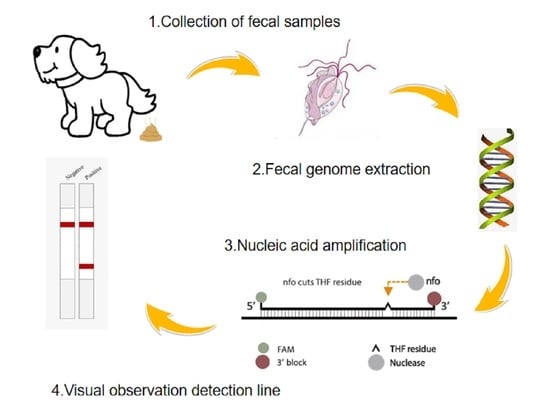
Development of an LFD-RPA Assay for Rapid Detection of Pentatrichomonas hominis Infection in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction
2.3. Primer and Probe Design
2.4. RPA Reaction and Lateral Flow Reading
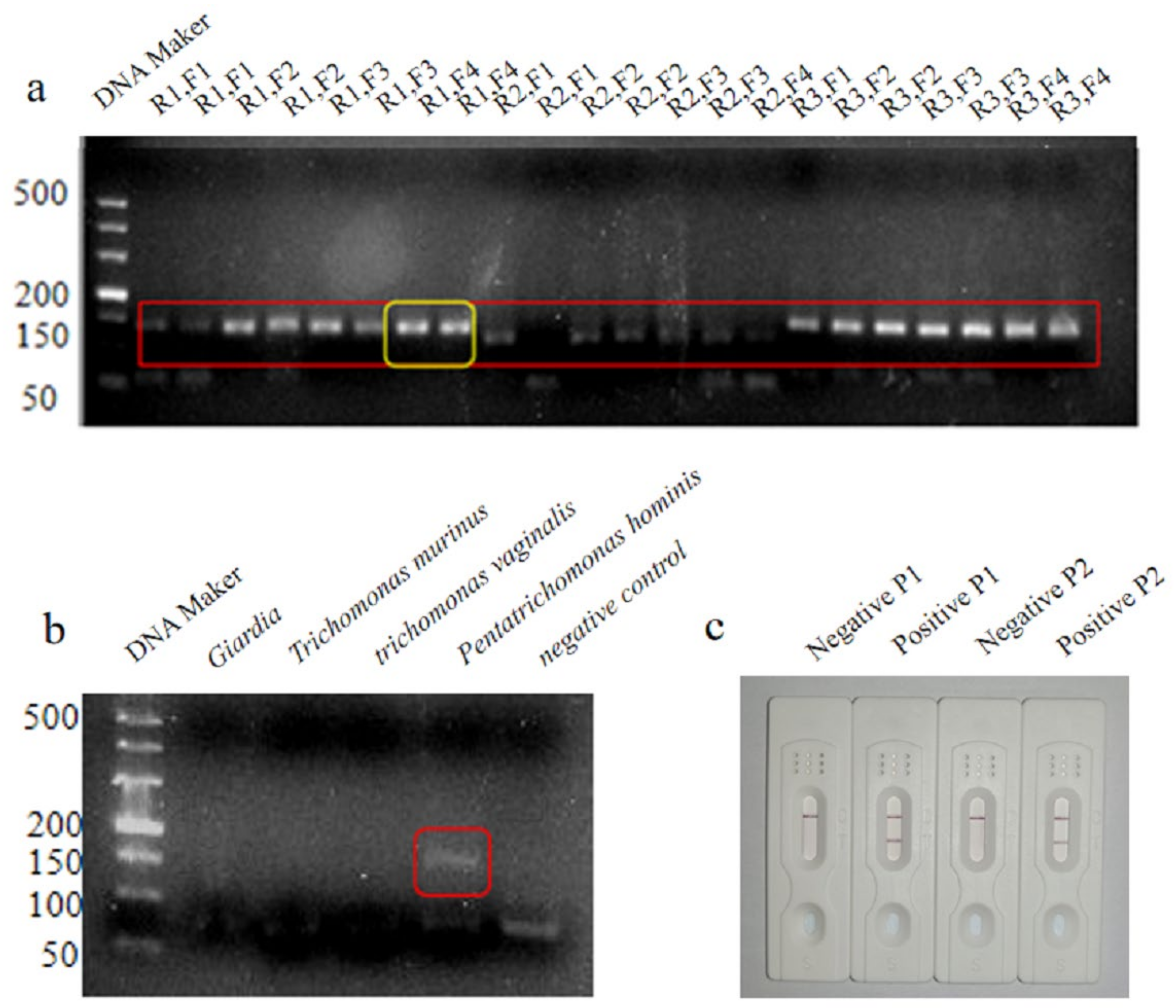
2.5. Specificity of the LFD-RPA Assay
2.6. Sensitivity of the LFD-RPA Assay
2.7. Applicability of the LFD-RPA Assay
3. Results
3.1. Primer and Probe Design
3.2. Optimization of the LFD-RPA Assay
3.3. Optimization of the LFD-RPA Assay
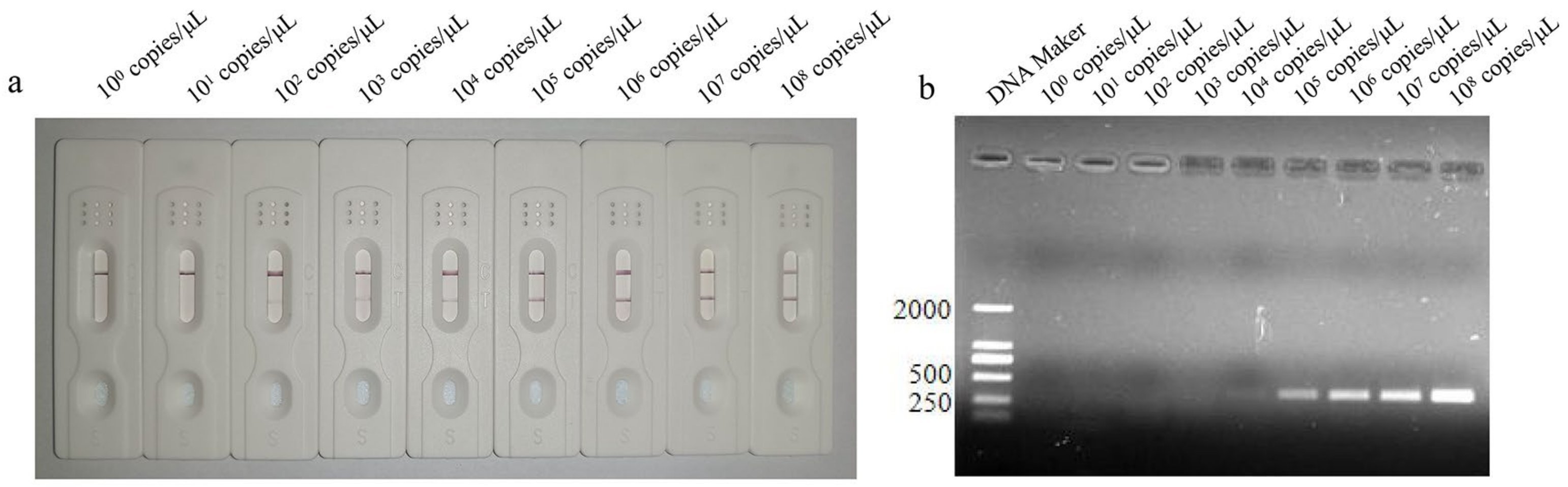
3.4. Sensitivity Analysis of LFD-RPA Assay
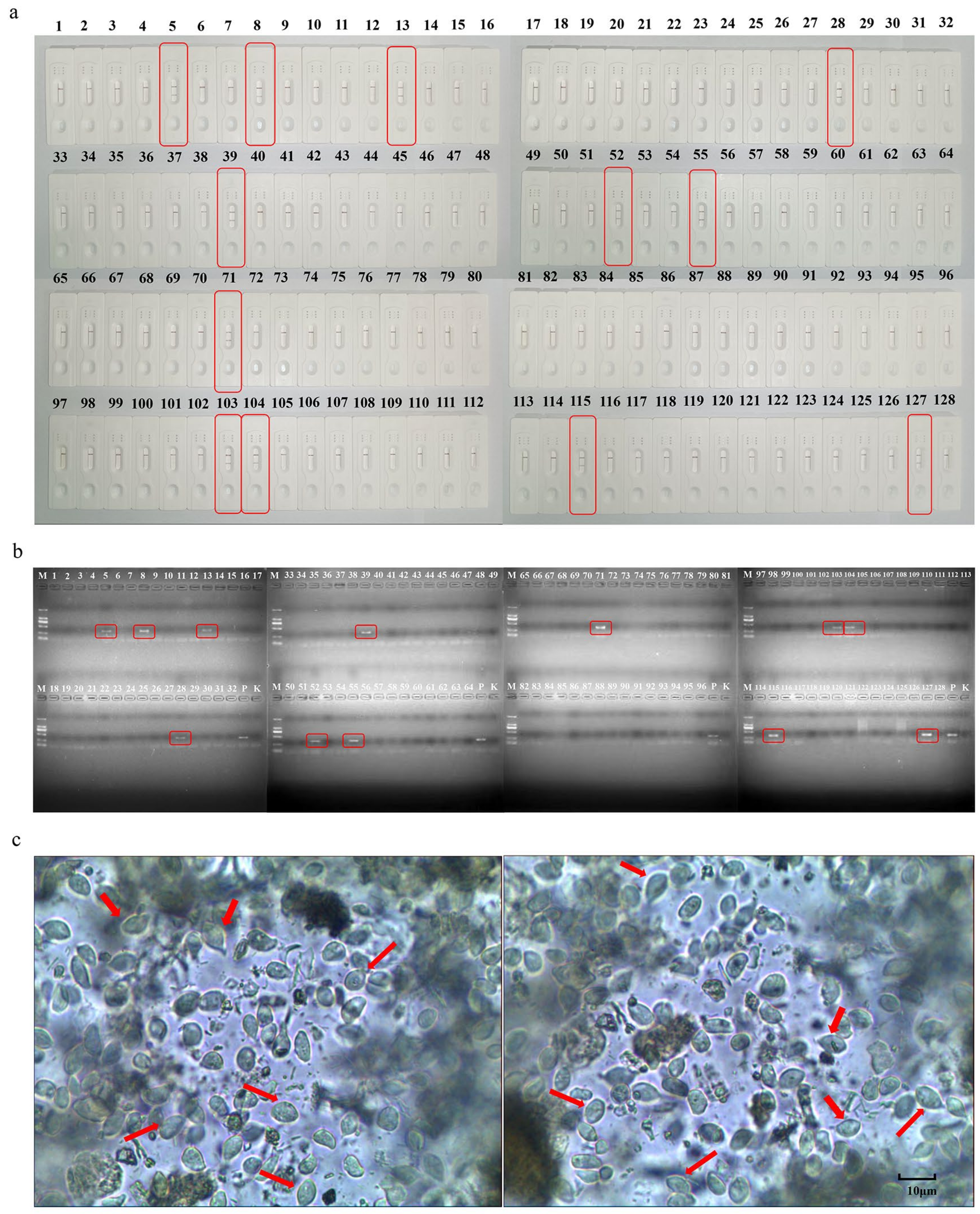
3.5. Comparative Clinical Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamaruddin, M.; Tokoro, M.; Rahman, M.M.; Arayama, S.; Hidayati, A.P.; Syafruddin, D.; Asih, P.B.; Yoshikawa, H.; Kawahara, E. Molecular characterization of various trichomonad species isolated from humans and related mammals in Indonesia. Korean J. Parasitol. 2014, 52, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, C.C.; McIntosh, D.; Dos Santos, D.J.; Lopes, S.d.P.; de Jesus, V.L.T. Morphological and molecular characterization of a species of Tetratrichomonas present in feces of Brazilian sheep (Ovis aries) and goats (Capra hircus). Parasitol. Res. 2019, 119, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Yu, Y.; Gong, P.; Li, J.; Li, Z.; Li, T.; Cong, Z.; Tian, C.; Liu, X.; et al. High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasites Vectors 2019, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Saksirisampant, W.; Nuchprayoon, S.; Wiwanitkit, V.; Yenthakam, S.; Ampavasiri, A. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J. Med. Assoc. Thai. 2003, 86 (Suppl. S2), S263–S270. [Google Scholar]
- Kim, Y.A.; Kim, H.Y.; Cho, S.H.; Cheun, H.-I.; Yu, J.-R.; Lee, S.-E. PCR detection and molecular characterization of Pentatrichomonas hominis from feces of dogs with diarrhea in the Republic of Korea. Korean J. Parasitol. 2010, 48, 9–13. [Google Scholar] [CrossRef]
- Grellet, A.; Brunopolack; Feugier, A.; Boucraut-Baralon, C.; Grandjean, D.; Vandewynckel, L.; Cian, A.; Meloni, D.; Viscogliosi, E. Prevalence, risk factors of infection and molecular characterization of trichomonads in puppies from French breeding kennels. Vet. Parasitol. 2013, 197, 418–426. [Google Scholar] [CrossRef]
- Bastos, B.F.; Brener, B.; de Figueiredo, M.A.; Leles, D.; Mendes-De-Almeida, F. Pentatrichomonas hominis infection in two domestic cats with chronic diarrhea. JFMS Open Rep. 2018, 4, 2055116918774959. [Google Scholar] [CrossRef]
- Li, W.C.; Ying, M.; Gong, P.T.; Li, J.-H.; Yang, J.; Li, H.; Zhang, X.-C. Pentatrichomonas hominis: Prevalence and molecular characterization in humans, dogs, and monkeys in Northern China. Parasitol. Res. 2016, 115, 569–574. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kon, M.; Saito, T.; Maeno, N.; Koyama, M.; Sunaoshi, K.; Yamaguchi, M.; Morishima, Y.; Kawanaka, M. Prevalence of intestinal canine and feline parasites in Saitama Prefecture, Japan. Kansenshogaku Zasshi. 2009, 83, 223–228. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R.; Socha, P. Detection of Pentatrichomonas hominis in dogs using real-time PCR. Pol. J. Vet. Sci. 2015, 18, 775–778. [Google Scholar] [CrossRef]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent advances in recombinase polymerase amplification: Principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; A Armes, N. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Gong, P.; Cheng, S.; Wang, X.; Li, X.; Hou, Z.; Liu, C.; Bi, T.; Wang, B.; et al. Prevalence and molecular characterization of Pentatrichomonas hominis in Siberian tigers (Panthera tigris altaica) in northeast China. Integr. Zool. 2022, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Morio, F.; Renard, B.T.; Poirier, A.-S.; Miegeville, M.; Chambreuil, G. Trichomonads in pleural effusion: Case report, literature review and utility of PCR for species identification. New Microbiol. 2012, 35, 83–87. [Google Scholar] [PubMed]
- Wang, H.K.; Jerng, J.S.; Su, K.E.; Chang, S.-C.; Jerng, J.S. Trichomonas empyema with respiratory failure. Am. J. Trop. Med. Hyg. 2006, 75, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Doherty, D.E.; Ribes, J.; Seabolt, J.P.; Bensadoun, E.S. Empyema Caused by Trichomonas. Chest 2003, 123, 291–292. [Google Scholar] [CrossRef]
- Gumaa, M.M.; Cao, X.; Li, Z.; Lou, Z.; Zhang, N.; Zhang, Z.; Zhou, J.; Fu, B. Establishment of a recombinase polymerase amplification (RPA) assay for the detection of Brucella spp. Infection. Mol. Cell. Probes 2019, 47, 101434. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Peng, B.; Wu, W.; Wang, X.; Liu, H.; Yuan, L.; Fang, S.; Lu, J. Rapid Detection of Avian Influenza A Virus (H7N9) by Lateral Flow Dipstick Recombinase Polymerase Amplification. Biol. Pharm. Bull. 2018, 41, 1804–1808. [Google Scholar] [CrossRef]
- Hou, P.; Wang, H.; Zhao, G.; He, C.; He, H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017, 13, 386. [Google Scholar] [CrossRef]
- Crannell, Z.A.; Cabada, M.M.; Castellanos-Gonzalez, A.; White, A.C.; Irani, A.; Richards-Kortum, R. Recombinase polymerase amplification-based assay to diagnose Giardia in stool samples. Am. J. Trop. Med. Hyg. 2015, 92, 583–587. [Google Scholar] [CrossRef]
- Li, T.T.; Wang, J.L.; Zhang, N.Z.; Li, W.-H.; Yan, H.-B.; Li, L.; Jia, W.-Z.; Fu, B.-Q. Rapid and Visual Detection of Trichinella Spp. Using a Lateral Flow Strip-Based Recombinase Polymerase Amplification (LF-RPA) Assay. Front. Cell. Infect. Microbiol. 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Giroux, C.N.; Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 1997, 88, 375–384. [Google Scholar] [CrossRef]
- Lambing, C.; Kuo, P.; Kim, J.; Osman, K.; Whitbread, A.L.; Yang, J.; Choi, K.; Franklin, F.C.H.; Henderson, I.R. Differentiated function and localisation of SPO11-1 and PRD3 on the chromosome axis during meiotic DSB formation in Arabidopsis thaliana. PLoS Genet. 2022, 18, e1010298. [Google Scholar] [CrossRef] [PubMed]
- Da Ines, O.; Michard, R.; Fayos, I.; Bastianelli, G.; Nicolas, A.; Guiderdoni, E.; White, C.; Sourdille, P. Bread wheat TaSPO11-1 exhibits evolutionarily conserved function in meiotic recombination across distant plant species. Plant J. 2020, 103, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Dernburg, A.F.; McDonald, K.; Moulder, G.; Barstead, R.; Dresser, M.; Villeneuve, A.M. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 1998, 94, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Greeshma, M.; Bhat, A.I.; Jeevalatha, A. Rapid onsite detection of piper yellow mottle virus infecting black pepper by recombinase polymerase amplification-lateral flow assay (RPA-LFA). J. Virol. Methods 2023, 315, 114695. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhou, D.H.; Zhang, L.X.; Zheng, W.-B.; Ma, J.-G.; Wang, M.; Zhu, X.-Q.; Xu, M.-J. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for equipment-free detection of Cryptosporidium spp. oocysts in dairy cattle feces. Parasitol. Res. 2016, 115, 3551–3555. [Google Scholar] [CrossRef]
- Ma, X.; Bai, X.; Li, H.; Ding, J.; Zhang, H.; Qiu, Y.; Wang, J.; Liu, X.; Liu, M.; Tang, B.; et al. A rapid and visual detection assay for Clonorchis sinensis based on recombinase polymerase amplification and lateral flow dipstick. Parasites Vectors 2023, 16, 165. [Google Scholar] [CrossRef]


| Name | Sequence (5′ to 3′) |
|---|---|
| Ph-F1-SPO11 | CAGATAGTGGTGGACGAGCCCTGGATTGCC |
| Ph-F2-SPO11 | AGATAGTGGTGGACGAGCCCTGGATTGCCT |
| Ph-F3-SPO11 | GATAGTGGTGGACGAGCCCTGGATTGCCTT |
| Ph-F4-SPO11 | ATAGTGGTGGACGAGCCCTGGATTGCCTTG |
| Ph-R1-SPO11 | CTTGTGCCAATCTCATAAAAACGCTCTCCT |
| Ph-R2-SPO11 | CGCTCTCCTTTTCAACAACAATAACCGCAA |
| Ph-R3-SPO11 | GATGCTTGTGCCAATCTCATAAAAACGCTC |
| Name | Sequence (5′ to 3′) |
|---|---|
| SPO11-F4-RPA | ATAGTGGTGGACGAGCCCTGGATTGCCTTG |
| SPO11-R1-RPA | Biotin-CTTGTGCCAATCTCATAAAAACGCTCTCCT |
| P1 | 6-FAM-gacgtgtagttccaataccagcttcaccagatg-THF-cataaaagcagttagatgtaca-C3 Spacer |
| P2 | 6-FAM-ccaataccagcttcaccagatggcataaaagcagtta-THF-atgtacaggaattgcggttatt-C3 Spacer |
| Reagent | Dosage |
|---|---|
| ddH2O | 37.75 μL |
| 10× PCR Buffer (Mg2+ plus) | 5 μL |
| dNTP Mixture | 4 μL |
| Forward Primer | 1 μL |
| Reverse Primer | 1 μL |
| rTaq | 0.25 μL |
| DNA | 1 μL |
| Name | Sequence (5′ to 3′) |
|---|---|
| 18SrRNA-F1 | ATGGCGAGTGGTGGAATA |
| 18SrRNA-R1 | CCCAACTACGCTAAGGATT |
| 18SrRNA-F2 | TGTAAACGATGCCGACAGAG |
| 18SrRNA-R2 | CAACACTGAAGCCAATGCGAGC |
| Reagent | Dosage |
|---|---|
| ddH2O | 37.75 μL |
| 10× PCR Buffer (Mg2+ plus) | 5 μL |
| dNTP Mixture | 4 μL |
| Forward Primer | 1 μL |
| Reverse Primer | 1 μL |
| rTaq | 0.25 μL |
| DNA | 1 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Y.; Zhang, X.; Chen, X.; Li, J.; Gong, P.; Wang, X.; Li, X.; Zhang, X.; Yue, T.; Zhang, H.; et al. Development of an LFD-RPA Assay for Rapid Detection of Pentatrichomonas hominis Infection in Dogs. Curr. Issues Mol. Biol. 2023, 45, 9252-9261. https://doi.org/10.3390/cimb45110579
Rong Y, Zhang X, Chen X, Li J, Gong P, Wang X, Li X, Zhang X, Yue T, Zhang H, et al. Development of an LFD-RPA Assay for Rapid Detection of Pentatrichomonas hominis Infection in Dogs. Current Issues in Molecular Biology. 2023; 45(11):9252-9261. https://doi.org/10.3390/cimb45110579
Chicago/Turabian StyleRong, Yao, Xichen Zhang, Xuejiao Chen, Jianhua Li, Pengtao Gong, Xiaocen Wang, Xin Li, Xu Zhang, Taotao Yue, Hongbo Zhang, and et al. 2023. "Development of an LFD-RPA Assay for Rapid Detection of Pentatrichomonas hominis Infection in Dogs" Current Issues in Molecular Biology 45, no. 11: 9252-9261. https://doi.org/10.3390/cimb45110579
APA StyleRong, Y., Zhang, X., Chen, X., Li, J., Gong, P., Wang, X., Li, X., Zhang, X., Yue, T., Zhang, H., Zhou, X., & Zhang, N. (2023). Development of an LFD-RPA Assay for Rapid Detection of Pentatrichomonas hominis Infection in Dogs. Current Issues in Molecular Biology, 45(11), 9252-9261. https://doi.org/10.3390/cimb45110579