Abstract
Pentatrichomonas hominis is a trichomonad protozoan that infects the cecum and colon of humans and other mammals. It is a zoonotic pathogen that causes diarrhea in both animals and humans. As companion animals, dogs infected with P. hominis pose a risk of transmitting it to humans. Current methods, such as direct smears and polymerase chain reaction (PCR), used for P. hominis detection have limitations, including low detection rates and the need for specialized equipment. Therefore, there is an urgent need to develop rapid, sensitive, and simple detection methods for clinical application. Recombinase polymerase amplification (RPA) has emerged as a technology for rapid pathogen detection. In this study, we developed a lateral flow dipstick (LFD)-RPA method based on the highly conserved SPO11-1 gene for detecting P. hominis infection by optimizing the primers, probes, and reaction conditions, and evaluating cross-reactivity with genomes of Giardia duodenalis and other parasites. The LFD-RPA method was then used to test 128 dog fecal samples collected from Changchun. The results confirmed the high specificity of the method with no cross-reactivity with the five other parasites. The lowest detection limit of the method was 102 copies/µL, and its sensitivity was 100 times higher than that of the conventional PCR method. Consistent with the positivity rate observed using nested PCR, 12 samples (out of 128) tested positive using this method (positivity rate, 9.38%). In conclusion, the LFD-RPA method developed in this study represents a simple and sensitive assay that allows for the rapid detection of P. hominis infection in dogs, especially in this field.
1. Introduction
Pentatrichomonas hominis (P. hominis) is a zoonotic parasite that primarily colonizes the colon and cecum in humans and other vertebrates and is distributed worldwide. It was previously considered to be an intestinal non-pathogenic symbiotic parasite or a conditional pathogenic protozoan. However, in recent years, P. hominis has been isolated from human and animal feces with diarrhea [1], indicating that it may be pathogenic. P. hominis infection causes mushy feces, diarrhea, constipation, bloody stools, and even rectal prolapse in animals [2]. Recent studies have shown that P. hominis infections are associated with gastrointestinal cancer [3].
P. hominis has a relatively wide host range, and has been detected in various animals, including dogs and cats, and in humans [4,5,6,7]. P. hominis that infects both dogs and humans belongs to the CC1 type [8]. This suggests that P. hominis can spread between humans and dogs. Dogs, as companion animals, are often in close contact with humans, and hence those infected with P. hominis may transmit it to humans. Current methods for detecting P. hominis infection in animals mainly include pathogen detection and polymerase chain reaction (PCR). Although the direct smear method is the “gold standard” for detecting infection, it has a low detection rate and pathogen detection is easy to miss [9]. PCR is more specific and sensitive than pathogen detection; however, it requires special equipment, and is not practical for field application [10]. Although rapid detection methods for some parasite infections including colloidal gold and RPA, etc., have been established, there are no rapid detection methods for P. hominis infection currently. Therefore, it is necessary to establish a rapid, sensitive, convenient, and suitable method for the clinical detection of P. hominis infection.
Recombinase polymerase amplification (RPA) is a relatively new and rapid diagnostic method that has been widely used for the detection of parasites [11,12]. Lateral flow dipstick (LFD)-RPA is a rapid, on-site detection system that combines RPA technology with LFD. Currently, there are no reports on an LFD-RPA-based detection method for P. hominis infection. Therefore, in this study, we developed an LFD-RPA method using the target gene SPO11-1 for the clinical detection of P. hominis infection in dogs.
2. Materials and Methods
2.1. Sample Collection
A P. hominis sample was collected from the feces of naturally-infected dogs from the endemic area of Changchun (Jilin Province, China). Several P. hominis-related and other common parasites, including Giardia duodenalis (G. duodenalis), Tritrichomonas foetus (T. foetus), Isospora canis (I. canis), Toxocara canis (T. canis), and Cryptosporidium parvum (C. parvum) (all maintained in our laboratory) were used as controls to evaluate the specificity of this method. Dog fecal samples used for analyses were randomly collected from a stray animal rescue station and an animal hospital in Changchun. The collection procedures of dog feces were conducted in strict accordance with the guidelines of the Animal Care and Welfare Committee of Jilin University (No: SY202201109).
2.2. DNA Extraction
Total genomic DNA was extracted from fecal samples (from dogs with P. hominis infection) using the TIANamp Genomic DNA Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. Genomic DNA was extracted from the other control samples using the same method and stored at −20 °C.
2.3. Primer and Probe Design
SPO11-1 (GenBank accession no. JF298016) was selected as the target gene for the detection of P. hominis infection using LFD-RPA. Twelve pairs of primers were designed using Primer 5.0 software (Table 1). PCR amplification followed by electrophoresis on a 3% agarose gel was used to screen for high-specificity primers. The genomes of G. duodenalis, Trichomonas murinus, Trichomonas vaginalis, and P. hominis were used to validate the primer specificity. Based on the target fragment amplified by the primers, the DNA probe was designed with the addition of 6-FAM modification at the 5′ end and C3-spacer modification at the 3′ end (Table 2). Using P. hominis as the positive template, the two probes were compared in the LFD-RPA assay via incubation in a 39 °C water bath for 30 min and observation of the color change of the LFD test line.

Table 1.
Primer design for SPO11-1 gene.

Table 2.
Primer probe sequence.
2.4. RPA Reaction and Lateral Flow Reading
RPA reactions were performed using the RAA-nfo Nucleic Acid Amplification Kit (Hangzhou ZC Bio-Sci & Tech Co., Ltd., Hangzhou, China) according to the manufacturer’s instructions. A reaction mixture consisting of 25 µL of A Buffer, 1.2 µL of 2 µM upstream primer, 1.2 µL of 2 µM downstream primer, 0.25 µL of 2 µM probe, and 15.1 µL of ddH2O was added to the reaction dry powder tube. After mixing, the mixture was divided into two tubes, and 2.5 µL of DNA sample and 1.25 µL of B Buffer were added to each tube. After centrifugation at low speed, the mixture was mixed and allowed to settle at the bottom.
The tubes were incubated in a water bath at six different temperatures (25, 30, 35, 40, 45, and 50 °C) for 20 min. The amplification product was diluted 8× to 225 µL, and 10 µL of the amplification product was placed in the sample pad of the disposable Nucleic Acid Detection Kit (Hangzhou ZC Bio-Sci & Tech Co., Ltd.). Then, 10 µL of LFD buffer was added, and the color change of the LFD was observed after 2–3 min. The optimal reaction temperature was determined based on the color intensity of the LFD test line.
To determine the optimal reaction time, the water bath was heated to the optimal reaction temperature, and the LFD-RPA reactions were performed for different periods (0, 2, 5, 10, 15, 20, 25, or 30 min). The color intensity of the LFD test line was observed.
2.5. Specificity of the LFD-RPA Assay
To verify the specificity of the LFD-RPA assay, the genomes of G. duodenalis, T. foetus, I. canis, T. canis, and C. parvum were used as RPA reaction templates, and LFD-RPA was performed at the optimal reaction time and temperature. The color intensity of the LFD test line was observed to determine the specificity of the detection method.
2.6. Sensitivity of the LFD-RPA Assay
Genomic DNA of P. hominis was serially diluted 10-fold (108–100 copies/μL), and different copy numbers of the genome were used as templates for LFD-RPA. The minimum detection limit of the method was determined via observation of the color intensity of the LFD test line, and nucleic acid templates were detected with traditional PCR using the designed RPA primers (Table 3). The PCR program was as follows: 95 °C for 3 min; thirty-four 3-step cycles consisting of denaturation at 95 °C for 30 s, annealing at 61.1 °C for 30 s, and extension at 72 °C for 20 s; followed by final extension at 72 °C for 10 min. The minimum detectable amount with this primer for P. hominis in the PCR assay was determined to compare the sensitivity of the LFD-RPA method.

Table 3.
PCR system.
2.7. Applicability of the LFD-RPA Assay
A total of 128 clinical fecal samples from dogs were obtained from an animal rescue station and an animal hospital in Changchun. P. hominis infection status was determined using the LFD-RPA method, based on the color intensity of the LFD test line. A nested PCR [13] and microscopic examination were performed simultaneously on these samples, and the results were analyzed and compared with those of the LFD-RPA method. The primers and program used for nested PCR are listed in Table 4 and Table 5. The reaction conditions were as follows: first round of nested PCR: 95 °C for 3 min, followed by 34 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 38 s, followed by final 72 °C extension for 7 min. Second round of nested PCR: 95 °C for 3 min, followed by 40 cycles at 95 °C for 45 s, 57 °C for 30 s, and 72 °C for 1 min, followed by final 72 °C extension for 7 min.

Table 4.
Primer sequences.

Table 5.
Nested PCR system.
3. Results
3.1. Primer and Probe Design
Twelve pairs of primers were designed for PCR, and the amplification products were screened using 3% agarose gel electrophoresis. Primers R1 and F4 were selected as the upstream and downstream primers, respectively, for LFD-RPA, as they produced bright bands, exhibited strong specificity, and did not generate primer dimers (Figure 1a). The results of the specificity verification assay showed that the primers amplified only the genome of P. hominis and not that of the other parasite genomes tested, thus confirming that the primers were highly specific (Figure 1b). Based on the target fragment amplified by the primers, two probes were designed and the intensity of the test line produced by the two probes was compared. Based on the results, probe P1 was selected as the final probe (Figure 1c).
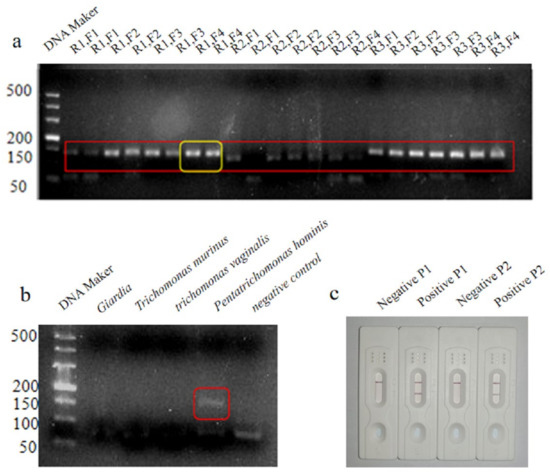
Figure 1.
Results of agarose gel electrophoresis with 12 pairs of designed primers, The red square is the target fragment and the yellow square is the best target fragment that we choose to use as the best primer pair. (a); primer specificity verification results, Shown in the red square is an amplified fragment of pentacomonas hominis. (b); results of the LFD assay for comparison of the two probes (c).
3.2. Optimization of the LFD-RPA Assay
The reaction temperature was optimized by performing the reaction for 20 min at different temperatures. The results showed the appearance of a faint test line at 30 °C, which increased in intensity at 35 °C. The color intensity started to reduce at 45 °C and became even fainter at 50 °C. Therefore, 35 °C was determined to be the optimal reaction temperature (Figure 2a).
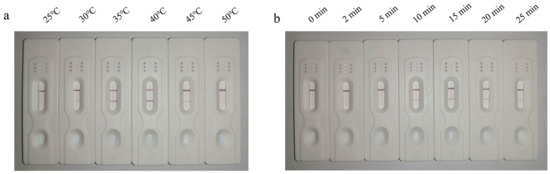
Figure 2.
LFD-RPA showing detectable test line at 35 °C that gradually faded at 45 °C (a). LFD-RPA reaction performed at the optimal reaction temperature for 10 min showing initiation of amplification as evidenced by clearly detectable test line in the LFD (b).
The reaction time was optimized by performing the reaction at the optimal reaction temperature of 35 °C for different time periods. The results showed an absence of amplification at 0–5 min, as the test line in the LFD was undetectable. The amplification reaction began at a reaction time of 10 min, as evidenced by the appearance of a detection line in the dipsticks. Therefore, the optimal reaction time was determined to be 10 min (Figure 2b).
3.3. Optimization of the LFD-RPA Assay
The genomes of P. hominis, G. duodenalis, T. foetus, I. foetus, T. canis, and C. parvum were used as templates to test the specificity of the LFD-RPA. The reaction was performed at the optimal reaction time and temperature. The results showed that only the genome was amplified using the test strip. No amplification was observed for any of the other parasites, indicating optimal specificity of the assay (Figure 3).

Figure 3.
Specificity analysis of LFD-RPA showing specific amplification of the P. hominis genome.
3.4. Sensitivity Analysis of LFD-RPA Assay
The sensitivity of the LFD-RPA was analyzed using the different copies of P. hominis genomic samples. The results showed that the lowest detection limit of the LFD-RPA method was 102 copies/μL (Figure 4a), whereas as that of PCR was 104 copies/μL (Figure 4b), showing that the sensitivity of the LFD-RPA is higher than that of PCR.
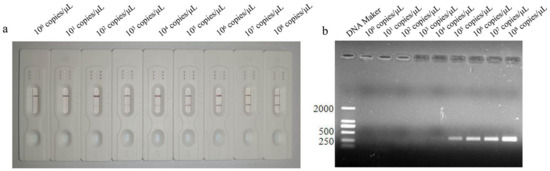
Figure 4.
Lowest detection limit of LFD-RPA method (appearance of test line) at 102 copies/μL (a), and the lowest detection limit of PCR method (appearance of corresponding band) at 104 copies/μL (b).
3.5. Comparative Clinical Samples
The LFD-RPA method was used to analyze 128 clinical dog fecal samples. The results showed that with the LFD-RPA method, 12 out of 128 fecal samples tested positive with a positivity rate of 9.38% (Figure 5a); that was consistent with the results of nested PCR (Figure 5b). Thus, the results of the LFD-RPA were consistent with those of nested PCR (Figure 5c), with a coincidence rate of 100%.
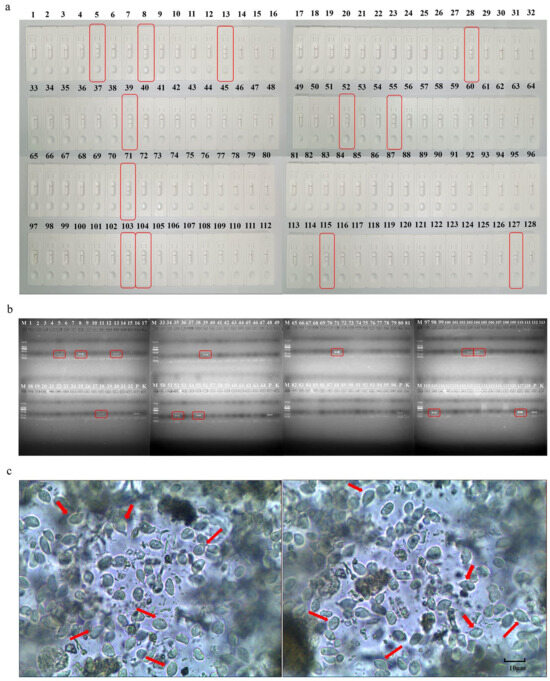
Figure 5.
Detection of 12 positive samples (out of 128 dog fecal samples tested) using LFD-RPA, the red squares show positive samples. (a); detection of 12 positive samples (out of 128 dog fecal samples tested) using nested PCR assay. P, positive control; K, negative control, the red squares show positive samples. (b). Partial results of stool microscopy, the red arrow points to the body of pentrichomonas hominis as seen under the microscope. (c).
4. Discussion
Recent molecular epidemiological studies indicate a high rate of P. hominis infection among dogs in many countries, with infection rates ranging from 6.45 to 47.4% [4,5,6]. This poses a high risk of zoonotic transmission to humans. In addition, P. hominis has been detected in the feces of immuno-compromised patients, including those with rheumatoid arthritis treated with adalimumab, irritable bowel syndrome, and empyema [14,15,16]. However, methods for the detection of P. hominis infection are limited to traditional pathogen- and PCR-based detection modalities.
LFD-RPA has been widely used for the detection of bacteria such as Brucella, viruses such as H7N9, IBRV [17,18,19], and parasites. Crannell et al. [20] developed an RPA assay for the detection of G. duodenalis infection based on the amplification of a fragment of the β-giardin gene. Li et al. [21] developed an LFD-RPA method using the highly conserved mitochondrial small-subunit ribosomal RNA as the target gene to detect Trichinella spiralis infection. SPO11 is a member of a highly conserved protein family, and its sequence, structure and functions are highly conserved [22]. SPO11 has been extensively studied in plants. Studies of Arabidopsis thaliana and wheat have shown that SPO11 controls the formation and repair of meiosis-specific DNA double-strand breaks (DSBs), and its function has been conserved during biological evolution [23,24]. It catalyzes specific DNA DSBs during meiosis in Saccharomyces cerevisiae, fission yeast, and Caenorhabditis elegans, and plays an important role in the sexual reproduction of organisms [25]. In this study, the SPO11-1 gene was selected as the specific target for the detection of P. hominis infection.
Screening of suitable primers and probes is crucial for the success of RPA. Unlike in basal-RPA systems, endonuclease Ⅳ (Nfo), nfo probes, and primers labeled with biotin or digoxin were used in the LFD-RPA reaction [26]. The probe contained a base sequence with a marker at the 5′ end, a tetrahydrofuran (THF), and a blocking modification group at the 3′ end. Nfo, a DNA repair enzyme, recognizes and cleaves this site. As the reaction proceeds, the cleaved probe and downstream primer form a double-labeled amplicon with both the probe and prime-specific labels. The RPA primer sequence selected for the method was from a highly differentiated region of SPO11-1, and 6-FAM (at the 5′ end) and C3-spacer (at the 3′ end) were selected as optimal modifications.
The sensitivity of RPA is markedly higher than that of PCR. A previous report showed that the minimum detection limit is 0.5 Cryptosporidium oocysts for LFA-RPA to detect Cryptosporidium infection in dairy cows, and its sensitivity is 6× that of nested PCR [27]. Another study used LFD-RPA to detect the three stages of Clonorchis sinensis, including adults, eggs, and cercariae, and reported a minimum detection limit of 10 fg for adults with a sensitivity 1000× that of PCR. The detection limit for purified eggs and metacercariae was one, and the sensitivity was 25× that of PCR [28]. We used SPO11-1 as the target gene to develop an LFD-RPA assay to detect P. hominis infection. The minimum detection limit of the assay was 102 copies/μL, whereas that of PCR using the same primers was 104 copies/μL. The sensitivity of the LFD-RPA assay was 100× that of PCR.
5. Conclusions
In this study, we successfully developed an LFD-RPA assay based on the SPO11-1 gene for the detection of P. hominis infection. Screening of clinical samples showed that the LFD-RPA method is fast, simple, and has an easy readout, which renders it useful for clinical application in the rapid detection of P. hominis infection in dogs.
Author Contributions
Conceptualization, N.Z.; methodology, Y.R. and X.Z. (Xichen Zhang); formal analysis, Y.R. and X.Z. (Xichen Zhang); investigation, Y.R., X.C. and X.Z. (Xichen Zhang); resources, X.L., X.Z. (Xu Zhang), X.Z. (Xiaofei Zhou) and X.C.; writing—original draft preparation, Y.R. and X.Z. (Xichen Zhang); writing—review and editing, J.L., P.G., X.W. and X.Z. (Xichen Zhang); visualization, T.Y., H.Z. and X.C.; funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant from the National Key Research and Development Program of China (No. 2021YFF0702900) and the National Natural Science Foundation of China (No. 32102696).
Institutional Review Board Statement
The collection procedures of dog feces were conducted in strict accordance with the guidelines of the Animal Care and Welfare Committee of Jilin University (No: SY202201109).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data have been included in the manuscript.
Acknowledgments
We thank Xiaojing Song of Jilin University for her help with the collection of clinical fecal samples of dogs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kamaruddin, M.; Tokoro, M.; Rahman, M.M.; Arayama, S.; Hidayati, A.P.; Syafruddin, D.; Asih, P.B.; Yoshikawa, H.; Kawahara, E. Molecular characterization of various trichomonad species isolated from humans and related mammals in Indonesia. Korean J. Parasitol. 2014, 52, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, C.C.; McIntosh, D.; Dos Santos, D.J.; Lopes, S.d.P.; de Jesus, V.L.T. Morphological and molecular characterization of a species of Tetratrichomonas present in feces of Brazilian sheep (Ovis aries) and goats (Capra hircus). Parasitol. Res. 2019, 119, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Yu, Y.; Gong, P.; Li, J.; Li, Z.; Li, T.; Cong, Z.; Tian, C.; Liu, X.; et al. High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasites Vectors 2019, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Saksirisampant, W.; Nuchprayoon, S.; Wiwanitkit, V.; Yenthakam, S.; Ampavasiri, A. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J. Med. Assoc. Thai. 2003, 86 (Suppl. S2), S263–S270. [Google Scholar]
- Kim, Y.A.; Kim, H.Y.; Cho, S.H.; Cheun, H.-I.; Yu, J.-R.; Lee, S.-E. PCR detection and molecular characterization of Pentatrichomonas hominis from feces of dogs with diarrhea in the Republic of Korea. Korean J. Parasitol. 2010, 48, 9–13. [Google Scholar] [CrossRef]
- Grellet, A.; Brunopolack; Feugier, A.; Boucraut-Baralon, C.; Grandjean, D.; Vandewynckel, L.; Cian, A.; Meloni, D.; Viscogliosi, E. Prevalence, risk factors of infection and molecular characterization of trichomonads in puppies from French breeding kennels. Vet. Parasitol. 2013, 197, 418–426. [Google Scholar] [CrossRef]
- Bastos, B.F.; Brener, B.; de Figueiredo, M.A.; Leles, D.; Mendes-De-Almeida, F. Pentatrichomonas hominis infection in two domestic cats with chronic diarrhea. JFMS Open Rep. 2018, 4, 2055116918774959. [Google Scholar] [CrossRef]
- Li, W.C.; Ying, M.; Gong, P.T.; Li, J.-H.; Yang, J.; Li, H.; Zhang, X.-C. Pentatrichomonas hominis: Prevalence and molecular characterization in humans, dogs, and monkeys in Northern China. Parasitol. Res. 2016, 115, 569–574. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kon, M.; Saito, T.; Maeno, N.; Koyama, M.; Sunaoshi, K.; Yamaguchi, M.; Morishima, Y.; Kawanaka, M. Prevalence of intestinal canine and feline parasites in Saitama Prefecture, Japan. Kansenshogaku Zasshi. 2009, 83, 223–228. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R.; Socha, P. Detection of Pentatrichomonas hominis in dogs using real-time PCR. Pol. J. Vet. Sci. 2015, 18, 775–778. [Google Scholar] [CrossRef]
- Tan, M.; Liao, C.; Liang, L.; Yi, X.; Zhou, Z.; Wei, G. Recent advances in recombinase polymerase amplification: Principle, advantages, disadvantages and applications. Front. Cell. Infect. Microbiol. 2022, 12, 1019071. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; A Armes, N. DNA detection using recombination proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Gong, P.; Cheng, S.; Wang, X.; Li, X.; Hou, Z.; Liu, C.; Bi, T.; Wang, B.; et al. Prevalence and molecular characterization of Pentatrichomonas hominis in Siberian tigers (Panthera tigris altaica) in northeast China. Integr. Zool. 2022, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Morio, F.; Renard, B.T.; Poirier, A.-S.; Miegeville, M.; Chambreuil, G. Trichomonads in pleural effusion: Case report, literature review and utility of PCR for species identification. New Microbiol. 2012, 35, 83–87. [Google Scholar] [PubMed]
- Wang, H.K.; Jerng, J.S.; Su, K.E.; Chang, S.-C.; Jerng, J.S. Trichomonas empyema with respiratory failure. Am. J. Trop. Med. Hyg. 2006, 75, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.L.; Doherty, D.E.; Ribes, J.; Seabolt, J.P.; Bensadoun, E.S. Empyema Caused by Trichomonas. Chest 2003, 123, 291–292. [Google Scholar] [CrossRef]
- Gumaa, M.M.; Cao, X.; Li, Z.; Lou, Z.; Zhang, N.; Zhang, Z.; Zhou, J.; Fu, B. Establishment of a recombinase polymerase amplification (RPA) assay for the detection of Brucella spp. Infection. Mol. Cell. Probes 2019, 47, 101434. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Peng, B.; Wu, W.; Wang, X.; Liu, H.; Yuan, L.; Fang, S.; Lu, J. Rapid Detection of Avian Influenza A Virus (H7N9) by Lateral Flow Dipstick Recombinase Polymerase Amplification. Biol. Pharm. Bull. 2018, 41, 1804–1808. [Google Scholar] [CrossRef]
- Hou, P.; Wang, H.; Zhao, G.; He, C.; He, H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017, 13, 386. [Google Scholar] [CrossRef]
- Crannell, Z.A.; Cabada, M.M.; Castellanos-Gonzalez, A.; White, A.C.; Irani, A.; Richards-Kortum, R. Recombinase polymerase amplification-based assay to diagnose Giardia in stool samples. Am. J. Trop. Med. Hyg. 2015, 92, 583–587. [Google Scholar] [CrossRef]
- Li, T.T.; Wang, J.L.; Zhang, N.Z.; Li, W.-H.; Yan, H.-B.; Li, L.; Jia, W.-Z.; Fu, B.-Q. Rapid and Visual Detection of Trichinella Spp. Using a Lateral Flow Strip-Based Recombinase Polymerase Amplification (LF-RPA) Assay. Front. Cell. Infect. Microbiol. 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Giroux, C.N.; Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 1997, 88, 375–384. [Google Scholar] [CrossRef]
- Lambing, C.; Kuo, P.; Kim, J.; Osman, K.; Whitbread, A.L.; Yang, J.; Choi, K.; Franklin, F.C.H.; Henderson, I.R. Differentiated function and localisation of SPO11-1 and PRD3 on the chromosome axis during meiotic DSB formation in Arabidopsis thaliana. PLoS Genet. 2022, 18, e1010298. [Google Scholar] [CrossRef] [PubMed]
- Da Ines, O.; Michard, R.; Fayos, I.; Bastianelli, G.; Nicolas, A.; Guiderdoni, E.; White, C.; Sourdille, P. Bread wheat TaSPO11-1 exhibits evolutionarily conserved function in meiotic recombination across distant plant species. Plant J. 2020, 103, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Dernburg, A.F.; McDonald, K.; Moulder, G.; Barstead, R.; Dresser, M.; Villeneuve, A.M. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 1998, 94, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Greeshma, M.; Bhat, A.I.; Jeevalatha, A. Rapid onsite detection of piper yellow mottle virus infecting black pepper by recombinase polymerase amplification-lateral flow assay (RPA-LFA). J. Virol. Methods 2023, 315, 114695. [Google Scholar] [CrossRef]
- Wu, Y.D.; Zhou, D.H.; Zhang, L.X.; Zheng, W.-B.; Ma, J.-G.; Wang, M.; Zhu, X.-Q.; Xu, M.-J. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for equipment-free detection of Cryptosporidium spp. oocysts in dairy cattle feces. Parasitol. Res. 2016, 115, 3551–3555. [Google Scholar] [CrossRef]
- Ma, X.; Bai, X.; Li, H.; Ding, J.; Zhang, H.; Qiu, Y.; Wang, J.; Liu, X.; Liu, M.; Tang, B.; et al. A rapid and visual detection assay for Clonorchis sinensis based on recombinase polymerase amplification and lateral flow dipstick. Parasites Vectors 2023, 16, 165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).