Cloning, Expression and Functional Characterization of a Novel α-Humulene Synthase, Responsible for the Formation of Sesquiterpene in Agarwood Originating from Aquilaria malaccensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of RNA and cDNA Synthesis
2.3. Identification of Sesquiterpene Synthase Gene from A. malaccensis and Isolation of Its Full-Length Sequence
2.4. Ligation and Transformation of the Recombinant Vector into Host Cell
2.5. In Silico Analysis of the Sesquiterpene Synthase Gene
2.6. Gene Synthesis and Expression of Sesquiterpene Synthase in E. coli
2.7. Enzyme Assay and Identification of Sesquiterpene Using GC-MS Analysis
3. Results and Discussion
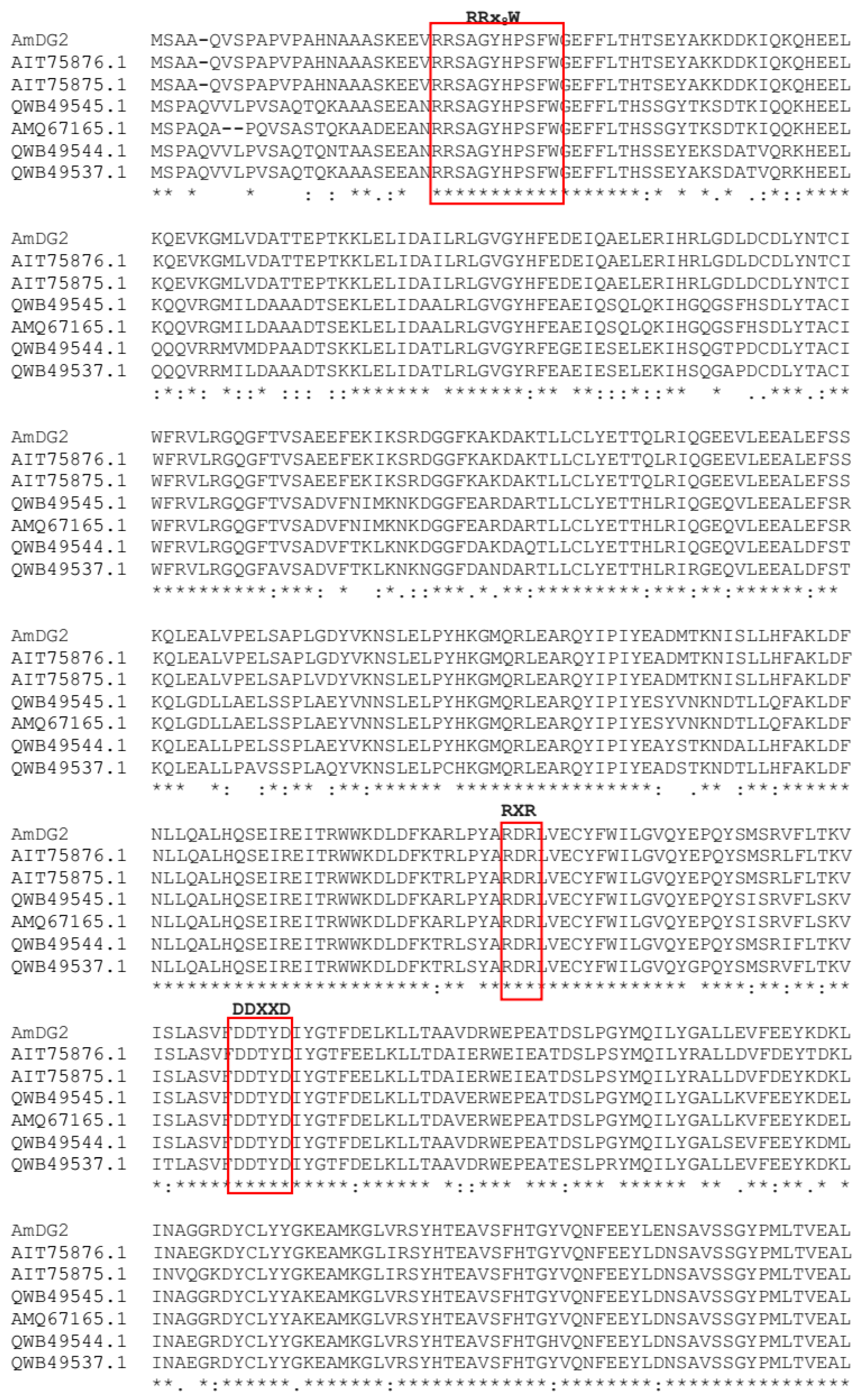
3.1. Full-Length Sesquiterpene Synthase (AmDG2) Sequence Analysis
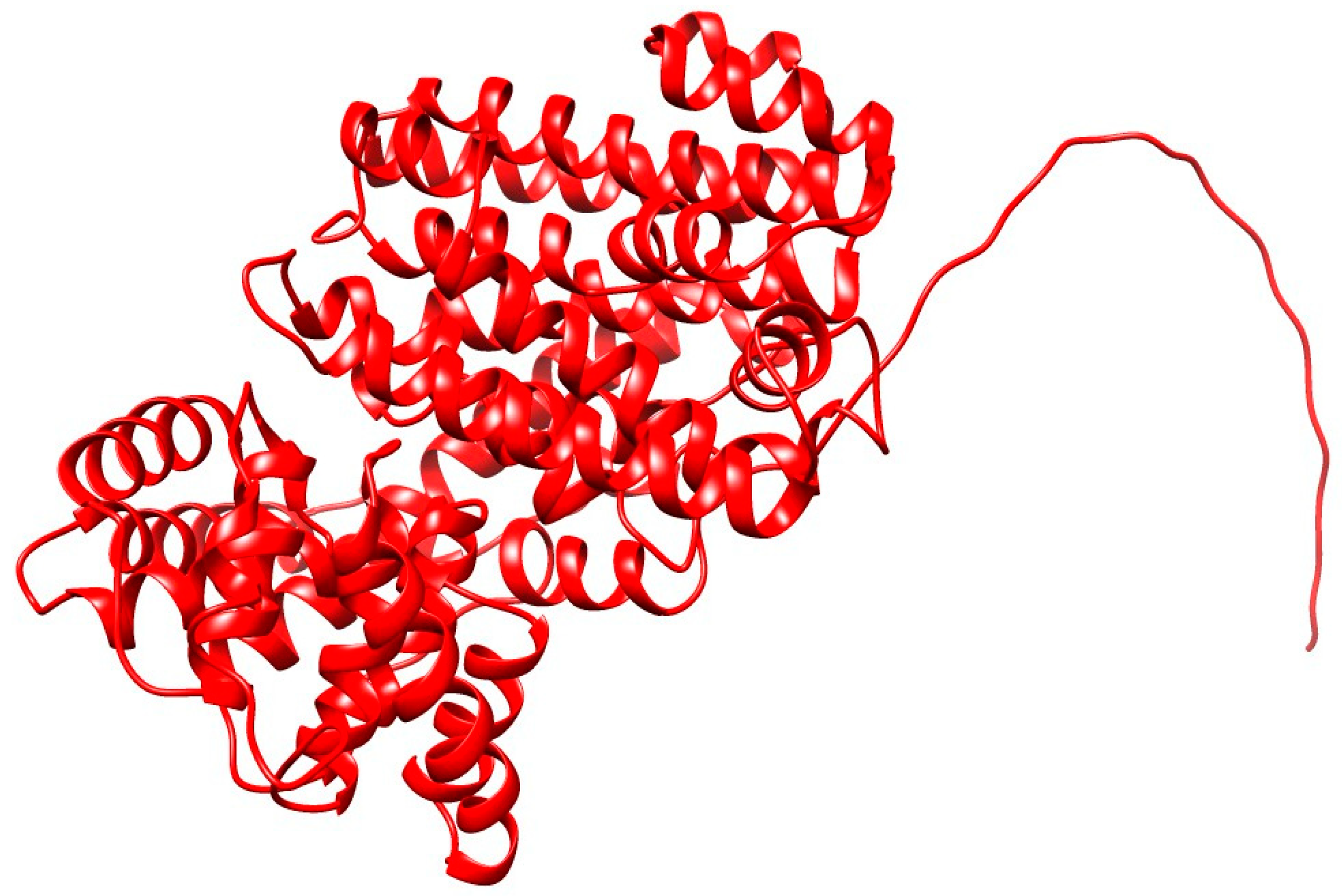
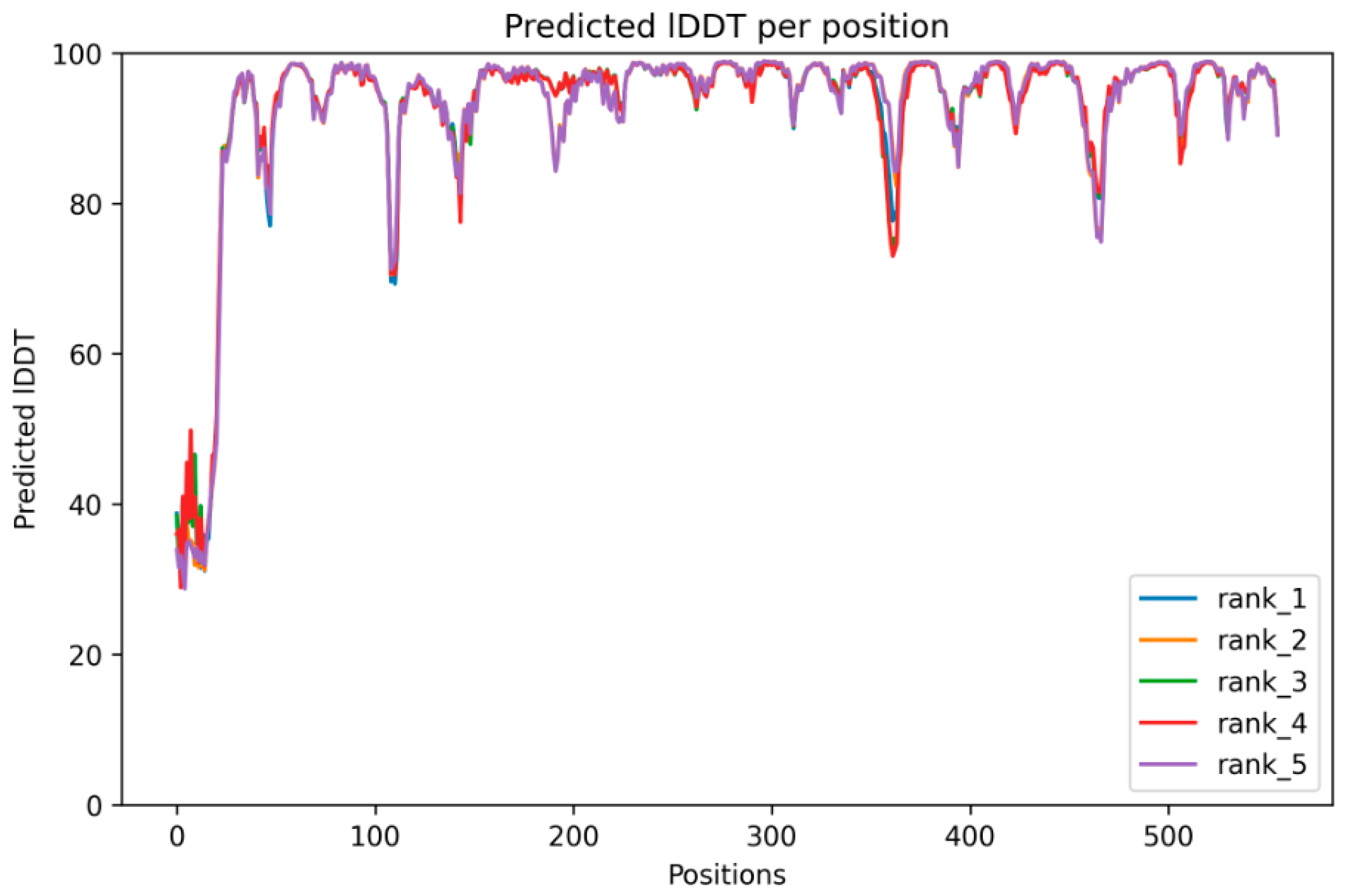
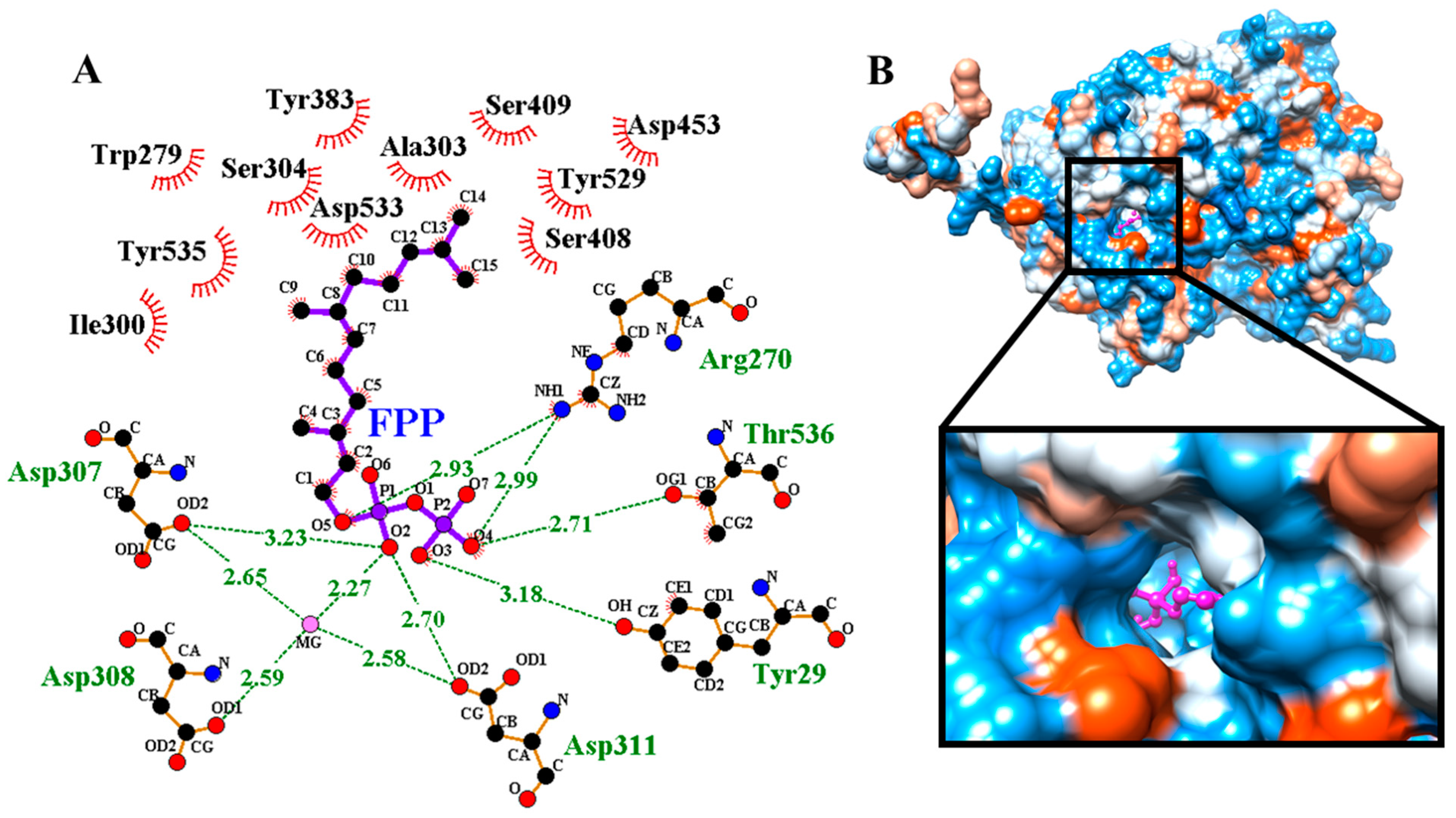
3.2. Structure Prediction, Validation and Site-Specific Molecular Docking
3.3. Expression of AmDG2 in E. coli
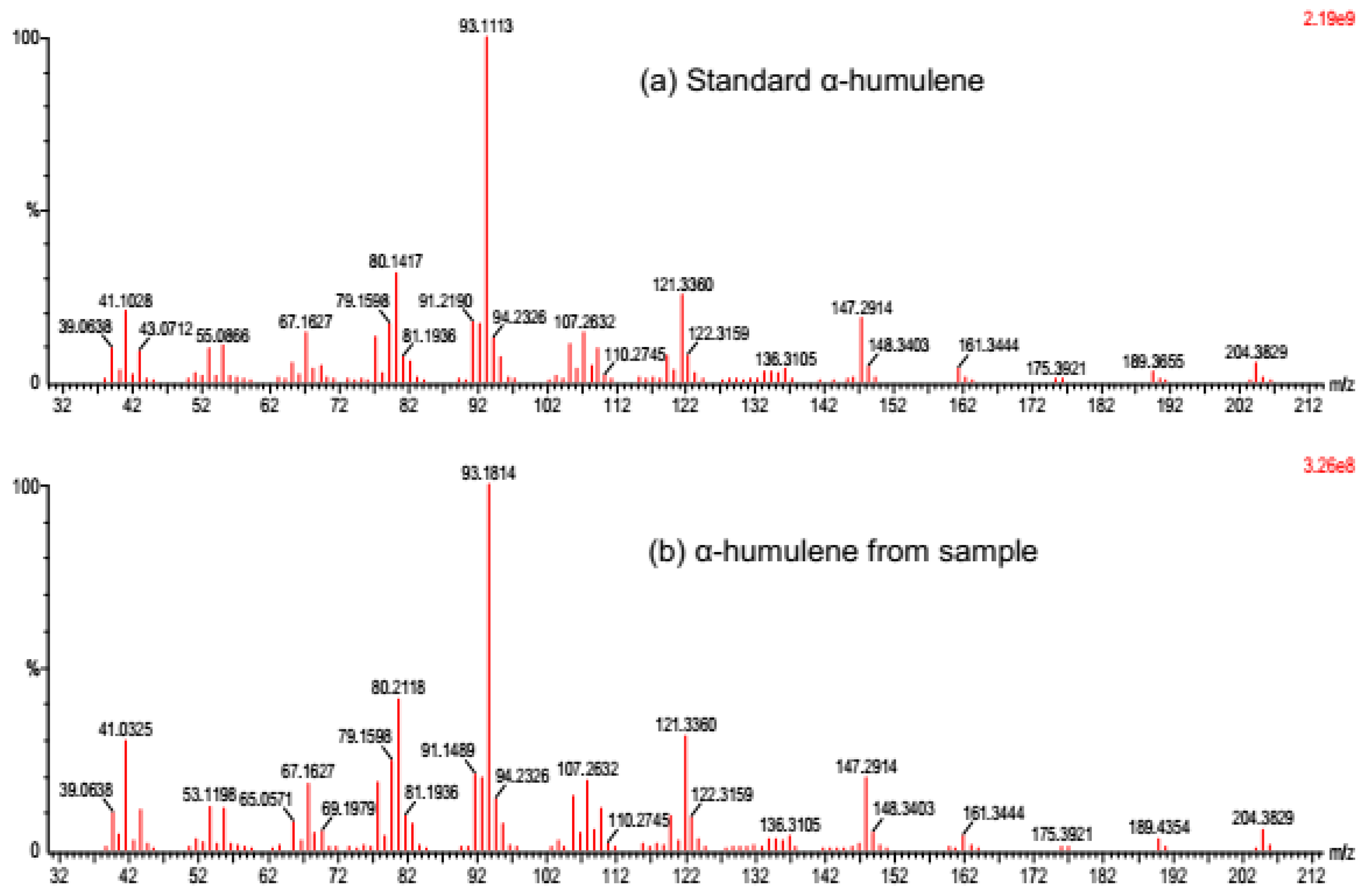
3.4. Enzyme Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Yu, C.; Cheng, B.; Han, Y.; Luo, L.; Pan, H.; Zhang, Q. Studies on the volatile compounds in flower extracts of Rosa odorata and R. chinensis. Ind. Crops Prod. 2020, 146, 112143. [Google Scholar] [CrossRef]
- Powers, J.M.; Seco, R.; Faiola, C.L.; Sakai, A.K.; Weller, S.G.; Campbell, D.R.; Guenther, A. Floral Scent Composition and FineScale Timing in Two Moth-Pollinated Hawaiian Schiedea (Caryophyllaceae). Front. Plant Sci. 2020, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbicvore-induced plantg volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Brosset, A.; Blande, J.E. Volatile-mediated plant–plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Derbassi, N.B.; Pedrosa, M.C.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Plant volatiles: Using Scented molecules as food additives. Trends Food Sci. 2022, 122, 97–103. [Google Scholar] [CrossRef]
- Hattan, J.I.; Shindo, K.; Sasaki, T.; Ohno, F.; Tokuda, H.; Ishikawa, K.; Misawa, N. Identification of novel sesquiterpene synthase genes that mediate the biosynthesis of valerianol, which was an unknown ingredient of tea. Sci. Rep. 2018, 8, 12474. [Google Scholar] [CrossRef]
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the production of isoprenoids. FEMS Microbiol. Lett. 2018, 365, fny079. [Google Scholar] [CrossRef]
- Mai, J.; Li, W.; Ledesma-Amaro, R.; Xiao-Jun, J. Engineering Plant Sesquiterpene Synthesis into Yeasts: A Review. J. Agric. Food Chem. 2021, 69, 9498–9510. [Google Scholar] [CrossRef]
- Rusdi, N.A.; Goh, H.H.; Sabri, S.; Ramzi, A.B.; Mohd Noor, N.; Baharum, S.N. Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum minus. Molecules 2018, 23, 1370. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Lee, S.Y. Keeping up appearances: Agarwood grades and quality. In Agarwood; Mohamed, R., Ed.; Springer: Singapore, 2016; pp. 149–167. [Google Scholar] [CrossRef]
- Jalil, A.M.; Abdul Hamid, H.; Lias, S.; Anwar Uyup, M.K.; Md Tahir, P.; Mohd Razali, S.; Mohd Noor, A.A.; Syazwan, S.A.; Shamsul Anuar, A.S.; Mohamad Kasim, M.R.; et al. Assessment of the Effects of Artificial Fungi Inoculations on Agarwood Formation and Sap Flow Rate of Aquilaria malaccensis Lam. Using Sonic Tomography (SoT) and Sap Flow Meter (SFM). Forests 2022, 13, 1731. [Google Scholar] [CrossRef]
- Deep, K.; Tajuddin, S.N. King of Scents—Agarwood. Perfum. Flavor 2019, 44, 42–56. [Google Scholar]
- Ran, J.; Li, Y.; Wen, X.; Geng, X.; Si, X.; Zhang, L.; Ma, Y.; Zhang, Z. Identification of sesquiterpene synthase genes in the genome of Aquilaria sinensis and characterization of an α-humulene synthase. J. For. Res. 2023, 34, 1117–1131. [Google Scholar] [CrossRef]
- Sundaraj, S.; Mediani, A.; Rodriques, K.F.; Baharum, S.N. GC-MS olfactometry reveals sesquiterpenes α-humulene and δ-cadinene significantly influence the aroma of treated Aquilaria malaccensis essential oil. Plant Omics 2023, forthcoming. [Google Scholar]
- Kumeta, Y.; Ito, M. Characterization of α-humulene synthases responsible for the production of sesquiterpenes induced by methyl jasmonate in Aquilaria cell culture. J. Nat. Med. 2016, 70, 452–459. [Google Scholar] [CrossRef]
- Jones, C.G.; Moniodis, J.; Zulak, K.G.; Scafdi, A.; Plummer, J.A.; Ghisalberti, E.L.; Barbour, E.L.; Bohlmann, J. Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J. Biol. Chem. 2011, 286, 17445–17454. [Google Scholar] [CrossRef]
- Keeling, C.I.; Weisshaar, S.; Ralph, S.G.; Jancsik, S.; Hamberger, B.; Dullat, H.K.; Bohlmann, J. Transcriptome mining, functional characterization, and phylogeny of a large terpene synthase gene family in spruce (Picea spp.). BMC Plant Biol. 2011, 11, 43. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Spyropoulou, E.A.; Diergaarde, P.J.; Volpin, H.; De Both, M.T.; Zerbe, P.; Bohlmann, J.; Falara, V.; Matsuba, Y.; Pichersky, E.; et al. RNA-seq discovery, functional characterization, and comparison of sesquiterpene synthases from Solanum lycopersicum and Solanum habrochaites trichomes. Plant Mol. Biol. 2011, 77, 323–336. [Google Scholar] [CrossRef]
- Yu, F.; Okamto, S.; Nakasone, K.; Adachi, K.; Matsuda, S.; Harada, H.; Misawa, N.; Utsumi, R. Molecular cloning and functional characterization of α-humulene synthase, a possible key enzyme of zerumbone biosynthesis in shampoo ginger (Zingiber zerumbet Smith). Planta 2008, 227, 1291–1299. [Google Scholar] [CrossRef]
- Wang, G.D.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.B.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene biosynthesis in glandular trichomes of hop. Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.M.; Omar Farouk, A.A.; Mohamad, A.S.; Sulaiman, M.R.; Perimal, E.K. Zerumbone alleviates chronic constriction injuryinduced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomed. Pharmacother. 2016, 83, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ren, M.Y.; Wang, Z.X.; Feng, S.J.; Li, S.; Cheng, Y.; Hu, C.X.; Gao, S.Q.; Zhang, G.Q. Zerumbone inhibits melanoma cell proliferation and migration by altering mitochondrial functions. Oncol. Lett. 2017, 13, 2397–2402. [Google Scholar] [CrossRef]
- Yeo, D.; Hwang, S.J.; Song, Y.S.; Lee, H.J. Humulene Inhibits Acute Gastric Mucosal Injury by Enhancing Mucosal Integrity. Antioxidants 2021, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, S.; König, J.C.; Hartwig, S.; Frister, T.; Scheper, T.; Beutel, S. Bioproduction of α-humulene in metabolically engineered Escherichia coli and application in zerumbone synthesis. Eng. Life Sci. 2017, 17, 900–907. [Google Scholar] [CrossRef]
- IPNI 2023 International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. Available online: http://www.ipni.org (accessed on 10 August 2023).
- Abdul Kadir, F.A.; Azizan, K.A.; Othman, R. Transcriptome of Aquilaria malaccensis containing agarwood formed naturally and induced artificially. BMC Res. Notes 2021, 25, 117. [Google Scholar] [CrossRef]
- Abdul Kadir, F.A.; Azizan, K.A.; Othman, R. Datasets of essential oils from naturally formed and artificially induced Aquilaria malaccensis agarwoods. Data Brief 2020, 28, 104987. [Google Scholar] [CrossRef]
- Abd Rasib, A.A.; Tong, F.X.; Mohemed Hussein, Z.A.; Othman, R. Sequence analysis of terpene synthase cDNA from transcriptome profile of infected Aquilaria malaccensis. Malays. J. Biochem. Mol. Biol. 2018, 21, 71–72. [Google Scholar]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Kumeta, Y.; Ito, M. Characterization of δ-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiol. 2010, 154, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Ashaari, N.S.; Ab Rahim, M.H.; Sabri, S.; Lai, K.S.; Song, A.A.; Rahim, R.A.; Wan Abdullah, W.M.A.N.; Abdullah, J.O. Functional Characterization of a New Terpene Synthase from Plectranthus amboinicus. PLoS ONE 2020, 15, e0235416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Jin, J.; Sarojam, R.; Ramachandran, S.A. Comprehensive Survey on the Terpene Synthase Gene Family Provides New Insight into Its Evolutionary Patterns. Genome Biol. Evol. 2019, 11, 2078–2098. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.C.; McGarvey, D.J.; Katahira, E.J.; Croteau, R. Truncation of limonene synthase preprotein provides a fully active “pseudomature” form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair. Biochemistry 1998, 37, 12213–12220. [Google Scholar] [CrossRef]
- Kersten, R.D.; Diedrich, J.K.; Yates, J.R.; Noel, J.P. Mechanism-Based Post-Translational Modification and Inactivation in Terpene Synthases. ACS Chem. Biol. 2015, 10, 2501–2511. [Google Scholar] [CrossRef]
- Aschenbrenner, A.K.; Kwon, M.; Conrad, J.; Ro, D.K.; Spring, O. Identification and Characterization of Two Bisabolene Synthases from Linear Glandular Trichomes of Sunflower (Helianthus annuus L., Asteraceae). Phytochemistry 2016, 124, 29–37. [Google Scholar] [CrossRef]
- Ker, D.S.; Pang, S.L.; Othman, N.F.; Kumaran, S.; Tan, E.F.; Krishnan, T.; Chan, K.G.; Othman, R.; Hassan, M.; Ng, C.L. Purification and Biochemical Characterization of Recombinant Persicaria Minor β-Sesquiphellandrene Synthase. PeerJ 2017, 5, e2961. [Google Scholar] [CrossRef]
- Karunanithi, P.S.; Zerbe, P. Terpene Synthases as Metabolic Gatekeepers in the Evolution of Plant Terpenoid Chemical Diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Ee, S.; Othman, R.; Shaharuddin, N.A.; Ismail, I.; Zainal, Z. Functional Characterization of Sesquiterpene Synthase from Polygonum minus. Sci. World J. 2014, 2014, 840592. [Google Scholar] [CrossRef]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef]
- Sala, D.; Engelberger, F.; McHaourab, H.S.; Meiler, J. Modeling conformational states of proteins with AlphaFold. Curr. Opin. Struct. Biol. 2023, 81, 102645. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Choudri, S. Bioinformatics for Beginners: Genes, Genomes, Molecular Evolution, Databases and Analysis Tools; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780124105102. [Google Scholar]
- Harris, G.G.; Lombardi, P.M.; Pemberton, T.A.; Matsui, T.; Weiss, T.M.; Cole, K.E.; Köksal, M.; Murphy, F.V., IV; Vedula, L.S.; Chou, W.K.; et al. Structural Studies of Geosmin Synthase, a Bifunctional Sesquiterpene Synthase with αα Domain Architecture That Catalyzes a Unique Cyclization–Fragmentation Reaction Sequence. Biochemistry 2015, 54, 7142–7155. [Google Scholar] [CrossRef] [PubMed]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Lin, H.; Yang, X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J. Ind. Microbiol. Biotechnol. 2012, 39, 383–399. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, T.; Pérez-Bermúdez, P.; Gavidia, I. Production of soluble eukaryotic recombinant proteins in E. coli is favoured in early log phase cultures induced at low temperature. Springerplus 2013, 2, 89. [Google Scholar] [CrossRef]
- Sorensen, H.P.; Mortensen, K.K. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb. Cell Fact. 2005, 4, 1. [Google Scholar] [CrossRef]
- Vasina, J.A.; Baneyx, F. Expression of aggregation-prone recombinant proteins at low temperatures: A comparative study of the Escherichia coli cspA and tac promoter systems. Protein Expr. Purif. 1997, 9, 211–218. [Google Scholar] [CrossRef]
- Haridhasapavalan, K.K.; Ranjan, S.H.; Bhattacharyya, S.; Thummer, R.P. Soluble expression, purification, and secondary structure determination of human MESP1 transcription factor. Appl. Microbiol. Biotechnol. 2021, 105, 2363–2376. [Google Scholar] [CrossRef]
- Gutiérrez-González, M.; Farías, C.; Tello, S.; Perez-Etcheverry, D.; Romero, A.; Zuniga, R.; Ribeiro, C.H.; Lorenzo-Ferreiro, C.; Molina, M.C. Optimization of culture conditions for the expression of three different insoluble proteins in Escherichia coli. Sci. Rep. 2019, 9, 16850. [Google Scholar] [CrossRef]
- Alemdar, S.; Hartwig, S.; Frister, T.; König, J.C.; Scheper, T.; Beutel, S. Heterologous Expression, Purification, and Biochemical Characterization of α-Humulene Synthase from Zingiber zerumbet Smith. Appl. Biochem. Biotechnol. 2016, 178, 474–489. [Google Scholar] [CrossRef] [PubMed]


| Description | Organism | Score | E-Value | Identity (%) | Accession |
|---|---|---|---|---|---|
| Sesquiterpene synthase | Aquilaria sinensis | 1048 | 0.0 | 94.84 | AIT75876.1 |
| Putative delta-guaiene synthase | Aquilaria sinensis | 1046 | 0.0 | 94.66 | AIT75875.1 |
| Terpene synthase 10 | Aquilaria sinensis | 968 | 0.0 | 86.92 | QWB49544.1 |
| Terpene synthase 3 | Aquilaria sinensis | 953 | 0.0 | 85.82 | QWB49537.1 |
| Terpene synthase 11 | Aquilaria sinensis | 945 | 0.0 | 84.71 | QWB49545.1 |
| Alpha-humulene synthase | Aquilaria crassna | 943 | 0.0 | 85.00 | AMQ67165.1 |
| Delta-guaiene synthase 4 | Aquilaria crassna | 626 | 0.0 | 52.40 | AEG77020.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaraj, Y.; Abdullah, H.; Nezhad, N.G.; Rasib, A.A.A.; Othman, R.; Rodrigues, K.F.; Sabri, S.; Baharum, S.N. Cloning, Expression and Functional Characterization of a Novel α-Humulene Synthase, Responsible for the Formation of Sesquiterpene in Agarwood Originating from Aquilaria malaccensis. Curr. Issues Mol. Biol. 2023, 45, 8989-9002. https://doi.org/10.3390/cimb45110564
Sundaraj Y, Abdullah H, Nezhad NG, Rasib AAA, Othman R, Rodrigues KF, Sabri S, Baharum SN. Cloning, Expression and Functional Characterization of a Novel α-Humulene Synthase, Responsible for the Formation of Sesquiterpene in Agarwood Originating from Aquilaria malaccensis. Current Issues in Molecular Biology. 2023; 45(11):8989-9002. https://doi.org/10.3390/cimb45110564
Chicago/Turabian StyleSundaraj, Yasotha, Hasdianty Abdullah, Nima Ghahremani Nezhad, Afiq Adham Abd Rasib, Roohaida Othman, Kenneth Francis Rodrigues, Suriana Sabri, and Syarul Nataqain Baharum. 2023. "Cloning, Expression and Functional Characterization of a Novel α-Humulene Synthase, Responsible for the Formation of Sesquiterpene in Agarwood Originating from Aquilaria malaccensis" Current Issues in Molecular Biology 45, no. 11: 8989-9002. https://doi.org/10.3390/cimb45110564
APA StyleSundaraj, Y., Abdullah, H., Nezhad, N. G., Rasib, A. A. A., Othman, R., Rodrigues, K. F., Sabri, S., & Baharum, S. N. (2023). Cloning, Expression and Functional Characterization of a Novel α-Humulene Synthase, Responsible for the Formation of Sesquiterpene in Agarwood Originating from Aquilaria malaccensis. Current Issues in Molecular Biology, 45(11), 8989-9002. https://doi.org/10.3390/cimb45110564







