Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy
Abstract
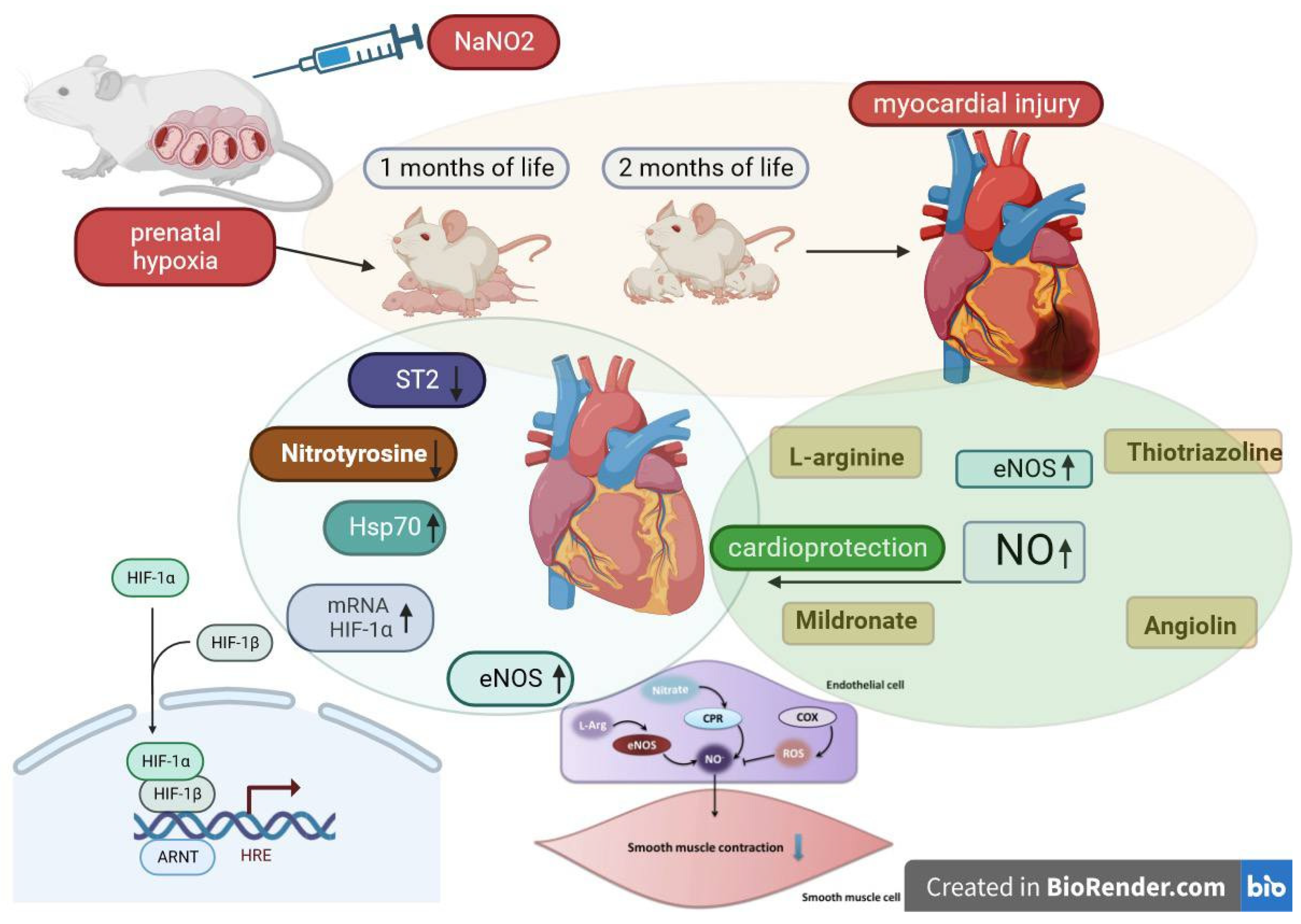
1. Introduction
2. Materials and Methods
2.1. PH Experimental Model and Laboratory Animal Characteristics
2.2. Justification for the Selected Drugs and Their Characteristics
- Intact group (rats born from rats with uncomplicated pregnancies), physiological solution.
- Control group (rats born after experiencing intrauterine hypoxia), physiological solution.
- Thiotriazoline (Morpholinium-3-methyl-1,2,4- triazolyl-5-thioacetic acid) (2.5% injection solution, “Arterium”, Ukraine), metabolitotropic cardioprotector and antioxidant, administered at 50 mg/kg via intraperitoneal injection [24].
- Angiolin ([S]-2,6-diaminohexane acid 3-methyl-1,2,4-triazolyl-5-thioacecate) (substance, RPA “Farmatron”, Ukraine), anti-ischemic, endothelium-protective drug, administered at 50 mg/kg via intraperitoneal injection [25].
- L-arginine (42% injection solution in vial, Tivortin, Yuria-pharm, Ukraine), a NO precursor; it mitigates disruptions in the nitroxidergic system in ischemia, administered at 200 mg/kg via intraperitoneal injection [26].
- Mildronate (2-(2-carboxyethyl)-1,1,1-trimethylhydrazinium) (10% injection solution in ampoules, Grindex (Latvia)), metabolitotropic agent, administered at 100 mg/kg via intraperitoneal injection [27].
2.3. Preparation of Biological Material
2.4. Enzyme-Linked Immunoassay
2.5. Polymerase Chain Reaction in Real-Time
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patterson, A.J.; Zhang, L. Hypoxia and Fetal Cardiac Development. Curr. Mol. Med. 2010, 10, 653–666. [Google Scholar] [CrossRef]
- Narohan, M.V.; Bazhenova, L.K.; Kapranova, E.I.; Melnikova, E.V.; Belousova, N.A. Posthypoxic Dysfunction of the Cardiovascular System in Newborns. Curr. Issues Pediatr. 2007, 6, 42–46. (In Ukranian) [Google Scholar]
- Zhang, L. Prenatal Hypoxia and Cardiac Programming. J. Gynecol. Investig. 2005, 12, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Wilkinson, J.D.; Messiah, S.E.; Miller, T.L. Clinical Research Directions in Pediatric Cardiology. Curr. Opin. Pediatr. 2009, 21, 585–593. [Google Scholar] [CrossRef]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental Programming of Cardiovascular Dysfunction by Prenatal Hypoxia and Oxidative Stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef] [PubMed]
- Hauton, D.; Ousley, V. Prenatal Hypoxia Induces Increased Cardiac Contractility on a Background of Decreased Capillary Density. BMC Cardiovasc. Disord. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Bak, P.G.; Popazova, O.O.; Bukhtiyarova, N.; Yadlovsky, O.E. Nitric Oxide-Dependent Mechanism of Endothelial Dysfunction Formation: A Promising Target for Pharmacological Management. Biopolym. Cell 2022, 38, 145–157. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. Adv. Exp. Med. Biol. 2021, 1332, 167–187. [Google Scholar]
- Lorin, J.; Zeller, M.; Guilland, J.C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and Nitric Oxide Synthase: Regulatory Mechanisms and Cardiovascular Aspects. Mol. Nutr. Food Res. 2014, 58, 101–116. [Google Scholar] [CrossRef]
- Le Melledo, J.M.; Baker, G.; Gyenes, G.; Tsuyuki, R.; Jurasz, P. L-Arginine and NOS Activity: A Complex Relationship. Psychopharmacology 2021, 238, 1223–1224. [Google Scholar] [CrossRef] [PubMed]
- Regeda-Furdychko, M.M. The Influence of Thiotriazoline on Nitric Oxide Indicators in Experimental Contact Dermatitis and Experimental Pneumonia. J. Educ. Health Sport 2020, 10, 35–40. [Google Scholar] [CrossRef]
- Bibik, V.V.; Bolgov, D.M. Thiotriazoline: Pharmacology and Pharmacotherapy. Ukr. Med. J. 2000, 3, 226–229. (In Ukranian) [Google Scholar]
- Tkachev, A.V.; Devlikamova, T.A.; Yandieva, Z.H.; Makarenko, A.S. Evaluation of Thiotriazoline in Patients with Alcoholic Hepatitis. Med. Her. South Russ. 2012, 1, 63–69. (In Russian) [Google Scholar]
- Goodyma, A.; Zachepa, O.; Sushko, Y. The Influence of Combined Abdominal and Thoracic Trauma on Liver Functions During the Early Manifestations of Traumatic Disease and Their Correction with Thiotriazoline. Ukr. J. Mil. Med. 2019, 19, 66–72. [Google Scholar]
- Nagorna, O. Angioline Influence on Cardio and Systemic Hemodynamics Markers in Rabbits with Chronic Cardiac Insufficiency. JMBS 2017, 2, 55–58. (In Ukranian) [Google Scholar] [CrossRef]
- Đurašević, S.; Stojković, M.; Sopta, J.; Pavlović, S.; Borković-Mitić, S.; Ivanović, A.; Jasnić, N.; Tosti, T.; Đurović, S.; Đorđević, J.; et al. The effects of meldonium on the acute ischemia/reperfusion liver injury in rats. Sci. Rep. 2021, 14, 1305. [Google Scholar] [CrossRef]
- Sjakste, N.; Gutcaits, A.; Kalvinsh, I. Mildronate: An antiischemic drug for neurological indications. CNS Drug Rev. 2005, 1, 151–168. [Google Scholar] [CrossRef]
- Liamina, N.P.; Kotel’nikova, E.V.; Karpova, É.S.; Biziaeva, E.A.; Senchikhin, V.N.; Lipchanskaia, T.P. Cardioprotective capabilities of drug meldonium in secondary prevention after percutaneous coronary intervention in patients with documented myocardial ischemia. Kardiologiia 2014, 54, 60–65. [Google Scholar] [CrossRef]
- Kuka, J.; Vilskersts, R.; Cirule, H.; Makrecka, M.; Pugovics, O.; Kalvinsh, I.; Dambrova, M.; Liepinsh, E. The Cardioprotective Effect of Mildronate is Diminished After Co-Treatment With l-Carnitine. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 215–222. [Google Scholar] [CrossRef]
- Zadnipryanyi, I.V.; Tret’yakova, O.S.; Sataeva, T.P. Morphological Changes in Rat Myocardium under Hemic Hypoxia and Cytoflavin Treatment. Eksp. Klin. Farmakol. 2016, 79, 20–25. (In Russian) [Google Scholar] [PubMed]
- Zadnipryanyy, I.V.; Tretiakova, O.S.; Sataieva, T.P. Cardio and cytoprotective effect of cytoflavin in terms of experimental perinatal hemic hypoxia. Patol. Fiziol. I Eksperimental’naia Ter. 2016, 60, 64–71. [Google Scholar]
- Cherkasova, D.U.; Magomedgadzhieva, D.N.; Rabadanova, A.I. Functional changes in the mother-fetus system in experimental chronic nitrite hypoxia. Cent. Rus. Acad. Sci. 2009, 11, 934–937. (In Russian) [Google Scholar]
- Mukhina, I.V.; (Nizhnij Novgorod, Russia). Report on the Independent Preclinical Evaluation of the Specific Activity of the Dosage Forms of Thiotriazoline (2.5% Injection and Tablets). Unpublished internal report. 2009; 112p. (In Russian) [Google Scholar]
- Kolesnik, Y.M. Report on preclinical study of specific biological activity (anti-ischemic, endothelioprotective) of the drug Lisinium (Angiolin) at parenteral administration. Zaporozhye 2018, 11, 2584. (In Ukranian) [Google Scholar]
- Ghotbeddin, Z.; Basir, Z.; Jamshidian, J.; Delfi, F. Modulation of behavioral responses and CA1 neuronal death by nitric oxide in the neonatal rat’s hypoxia model. Brain Behav. 2020, 10, e01841. [Google Scholar] [CrossRef]
- Berlato, D.G.; de Bairros, A.V. Meldonium: Pharmacological, toxicological, and analytical aspects. Toxicol. Res. Appl. 2020, 4, 2397847320915143. [Google Scholar] [CrossRef]
- Henry, J.B. Clinical Diagnosis and Management by Laboratory Methods; W.B Saunders Company: Philadelphia, PA, USA, 1979; Volume 1, 1233p. [Google Scholar]
- Pierce, E. (Ed.) Histochemistry; Moscow University Foreign Literature; Moscow University: Moscow, Russia, 1962; 962p. (In Russian) [Google Scholar]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [Google Scholar] [CrossRef]
- Popazova, O.O.; Belenichev, I.F.; Abramov, A.V.; Bukhtiyarova, N.V.; Chereshniuk, I.L.; Skoryna, D.Y. Indicators of Bioelectrical Activity of the Rat Heart after Prenatal Hypoxia and Pharmacological Correction. Innov. Biosyst. Bioeng. 2022, 6, 148–160. [Google Scholar] [CrossRef]
- Pyatyshkina, N.A.; Yushkov, B.G.; Brykina, I.A.; Sheshenina, A.V.; Volodarskaya, A.V. Changes in macrometric indices of rat placentas under hypobaric hypoxia and posthemorrhagic anemia in different embryonic periods. Bull. Sib. Med. 2005, T4, 14. [Google Scholar]
- Ivanitskaya, N.F. Methods of obtaining different stages of hemic hypoxia in rats by sodium nitrite administration. Pathol. Physiol. Exp. Ther. 1976, 3, 69–71. [Google Scholar]
- Ivanchenko, M.V.; Tverdokhleb, I.V. The Impact of Intrauterine Hypoxia on Mitochondrial Heterogeneity Controlled by Alterations in Rat Ventricular Myocardium. Bull. Volgogr. State Med. Univ. 2014, 4, 101–106. [Google Scholar]
- Petruk, N.S. Influence of chronic prenatal hypoxia on the specialized contact apparatus of rat heart ventricles during ontogeny. Pathologia 2014, 2, 30–33. [Google Scholar] [CrossRef]
- Shevchenko, K.M. Morphological changes of rat atrial cardiomyocytes contractile apparatus in normal conditionsand under the influence of acute and chronic prenatal hypoxia. Role of α-smooth muscle actin in myofibriloge-nesis. Morphologia 2016, 10, 85–92. (In Ukrainian) [Google Scholar] [CrossRef]
- Evidence on Developmental and Reproductive Toxicity of Sodium Nitrite. Reproductive and Cancer Hazard Assessment Section (RCHAS) Office of Environmental Health Hazard Assessment (OEHHA) California Environmental Protection Agency (CAL/EPA) DRAFT. USA. 3 March 2000. Available online: https://oehha.ca.gov/proposition-65/crnr/draft-hid-available-sodium-nitrite (accessed on 27 October 2023).
- Sutovska, H.; Babarikova, K.; Zeman, M.; Molcan, L. Prenatal Hypoxia Affects Foetal Cardiovascular Regulatory Mechanisms in a Sex- and Circadian-Dependent Manner: A Review. Int. J. Mol. Sci. 2022, 23, 2885. [Google Scholar] [CrossRef]
- Granne, I.; Southcombe, J.H.; Snider, J.V.; Tannetta, D.S.; Child, T.; Redman, C.W.; Sargent, I.L. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS ONE 2011, 6, e24463. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.M.; Swiderska, A.; Lock, M.C.; Graham, L.; Iswari, W.; Choudhary, T.; Thomas, D.; Kowash, H.M.; Desforges, M.; Cottrell, E.C.; et al. Chronic developmental hypoxia alters mitochondrial oxidative capacity and reactive oxygen species production in the fetal rat heart in a sex-dependent manner. J. Pineal. Res. 2022, 73, e12821. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Bilous, I.I.; Pavlovych, L.L.; Kamyshnyi, A.M. Primary hypothyroidism and autoimmune thyroiditis alter the transcriptional activity of genes regulating neurogenesis in the blood of patients. Endocr. Regul. 2021, 55, 5–15. [Google Scholar] [CrossRef]
- Topol, I.; Kamyshny, A. Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian Med. News 2013, 225, 115–122. [Google Scholar]
- Fike, C.D.; Dikalova, A.; Slaughter, J.C.; Kaplowitz, M.R.; Zhang, Y.; Aschner, J.L. Reactive oxygen species-reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid. Redox Signal. 2013, 18, 1727–1738. [Google Scholar] [CrossRef]
- Le Brocq, M.; Leslie, S.J.; Milliken, P.; Megson, I.L. Endothelial Dysfunction: From Molecular Mechanisms to Measurement, Clinical Implications, and Therapeutic Opportunities. Antioxid. Redox Signal. 2008, 10, 1631–1674. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Westenskow, P.D.; Friedlander, M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv. Exp. Med. Biol. 2014, 801, 275–281. [Google Scholar]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Marchiq, I.; Pouysségur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J. Mol. Med. 2016, 94, 155–171. [Google Scholar] [CrossRef]
- Brüne, B.; Zhou, J. The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1alpha (HIF-1alpha). Curr. Med. Chem. 2003, 10, 845–855. [Google Scholar] [CrossRef]
- Olson, N.; van der Vliet, A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 2011, 25, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of Heat Shock Proteins HSP70 in the Mechanisms of Endogenous Neuroprotection: The Prospect of Using HSP70 Modulators. Front. Cell Neurosci. 2023, 17, 1131683. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Kolesnik, Y.M.; Pavlov, S.V.; Sokolik, E.P.; Bukhtiyarova, N.V. Malate-aspartate shunt in neuronal adaptation to ischemic conditions: Molecular-biochemical mechanisms of activation and regulation. Neurochem. J. 2012, 6, 22–28. [Google Scholar] [CrossRef]
- Kucherenko, L.I.; Hromyleva, O.V.; Mazur, I.A.; Shishkina, S.V. Theoretical study about L-arginine complexes formation with thiotriazolin. Zaporozhye Med. J. 2017, 19, 108–112. [Google Scholar] [CrossRef][Green Version]
- Kastanayan, A.A.; Kartashova, E.A.; Zheleznyak, E.I. The effect of thiotriazoline on energy production in conditions ofchronic myocardial ischemia. South Russ. J. Ther. Pract. 2020, 1, 84–90. (In Russian) [Google Scholar] [CrossRef]
- Koltsov, A.V.; Tyrenko, V.V. Cardioprotective effect of thiotriazoline in cancer patients. Russ. J. Cardiol. 2023, 28, 5304. (In Russian) [Google Scholar] [CrossRef]
- Dunaev, V.V.; Kraĭdashenko, O.V.; Berezin, A.E. The use of the new cardioprotective agent thiotriazoline in the therapy of ischemic heart disease in older patients. Eksp. Klin. Farmakol. 1996, 59, 21–23. (In Russian) [Google Scholar]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Kappou, D.; Sifakis, S.; Konstantinidou, A.; Papantoniou, N.; Spandidos, D.A. Role of the angiopoietin/Tie system in pregnancy (Review). Exp. Ther. Med. 2015, 9, 1091–1096. [Google Scholar] [CrossRef]
- Hellgren, K.T. Long-Term Effects of Prenatal Hypoxia on Cardiomyocyte Function; School of Medical Sciences, Division of Cardiovascular Sciences: Manchester, UK, 2020. [Google Scholar]
- Todorović, Z.; Đurašević, S.; Stojković, M.; Grigorov, I.; Pavlović, S.; Jasnić, N.; Tosti, T.; Macut, J.B. Lipidomics Provides New Insight into Pathogenesis and Therapeutic Targets of the Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 2798. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Chlopicki, S.; Liepinsh, E.; Kirjanova, O.; Gorshkova, O.; Kozlovski, V.I.; Uhlen, S.; Liepina, I.; Petrovska, R.; Kalvinsh, I. The methylester of gamma-butyrobetaine, but not gamma-butyrobetaine itself, induces muscarinic receptor-dependent vasodilatation. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 533–539. [Google Scholar] [CrossRef] [PubMed]
| Experimental Groups n = 10 | 3-Nitrotyrosine nM/mL | ST2 ng/mL | eNOS, pg/mL | HSP70, ng/mL | mRNA HIF-1, o.u. |
|---|---|---|---|---|---|
| Intact | 4.5 ± 0.82 | 16.7 ± 0.08 | 37.41 ± 0.88 | 14.42 ± 0.21 | 1.00 ± 0.0016 |
| PH (control) | 23.7 ± 1.23 1 | 105.0 ± 3.94 1 | 14.6 ± 0.26 1 | 2.57 ± 0.12 1 | 0.211 ± 0.0001 1 |
| PH + Angiolin | 10.3 ± 0.87 1,* | 24.33 ± 2.35 1,* | 54.73 ± 1.02 1,* | 5.37 ± 0.17 1,* | 4.87 ± 0.005 1,* |
| PH + Thiotriazoline | 14.1 ± 1.18 1,* | 33.4 ± 0.56 1,* | 25.63 ± 1.31 1,* | 2.27 ± 0.12 1 | 1.89 ± 0.001 1,* |
| PH + L-arginine | 18.4 ± 1.12 1,* | 28.33 ± 1.08 1,* | 32.4 ± 1.08 * | 5.76 ± 0.19 1,* | 1.88 ± 0.001 1,* |
| PH + Mildronate | 20.2 ± 1.85 1 | 32.17 ± 0.87 1,* | 28.4 ± 0.76 1,* | 3.73 ± 0.15 1 | 0.37 ± 0.001 1 |
| Experimental Groups n = 10 | 3-Nitrotyrosine nM/mL | ST2 ng/mL | eNOS, pg/mL | HSP70, ng/mL | mRNA HIF-1, o.u. |
|---|---|---|---|---|---|
| Intact | 4.8 ± 0.77 | 14.7 ± 0.50 | 54.03 ± 0.47 | 14.9 ± 0.37 1 | 1.00 ± 0.0003 |
| PH (control) | 18.2 ± 1.65 1 | 53.33 ± 0.62 1 | 26.43 ± 0.90 1 | 4.33 ± 0.11 | 0.389 ± 0.0005 1 |
| PH + Angiolin | 6.23 ± 0.76 * | 12.1 ± 0.54 * | 65.9 ± 0.65 1,* | 8.07 ± 0.15 1,* | 3.19 ± 0.001 1,* |
| PH + Thiotriazoline | 9.47 ± 1.14 1,* | 18.07 ± 0.88 1,* | 36.5 ± 1.04 1,* | 6.63 ± 0.13 1,* | 2.41 ± 0.0002 1,* |
| PH + L-arginine | 15.7 ± 1.35 1,* | 23.8 ± 1.00 1,* | 43.7 ± 1.30 1,* | 4.5 ± 0.19 1 | 1.08 ± 0.00 1,* |
| PH + Mildronate | 16.4 ± 1.42 1 | 26.33 ± 1.29 1,* | 26.2 ± 1.27 1 | 6.33 ± 0.13 1,* | 1.88 ± 0.0005 1,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popazova, O.; Belenichev, I.; Yadlovskyi, O.; Oksenych, V.; Kamyshnyi, A. Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Curr. Issues Mol. Biol. 2023, 45, 8704-8715. https://doi.org/10.3390/cimb45110547
Popazova O, Belenichev I, Yadlovskyi O, Oksenych V, Kamyshnyi A. Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Current Issues in Molecular Biology. 2023; 45(11):8704-8715. https://doi.org/10.3390/cimb45110547
Chicago/Turabian StylePopazova, Olena, Igor Belenichev, Oleh Yadlovskyi, Valentyn Oksenych, and Aleksandr Kamyshnyi. 2023. "Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy" Current Issues in Molecular Biology 45, no. 11: 8704-8715. https://doi.org/10.3390/cimb45110547
APA StylePopazova, O., Belenichev, I., Yadlovskyi, O., Oksenych, V., & Kamyshnyi, A. (2023). Altered Blood Molecular Markers of Cardiovascular Function in Rats after Intrauterine Hypoxia and Drug Therapy. Current Issues in Molecular Biology, 45(11), 8704-8715. https://doi.org/10.3390/cimb45110547








