Neuroprotective Effects of Water Extract from Brown Algae Petalonia binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PB Extracts
2.2. Cell Culture and Treatment
2.3. Liquid Chromatography–Mass Spectrometry (LC-MS)
2.4. Cell Viability Assay
2.5. ROS Measurement
2.6. Immunocytochemistry
2.7. Western Blot
2.8. Hoechst33258 Staining
2.9. Flow Cytometry
2.10. Animals and Treatments
2.11. MCAO/R
2.12. Neurological Deficit Scoring
2.13. Grip Strength Test
2.14. Rotarod Test
2.15. Infarct Volume Measurement
2.16. Cresyl-Violet Staining and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay
2.17. In Vivo ROS Measurement
2.18. Statistical Analyses
3. Results
3.1. wPB Protects HT-22 Cells against Glutamate-Induced Excitotoxicity
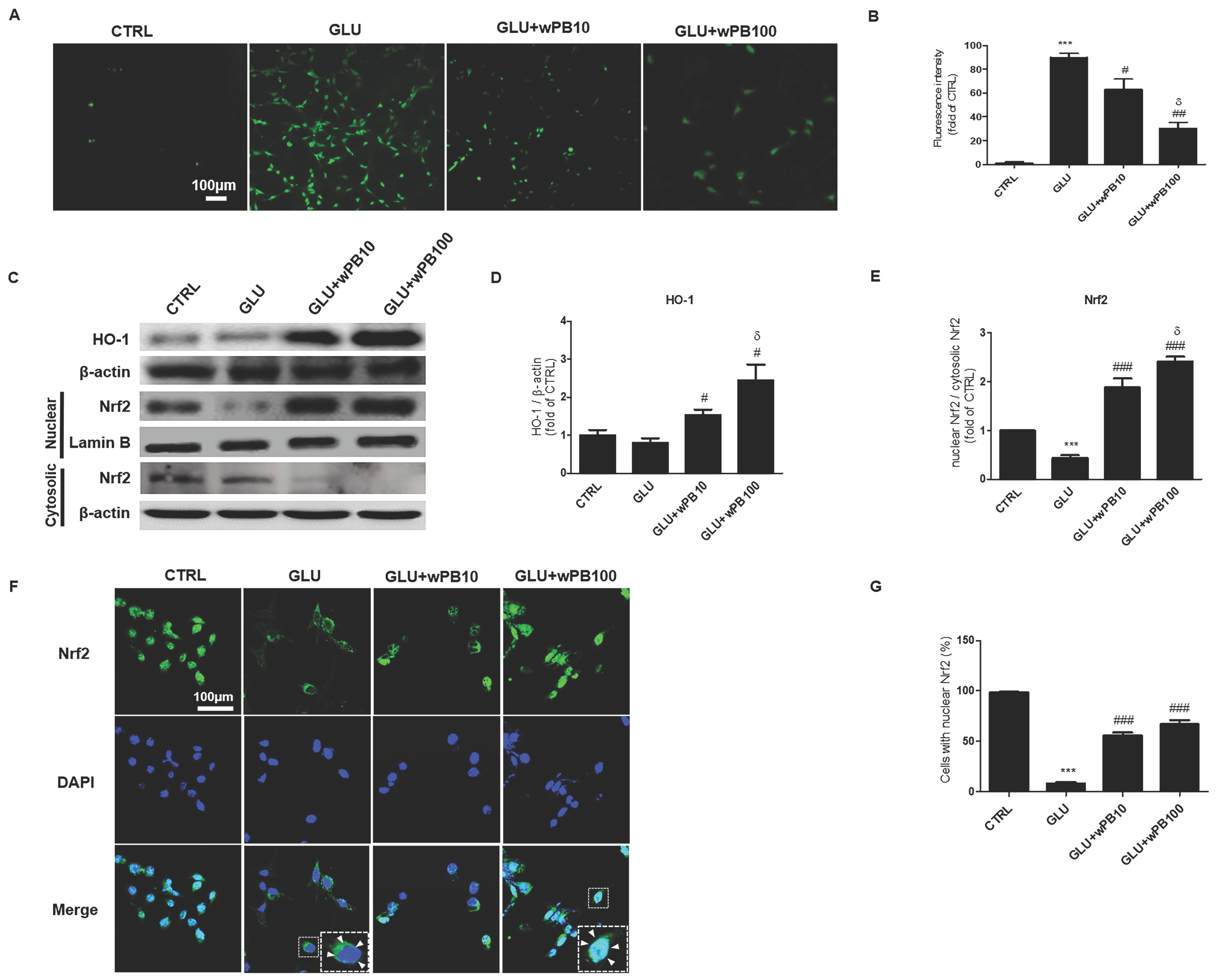
3.2. Antioxidant Activities of wPB Contribute to the Protection of HT-22 Cells against Glutamate-Induced Excitotoxicity
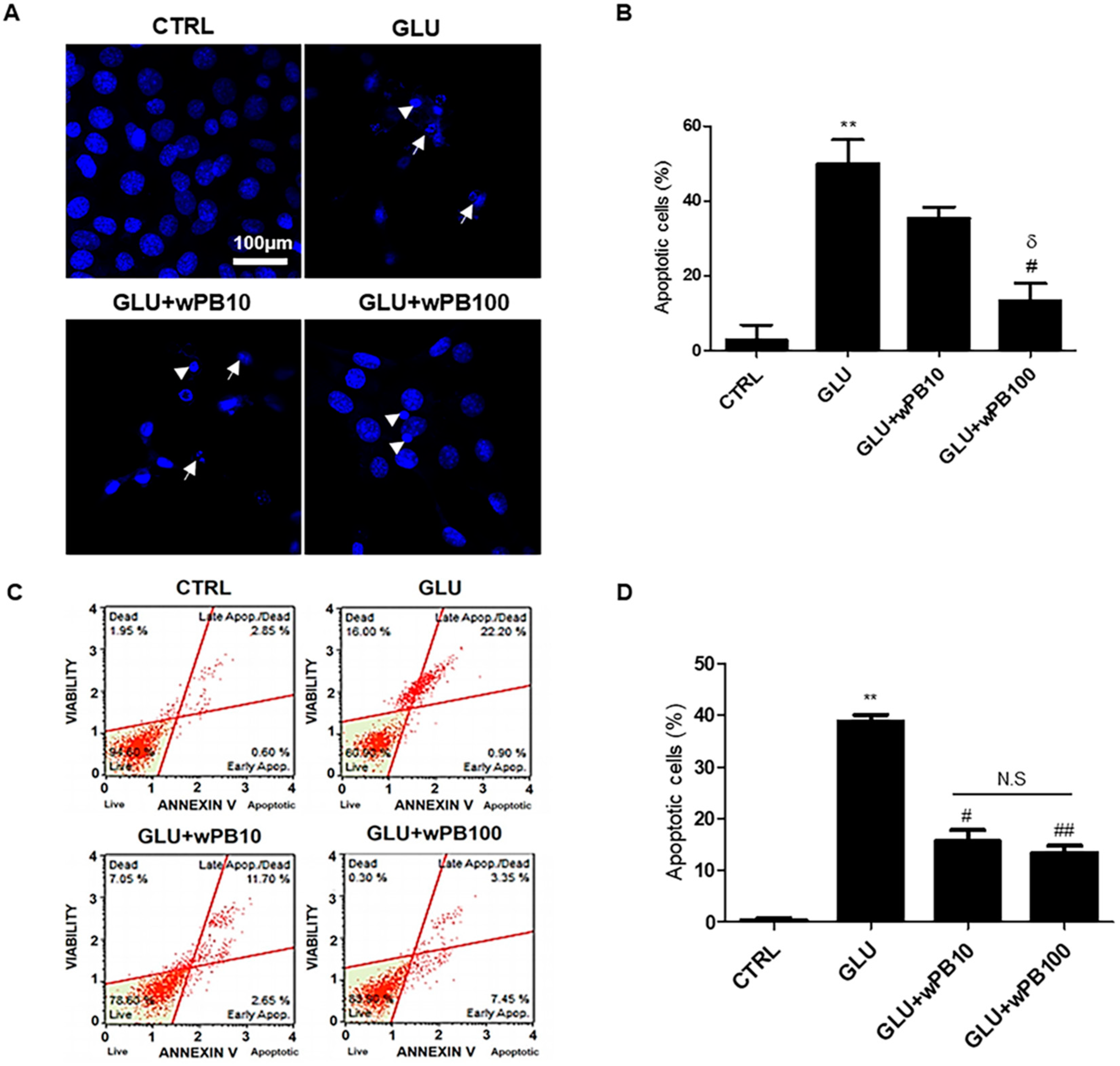
3.3. wPB Inhibits Excitotoxicity-Associated Apoptosis of HT-22 Cells
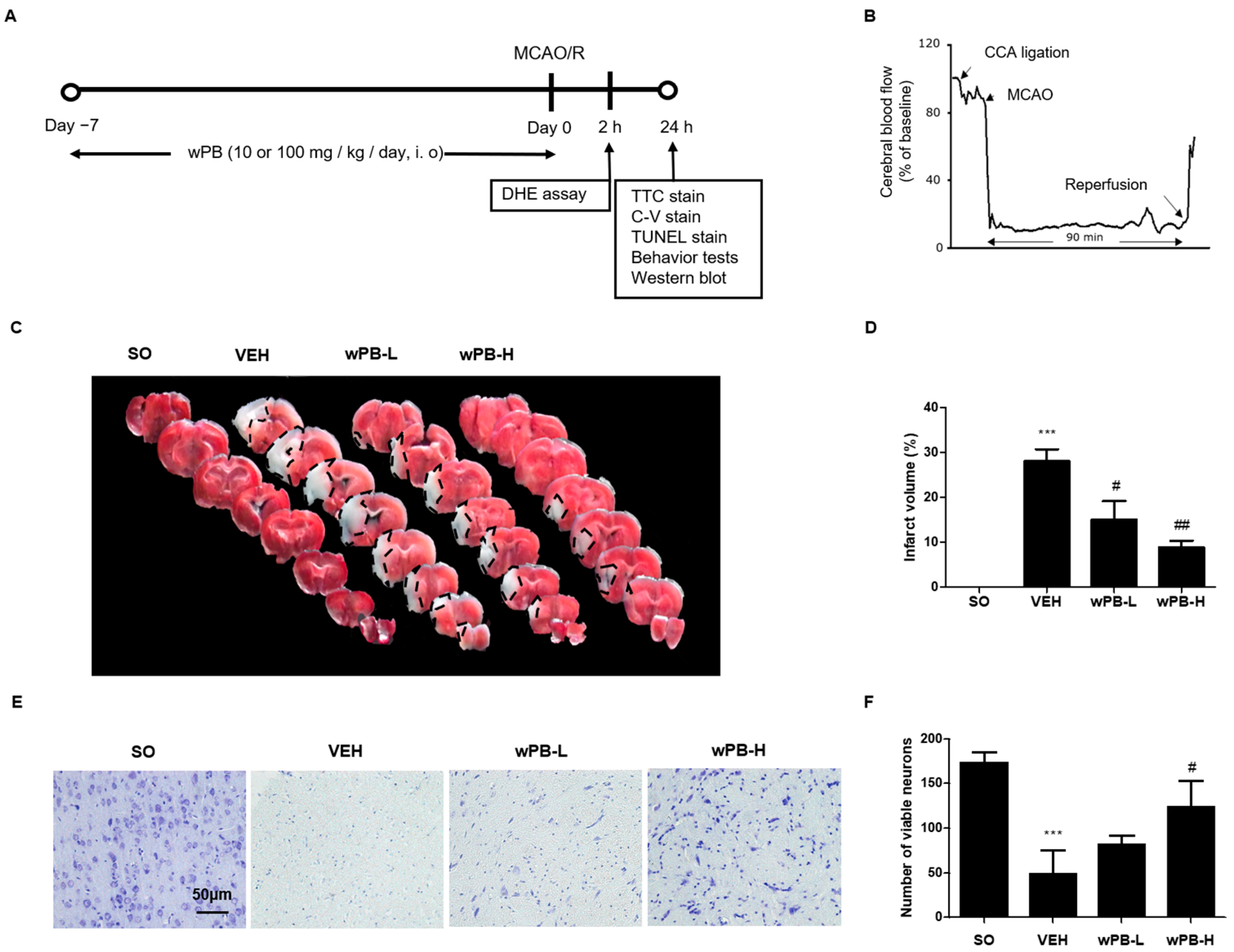
3.4. wPB Can Minimize MCAO/R-Triggered Infarct Volume and Neuronal Death
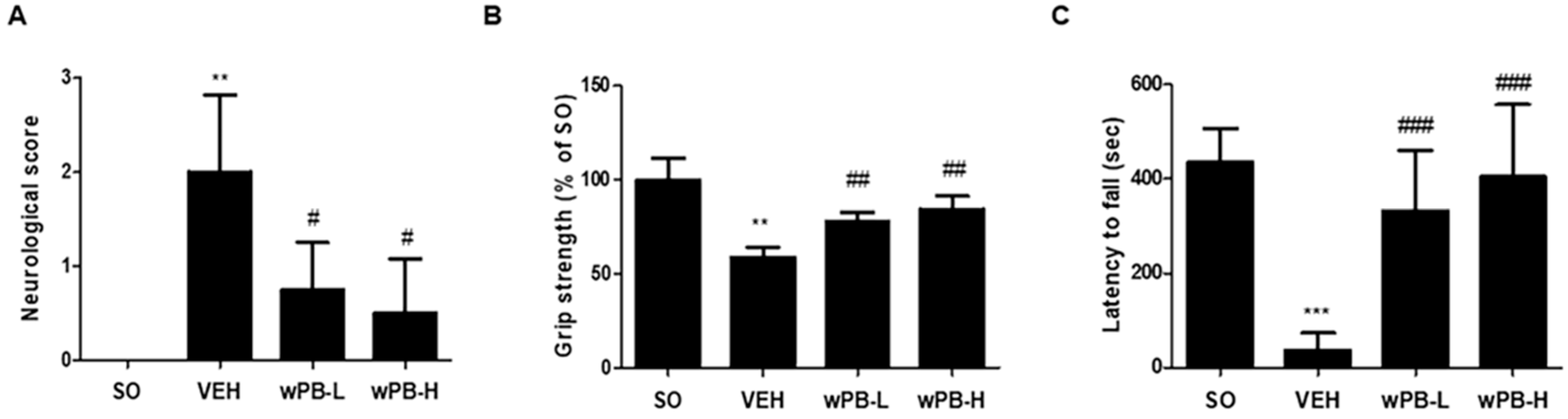
3.5. wPB Can Attenuate fCI-Associated Sensorimotor Deficits
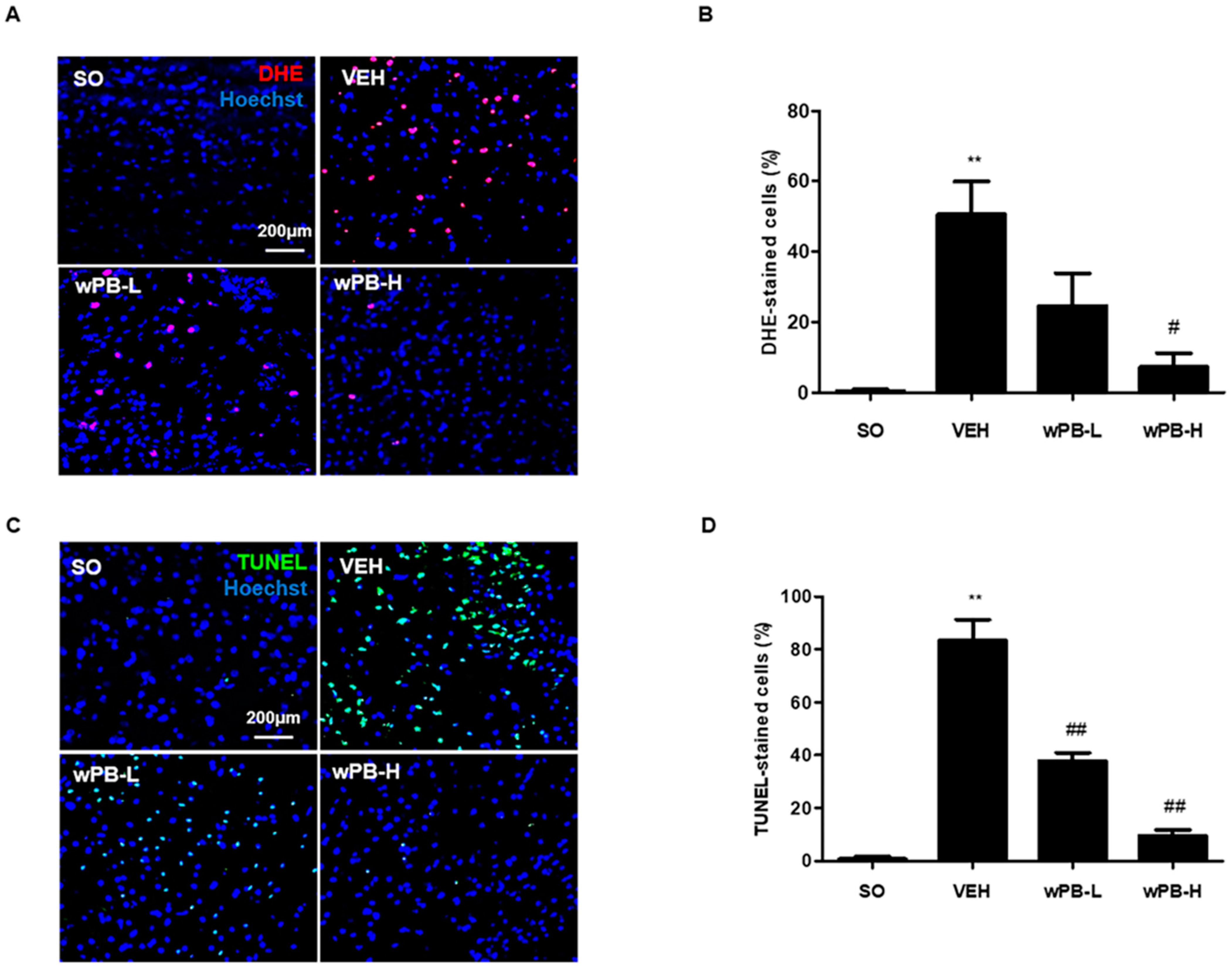
3.6. wPB Protects Neurons against fCI via Antioxidative and Antiapoptotic Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beal, C.C. Gender and stroke symptoms: A review of the current literature. J. Neurosci. Nurs. 2010, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Heron, M. Deaths: Leading causes for 2004. Natl. Vital. Stat. Rep. 2007, 56, 1–95. [Google Scholar] [PubMed]
- Besancon, E.; Guo, S.; Lok, J.; Tymianski, M.; Lo, E.H. Beyond NMDA and AMPA glutamate receptors: Emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008, 29, 268–275. [Google Scholar] [CrossRef]
- Ramon, R.; Julio César, G. Chapter 2—Excitotoxicity and Oxidative Stress in Acute Stroke. In Ischemic Stroke; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Ouyang, Y.B.; Voloboueva, L.A.; Xu, L.J.; Giffard, R.G. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J. Neurosci. 2007, 27, 4253–4260. [Google Scholar] [CrossRef]
- Xu, L.; Emery, J.F.; Ouyang, Y.B.; Voloboueva, L.A.; Giffard, R.G. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 2010, 58, 1042–1049. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79 (Suppl. S1), S52–S57. [Google Scholar] [CrossRef]
- Castilho, R.F.; Hansson, O.; Ward, M.W.; Budd, S.L.; Nicholls, D.G. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 1998, 18, 10277–10286. [Google Scholar] [CrossRef]
- Orellana-Urzua, S.; Rojas, I.; Libano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Ridder, D.A.; Schwaninger, M. NF-kappaB signaling in cerebral ischemia. Neuroscience 2009, 158, 995–1006. [Google Scholar] [CrossRef]
- Culmsee, C.; Zhu, C.; Landshamer, S.; Becattini, B.; Wagner, E.; Pellecchia, M.; Blomgren, K.; Plesnila, N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J. Neurosci. 2005, 25, 10262–10272. [Google Scholar] [CrossRef]
- Huang, X.; He, D.; Pan, Z.; Deng, J. Reactive-oxygen-species-scavenging nanomaterials for resolving inflammation. Mater. Today Bio. 2021, 11, 100124. [Google Scholar] [CrossRef] [PubMed]
- Polaka, S.; Katare, P.; Pawar, B.; Vasdev, N.; Gupta, T.; Rajpoot, K.; Sengupta, P.; Tekade, R.K. Emerging ROS-Modulating Technologies for Augmentation of the Wound Healing Process. ACS Omega 2022, 7, 30657–30672. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Cochemé, H.; Murphy, M. Can antioxidants be effective therapeutics? Curr Opin Investig Drugs. Curr. Opin. Investig. Drugs 2010, 11, 426–431. [Google Scholar]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds from Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.d.; Carneiro, J.A.; Costa, R.A.; Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Catanesi, M.; Caioni, G.; Castelli, V.; Benedetti, E.; Angelo, M.; Cimini, A. Benefits under the Sea: The Role of Marine Compounds in Neurodegenerative Disorders. Mar. Drugs 2021, 19, 24. [Google Scholar] [CrossRef]
- Morris, J.J.; Kirkegaard, R.; Szul, M.J.; Johnson, Z.I.; Zinser, E.R. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by "helper" heterotrophic bacteria. Appl. Environ. Microbiol. 2008, 74, 4530–4534. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kwon, K.K.; Kang, S.G.; Cha, S.S.; Kim, S.J.; Lee, J.H. Approaches for novel enzyme discovery from marine environments. Curr. Opin. Biotechnol. 2010, 21, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Nalini, S.; Sandy Richard, D.; Mohammed Riyaz, S.U.; Kavitha, G.; Inbakandan, D. Antibacterial macro molecules from marine organisms. Int. J. Biol. Macromol. 2018, 115, 696–710. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.; Moustafa, M.; Washed, A.A.; Mousawi, S.M.; Musharraf, S.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Buchanan, J. The Crustose Brown Algae of New Zealand: A Taxonomic Study. Master’s Thesis, Victoria University of Wellington, Wellington, New Zealand, 2007. [Google Scholar]
- Kang, S.I.; Jin, Y.J.; Ko, H.C.; Choi, S.-Y.; Hwang, J.H.; Whang, I.; Kim, M.H.; Shin, H.S.; Jeong, H.B.; Kim, S.J. Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem. Biophys. Res. Commun. 2008, 373, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Anti-inflammatory Effect of Petalonia binghamiae in LPS- Induced Macrophages is Mediated by Suppression of iNOS and COX-2. Int. J. Agric. Biol. 2010, 12, 754–758. [Google Scholar]
- Kang, J.S.; Choi, I.W.; Han, M.H.; Lee, D.S.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2. Mar. Drugs 2015, 13, 2666–2679. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, S.H.; Park, M.N.; Park, J.Y.; Park, H.Y.; Song, C.E.; Moon, J.H.; Choi, A.L.; Kim, K.D.; Lee, N.S.; et al. Water Extract of Mixed Mushroom Mycelia Grown on a Solid Barley Medium Is Protective against Experimental Focal Cerebral Ischemia. Curr. Issues Mol. Biol. 2021, 43, 365–383. [Google Scholar] [CrossRef]
- Wei, E.P.; Kontos, H.A.; Beckman, J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996, 271 Pt 2, H1262–H1266. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Lee, J.; Jeong, J.; Jeong, Y.-G.; Kim, D.-K.; Lee, N.S.; Na, C.S.; Doh, E.S.; Han, S.Y. Platycarya strobilacea leaf extract protects mice brain with focal cerebral ischemia by antioxidative property. Anat. Cell Biol. 2019, 52, 486. [Google Scholar] [CrossRef]
- Margaill, I.; Plotkine, M.; Lerouet, D. Antioxidant strategies in the treatment of stroke. Free Radic. Biol. Med. 2005, 39, 429–443. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, C.; Lv, J.; Fan, C.; Hu, W.; Di, S.; Yan, X.; Ma, Z.; Liang, Z.; Yang, Y. Nrf2 Weaves an Elaborate Network of Neuroprotection Against Stroke. Mol. Neurobiol. 2017, 54, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Jin, H.; Nan, D.; Yu, W.; Huang, Y. New insights into targeting mitochondria in ischemic injury. Apoptosis 2021, 26, 163–183. [Google Scholar] [CrossRef]
- Parente, M.; Neto, A.I.; Fletcher, R. Morphology and life history studies of Endarachne binghamiae (Scytosiphonaceae, Phaeophyta) from the Azores. Aquat. Bot. 2003, 76, 109–116. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, L.; Kong, A.N. MicroRNAs: New players in cancer prevention targeting Nrf2, oxidative stress and inflammatory pathways. Curr. Pharmacol. Rep. 2015, 1, 21–30. [Google Scholar] [CrossRef]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Lipton, S.A. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid. Redox Signal. 2011, 14, 1421–1424. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Hiraganahalli, B.D.; Chinampudur, V.C.; Dethe, S.; Mundkinajeddu, D.; Pandre, M.K.; Balachandran, J.; Agarwal, A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 2012, 8, 116–123. [Google Scholar] [PubMed]
- Ambreen Bano, A.H.; Swati Sharma Snober, S. Mir. Exploring Poisonous Plants Medicinal Values, Toxicity Responses, and Therapeutic Uses; CRC Press: Boca Raton, FL, USA, 2023; Volume 18. [Google Scholar]
- Matos, P.; Batista, M.T.; Figueirinha, A. A review of the ethnomedicinal uses, chemistry, and pharmacological properties of the genus Acanthus (Acanthaceae). J. Ethnopharmacol. 2022, 293, 115271. [Google Scholar] [CrossRef] [PubMed]
- Pabis, S.; Kula, J. Synthesis and Bioactivity of (R)-Ricinoleic Acid Derivatives: A Review. Curr. Med. Chem. 2016, 23, 4037–4056. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Fetzer, S.; Sauer, S.K.; Evangelista, S.; Averbeck, B.; Kress, M.; Reeh, P.W.; Cirillo, R.; Lippi, A.; Maggi, C.; et al. Pro- and anti-inflammatory actions of ricinoleic acid: Similarities and differences with capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, D.; Zhuo, Y.; Zhang, S.; Wang, X.; Gao, H. Protective Role of Liriodendrin in Sepsis-Induced Acute Lung Injury. Inflammation 2016, 39, 1805–1813. [Google Scholar] [CrossRef]
- Li, X.J.; Ye, Q.F. Fucoidan reduces inflammatory response in a rat model of hepatic ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2015, 93, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Slim, C.; Nassrallah, H.; Zaouali, M.A.; Amara, F.; Majdoub, H.; Morin, D.; Abdennebi, H.B. Fucoidan alleviates the mitochondria and endoplasmic reticulum stresses in ischemic rat livers. Phytomedicine Plus 2022, 2, 100250. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, J.H.; Lee, T.K.; Par, C.W.; Kim, B.; Lee, J.-C.; Kim, D.W.; Shin, M.G.; Cho, J.H.; Lee, C.-H.; et al. Laminarin Pretreatment Provides Neuroprotection against Forebrain Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Neuroinflammation in Aged Gerbils. Mar. Drugs 2020, 18, 213. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, K.; Li, P.; Zhu, L.; Xu, J.; Yang, B.; Hu, X.; Lu, Z.; Chen, J. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res. Rev. 2017, 34, 77–87. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef]
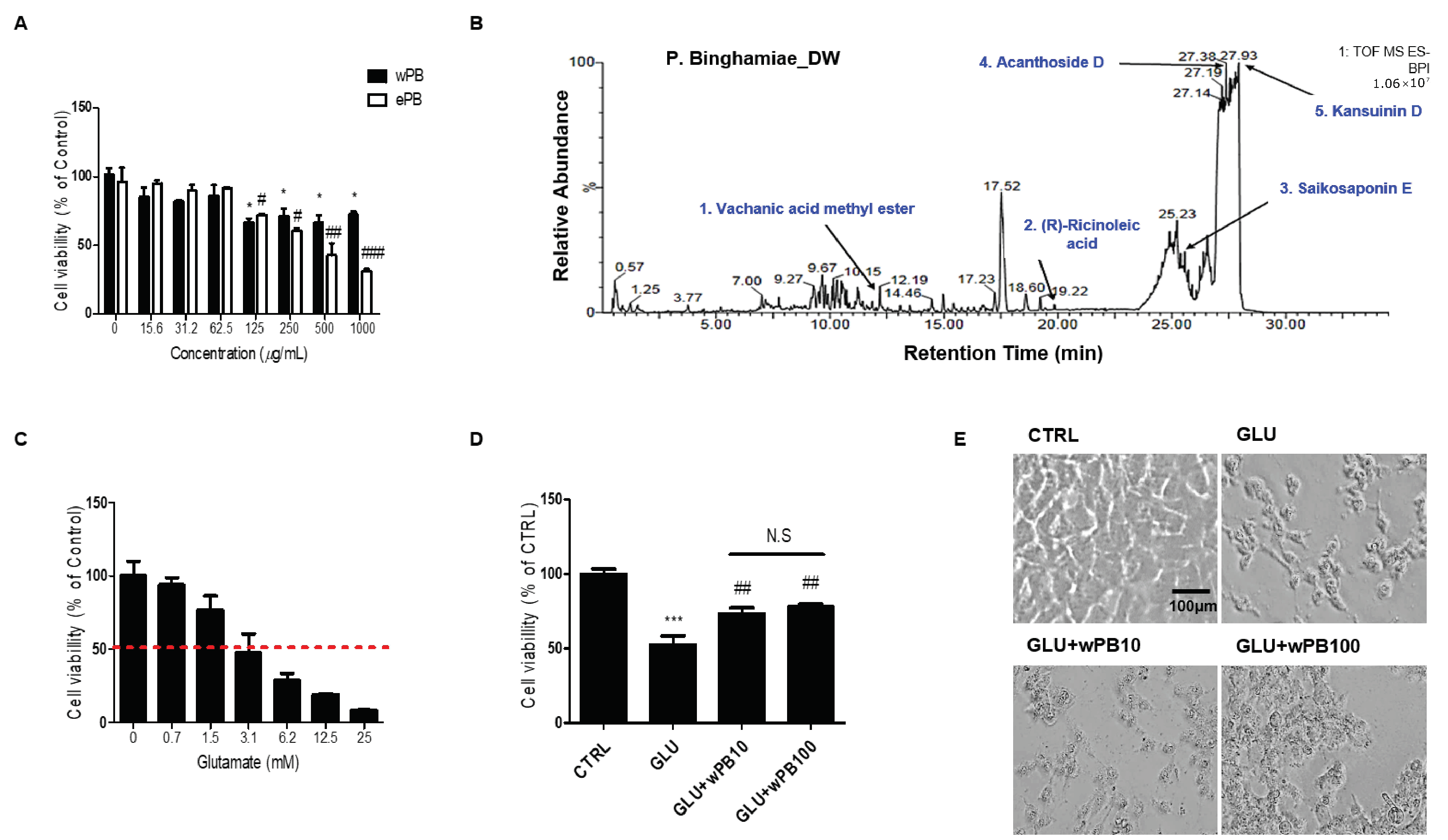
| Retention Time (min) | Molecular Formula | Molecular Weight (Da) | [M-H]− (m/z) | Compound | |
|---|---|---|---|---|---|
| 1 | 12.20 | C16H26O3 | 266.2 | 311.1865 | Vachanic acid methyl ester |
| 2 | 19.78 | C18H34O3 | 298.5 | 301.3041 | (R)-ricinoleic acid |
| 3 | 25.55 | C34H46O18 | 764.5 | 763.4675 | Saikosaponin E |
| 4 | 27.38 | C34H46O18 | 742.7 | 789.4834 | Acanthoside D |
| 5 | 27.90 | C41H47NO15 | 793.3 | 792.2906 | Kansuinin D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, S.H.; Hong, G.-L.; Kang, H.B.; Lee, N.-S.; Kim, D.K.; Jeong, Y.G.; Kim, C.-S.; Yoo, Y.C.; Lee, B.H.; Jung, J.-Y.; et al. Neuroprotective Effects of Water Extract from Brown Algae Petalonia binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo. Curr. Issues Mol. Biol. 2023, 45, 8427-8443. https://doi.org/10.3390/cimb45100531
Eom SH, Hong G-L, Kang HB, Lee N-S, Kim DK, Jeong YG, Kim C-S, Yoo YC, Lee BH, Jung J-Y, et al. Neuroprotective Effects of Water Extract from Brown Algae Petalonia binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo. Current Issues in Molecular Biology. 2023; 45(10):8427-8443. https://doi.org/10.3390/cimb45100531
Chicago/Turabian StyleEom, Sun Ho, Geum-Lan Hong, Hyun Bae Kang, Nam-Seob Lee, Do Kyung Kim, Young Gil Jeong, Chun-Sung Kim, Yung Choon Yoo, Bong Ho Lee, Ju-Young Jung, and et al. 2023. "Neuroprotective Effects of Water Extract from Brown Algae Petalonia binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo" Current Issues in Molecular Biology 45, no. 10: 8427-8443. https://doi.org/10.3390/cimb45100531
APA StyleEom, S. H., Hong, G.-L., Kang, H. B., Lee, N.-S., Kim, D. K., Jeong, Y. G., Kim, C.-S., Yoo, Y. C., Lee, B. H., Jung, J.-Y., Kim, D.-S., & Han, S. Y. (2023). Neuroprotective Effects of Water Extract from Brown Algae Petalonia binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo. Current Issues in Molecular Biology, 45(10), 8427-8443. https://doi.org/10.3390/cimb45100531







