In Vitro Anticancer Properties of Novel Bis-Triazoles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of MS47 and MS49
2.2. Cell Culturing
2.3. Measuring Cell Viability (MTT Assay)
2.4. Clonogenic Assay
2.5. Western Blots
2.6. Cell Cycle Analysis by Flow Cytometry
2.7. Annexin V-FITC and Propidium Iodide Apoptosis Assay
2.8. Senescence-Associated β-Galactosidase Staining
2.9. Statistical Analyses
3. Results and Discussion
3.1. NCI 60 Cell Line Panel In Vitro Screening

| Panel/Cell Line | Response Parameters of Ligand MS47 (µM) | ||
|---|---|---|---|
| GI50 | TGI | LC50 | |
| Leukaemia | |||
| CCRF-CEM | 0.251 | >100 | >100 |
| HL-60(TB) | 0.226 | 0.643 | >100 |
| K-562 | 0.597 | 14.4 | >100 |
| MOLT-4 | 0.226 | 0.600 | >100 |
| RPMI-8226 | 0.629 | 3.86 | >100 |
| SR | 0.457 | 5.35 | >100 |
| Non-Small Cell Lung Cancer | |||
| A549/ATCC | 1.43 | 3.17 | 7.04 |
| EKVX | 1.54 | 3.05 | 6.04 |
| HOP-62 | 1.54 | 2.91 | 5.52 |
| HOP-92 | 0.423 | 1.40 | 5.88 |
| NCI-H226 | 1.56 | 3.12 | 6.24 |
| NCI-H23 | 0.267 | 0.794 | 24.8 |
| NCI-H322M | 0.289 | 1.04 | 3.45 |
| NCI-H460 | 0.344 | 1.38 | 6.24 |
| NCI-H522 | 0.194 | 0.481 | 4.76 |
| Colon Cancer | |||
| COLO 205 | 0.174 | 0.335 | 0.644 |
| HCC-2998 | 0.317 | 1.12 | 4.49 |
| HCT-116 | 0.283 | 1.14 | 3.57 |
| HCT-15 | 22.9 | >100 | >100 |
| HT29 | 0.321 | 0.900 | 33.8 |
| KM12 | 0.544 | 2.17 | 6.39 |
| SW-620 | 0.407 | 1.73 | 7.40 |
| CNS Cancer | |||
| SF-268 | 0.326 | 1.45 | 6.88 |
| SF-295 | 1.36 | 2.72 | 5.46 |
| SF-539 | 0.202 | 0.420 | 0.875 |
| SNB-19 | 0.182 | 0.371 | 0.755 |
| SNB-75 | 0.176 | 0.421 | 1.01 |
| U251 | 0.175 | 0.348 | 0.694 |
| Melanoma | |||
| LOX IMVI | 0.187 | 0.489 | 2.62 |
| MALME-3M | 0.111 | 0.265 | 0.636 |
| M14 | 1.58 | 3.38 | 7.24 |
| MDA-MB-435 | 0.176 | 0.326 | 0.602 |
| SK-MEL-2 | 0.219 | 0.553 | 8.81 |
| SK-MEL-28 | 0.913 | 2.46 | 6.24 |
| SK-MEL-5 | 0.227 | 0.705 | 2.70 |
| UACC-257 | 0.711 | 2.42 | 7.07 |
| UACC-62 | 1.05 | 2.56 | 6.26 |
| Ovarian Cancer | |||
| IGROV1 | 0.233 | 0.687 | 3.79 |
| OVCAR-3 | 0.261 | 0.761 | 7.37 |
| OVCAR-4 | 0.131 | 0.260 | 0.515 |
| OVCAR-5 | 0.592 | 2.52 | 9.07 |
| OVCAR-8 | 1.56 | 3.66 | - |
| NCI/ADR-RES | >100 | >100 | >100 |
| SK-OV-3 | 1.99 | 5.32 | 36.9 |
| Renal Cancer | |||
| 786-0 | 24.1 | >100 | >100 |
| A498 | 1.61 | 6.02 | 26.0 |
| ACHN | 9.56 | 23.2 | 54.6 |
| CAKI-1 | >100 | >100 | >100 |
| RXF 393 | 3.60 | 15.2 | 54.4 |
| SN12C | 0.185 | 0.371 | 0.745 |
| TK-10 | 11.1 | 30.1 | 81.3 |
| UO-31 | 16.9 | 38.1 | 86.1 |
| Prostate Cancer | |||
| PC-3 | 2.40 | 7.52 | >100 |
| DU-145 | 1.03 | 2.23 | 4.83 |
| Breast Cancer | |||
| MCF7 | 0.144 | 0.358 | 0.894 |
| MDA-MB-231/ATCC | 0.253 | 10.2 | 61.4 |
| HS 578T | 2.88 | 9.17 | >100 |
| BT-549 | 1.73 | 3.16 | 5.78 |
| MDA-MB-468 | 0.173 | 0.382 | 0.843 |
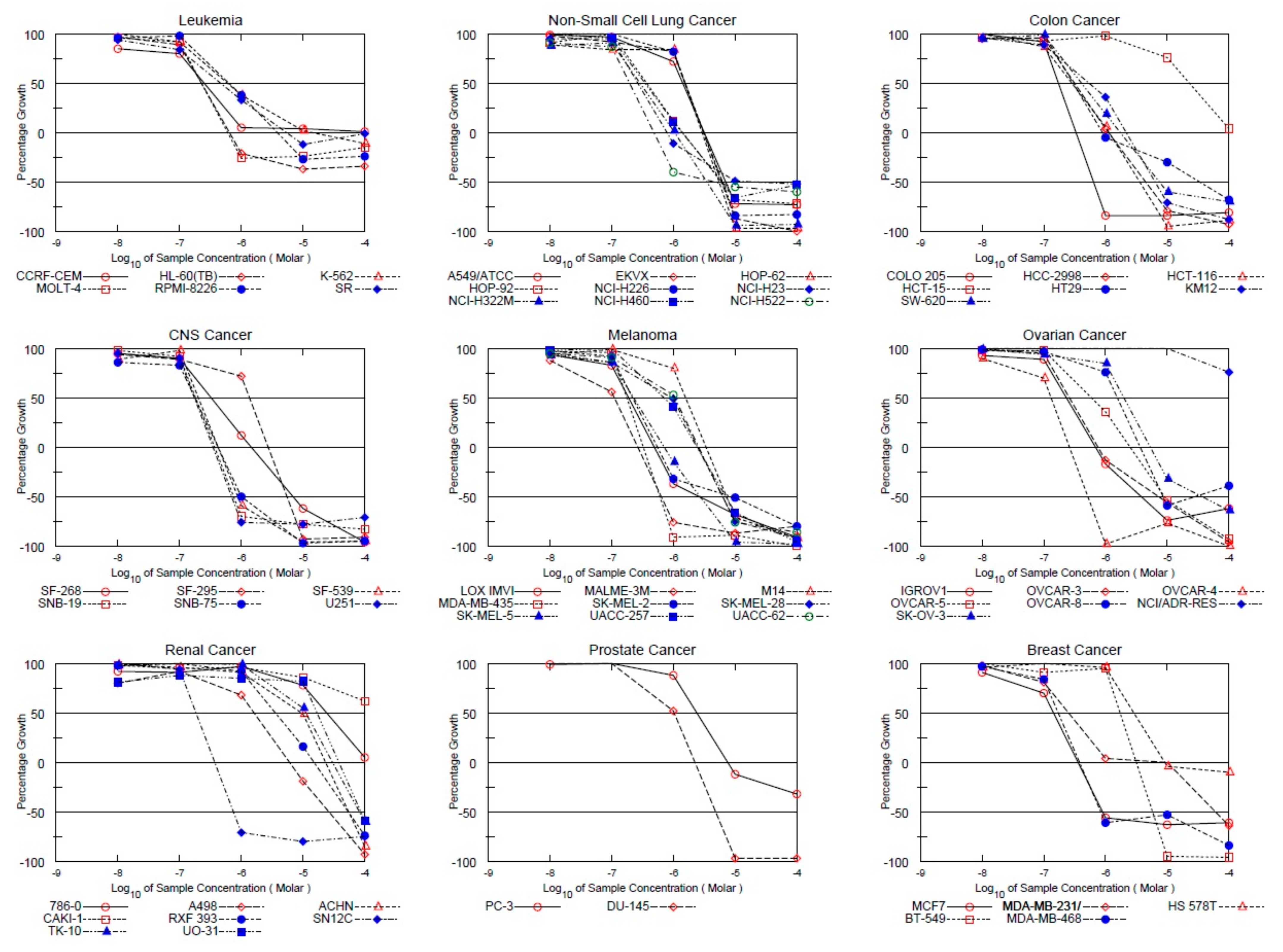
3.2. MTT Assay
| Ligand | GI50 (μM) ± S.D. | SI | |
|---|---|---|---|
| MDA-MB-435 Cell Line | MRC-5 Cell Line | ||
| MS47 | 0.226 ± 0.057 | 2.219 ± 0.076 | 9.8 |
| MS49 | 0.075 ± 0.010 | 1.325 ± 0.137 | 17.7 |
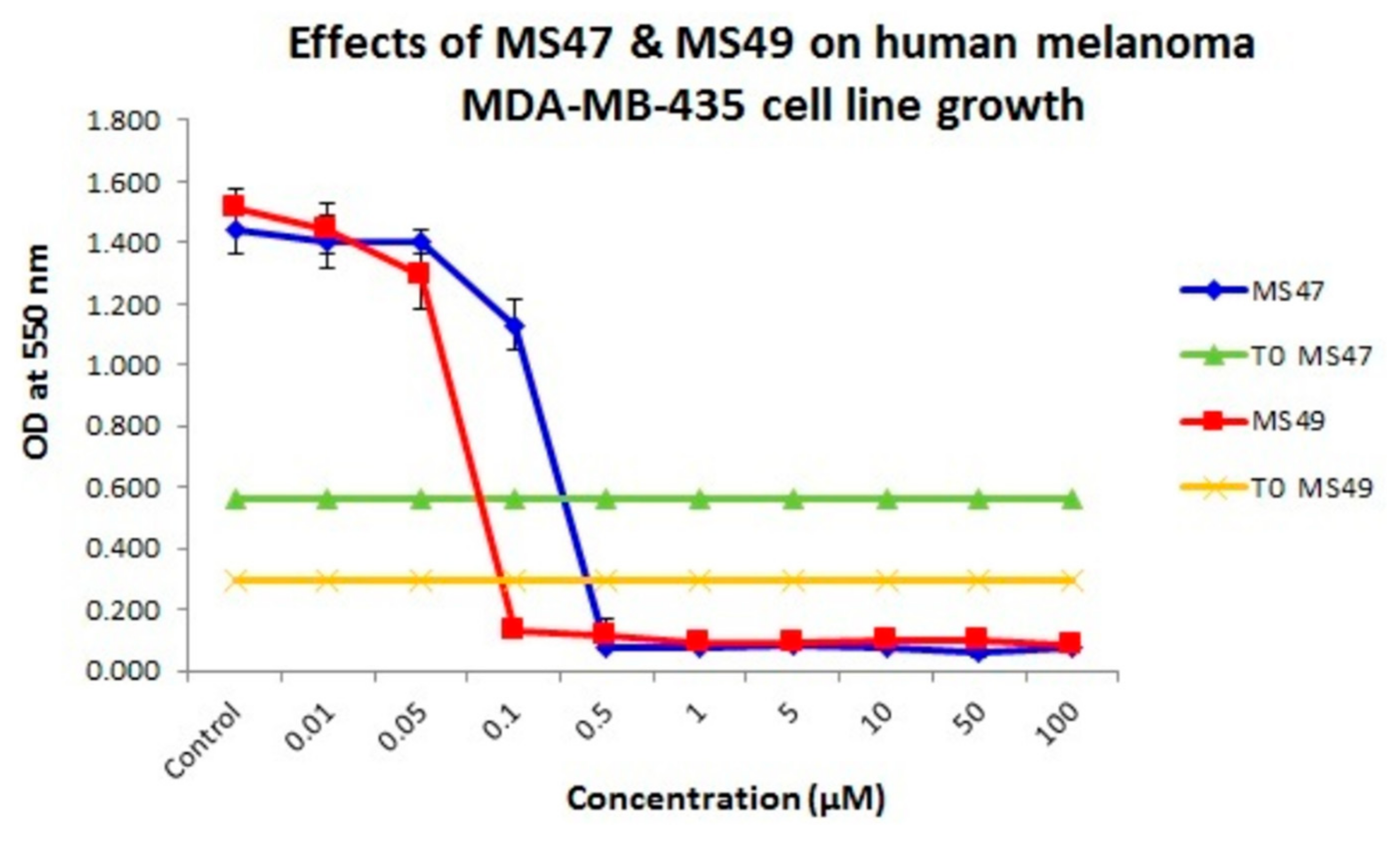
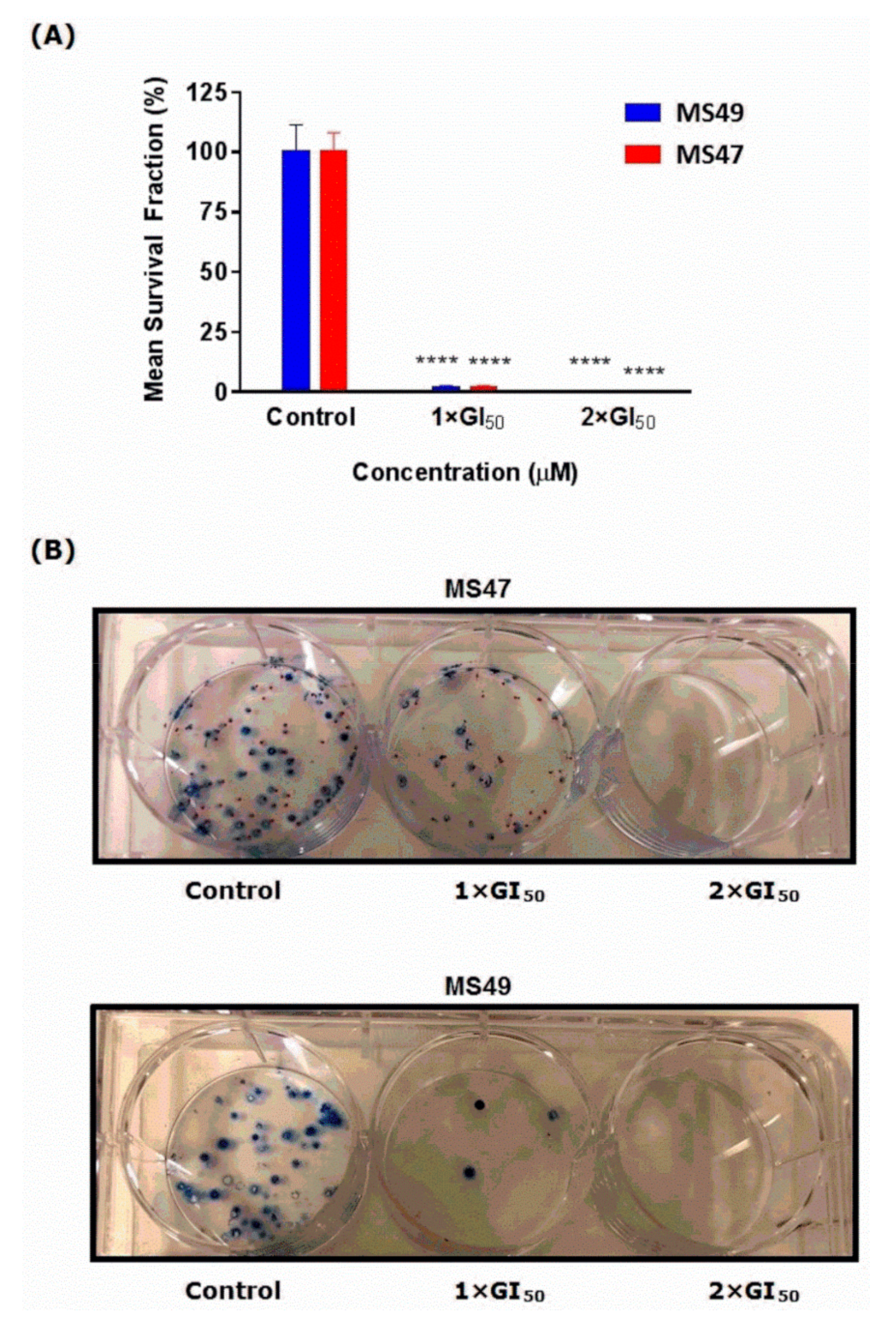
3.3. Clonogenic Assay
3.4. Cell Cycle Analysis
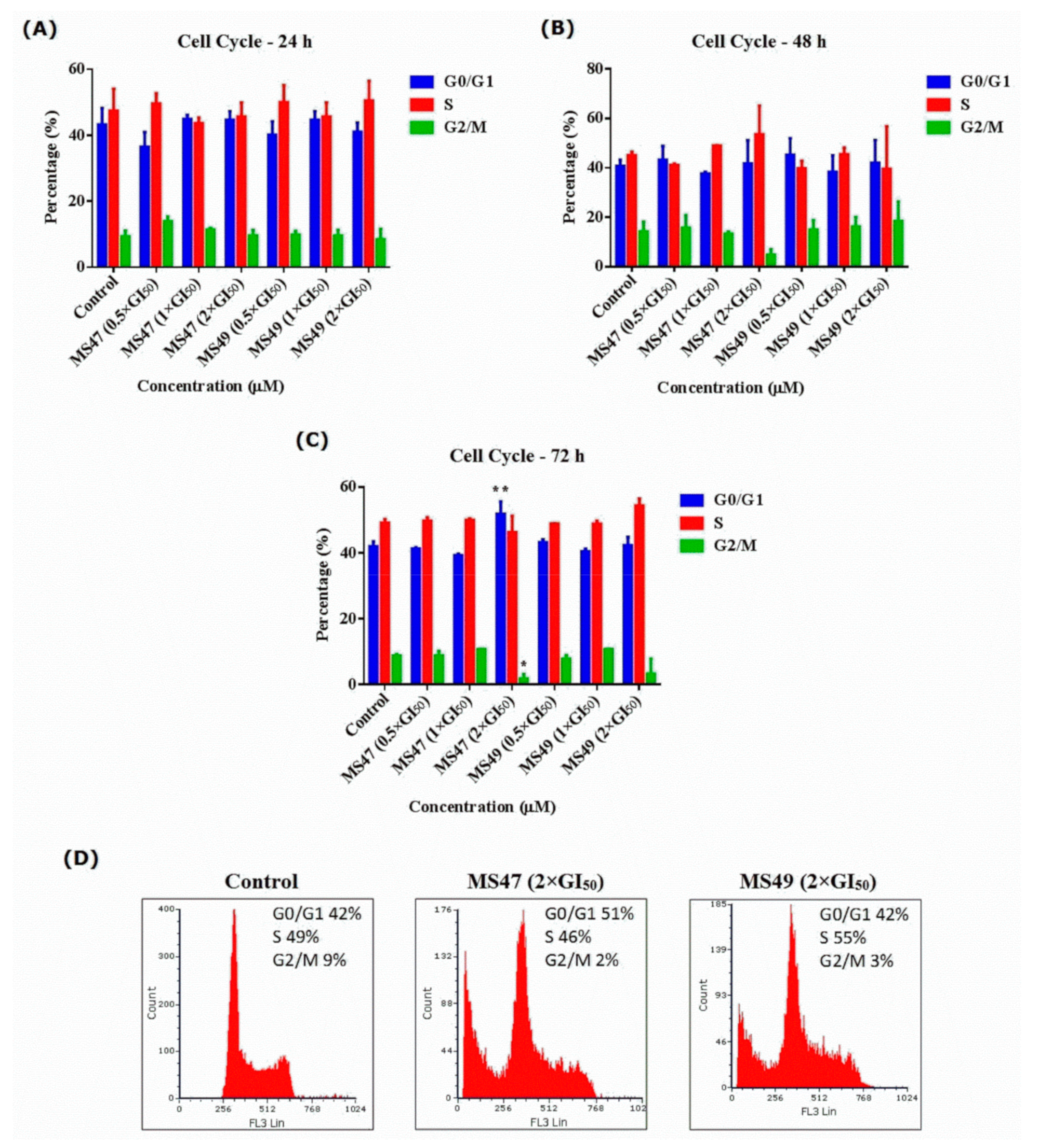
3.5. Annexin V-FITC and Propidium Iodide Apoptosis Assay

3.6. Western Blots
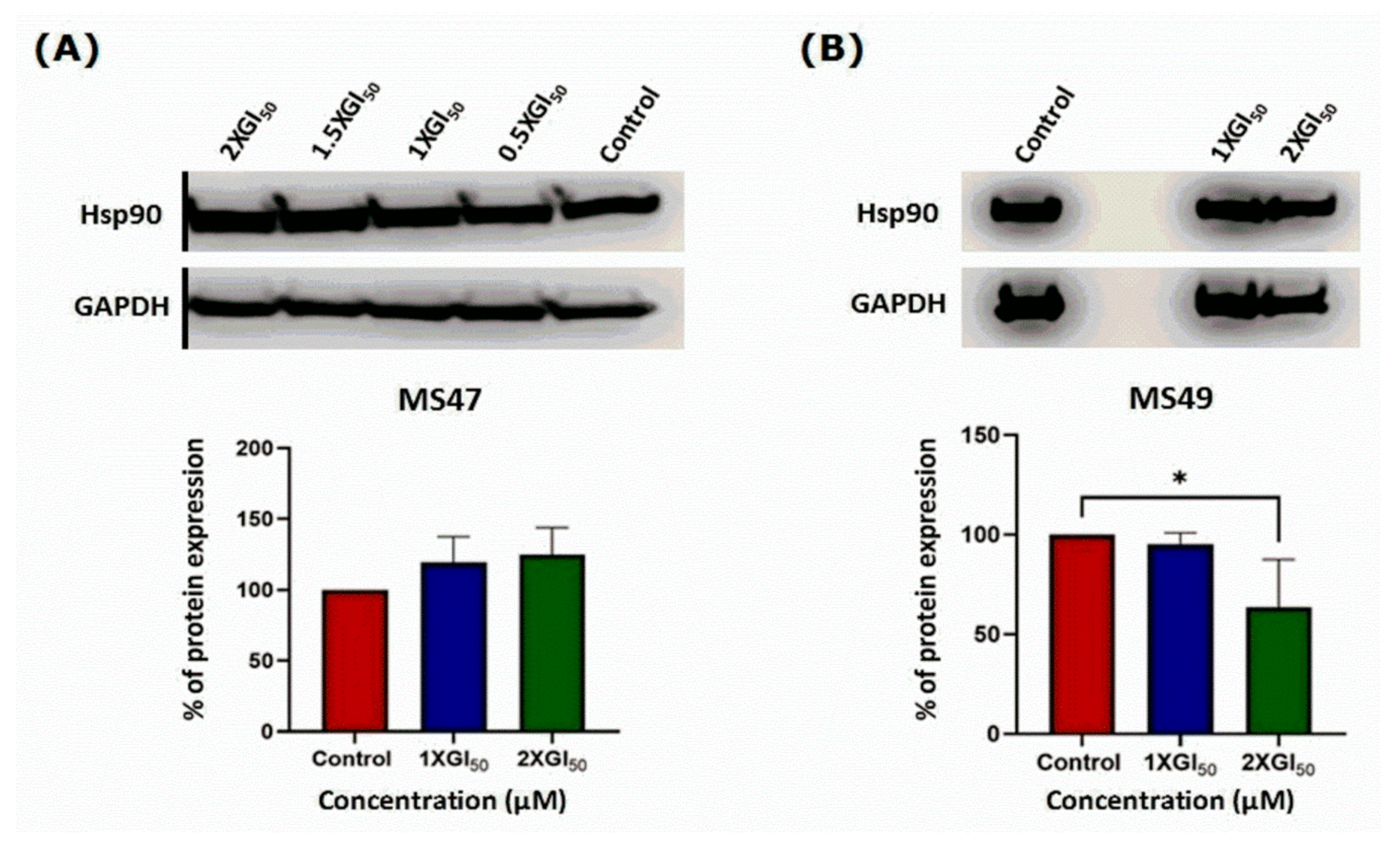
3.7. Senescence-Associated β-Galactosidase Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| FITC | Fluorescein isothiocyanate |
| GI50 | 50% Growth inhibition |
| HCT-116 | Human colorectal carcinoma cell line |
| Hsp90 | Heat shock protein 90 |
| Hsp90a | Labelled G-quadruplex forming oligomer of the promoter sequence Hsp90a oncogene |
| LC50 | 50% Lethal concentration |
| MDA-MB-435 | Human melanoma cell line |
| MiaPaCa-2 | Human pancreatic carcinoma cell line |
| MRC-5 | Medical Research Council cell strain 5; embryonic lung fibroblast cell line |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NCI | National Cancer Institute |
| OD | Optical density |
| PG | Percentage growth |
| PI | Propidium iodide |
| TGI | Total growth inhibition |
References
- Cancer Facts & Figures 2021. American Cancer Society. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 20 January 2022).
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008-2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Hossan, M.S.; Chan, Z.-Y.; Collins, H.M.; Shipton, F.N.; Butler, M.S.; Rahmatullah, M.; Lee, J.B.; Gershkovich, P.; Kagan, L.; Khoo, T.-J.; et al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. 2019, 453, 57–73. [Google Scholar] [CrossRef]
- Twelves, C.; Jove, M.; Gombos, A.; Awada, A. Cytotoxic chemotherapy: Still the mainstay of clinical practice for all subtypes metastatic breast cancer. Crit. Rev. Oncol. Hematol. 2016, 100, 74–87. [Google Scholar] [CrossRef]
- Patel, A.; Lewis, W.; Searle, M.S.; Stevens, M.F.G.; Moody, C.J. Synthesis of 6-arylisocytosines and their potential for hydrogen-bonding interactions. Tetrahedron 2015, 71, 7339–7343. [Google Scholar] [CrossRef]
- Parkinson, G.N.; Lee, M.P.H.; Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 2002, 417, 876–880. [Google Scholar] [CrossRef]
- Kuballa, P.; Baumann, A.-L.; Mayer, K.; Bär, U.; Burtscher, H.; Brinkmann, U. Induction of heat shock protein HSPA6 (HSP70B0) upon HSP90 inhibition in cancer cell lines. FEBS Lett. 2015, 589, 1450–1458. [Google Scholar] [CrossRef]
- Neidle, S. Quadruplex Nucleic Acids as Novel Therapeutic Targets. J. Med. Chem. 2016, 59, 5987–6011. [Google Scholar] [CrossRef]
- De Cian, A.; Lacroix, L.; Douarre, C.; Temime-Smaali, N.; Trentesaux, C.; Riou, J.-F.; Mergny, J.-L. Targeting Telomeres and Telomerase. Biochimie 2008, 90, 131–155. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. Hsp90 and the Chaperoning of Cancer. Reviews 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Campbell, N.H.; Patel, M.; Tofa, A.B.; Ghosh, R.; Parkinson, G.N.; Neidle, S. Selectivity in Ligand Recognition of G-Quadruplex Loops. Biochemistry 2009, 48, 1675–1680. [Google Scholar] [CrossRef]
- Gunaratnam, M.; Fuente, M.D.L.; Hampel, S.M.; Todd, A.K.; Reszka, A.P.; Schätzlein, A.; Neidle, S. Targeting pancreatic cancer with a G-quadruplex ligand. Bioorg. Med. Chem. 2011, 19, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Dobo, C.; Stavale, J.N.; Lima, F.O.; Ribeiro, D.A.; Arias, V.; Gomes, T.S.; Oshima, C.T. HSP27 is commonly expressed in cervical intraepithelial lesions of Brazilian women. Asian Pac. J. Cancer 2013, 14, 5007–5010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mamede, A.C.; Laranjo, M.; Carvalho, M.J.; Abrantes, A.M.; Pires, A.S.; Brito, A.F.; Moura, P.; Maia, C.J.; Botelho, M.F. Effect of amniotic membrane proteins in human cancer cell lines: An exploratory study. J. Membr. Biol. 2014, 247, 357–360. [Google Scholar] [CrossRef]
- Niknejad, H.; Khayat-Khoei, M.; Peirovi, H.; Abolghasemi, H. Human amniotic epithelial cells induce apoptosis of cancer cells: A new anti-tumor therapeutic strategy. Cytotherapy 2014, 16, 33–40. [Google Scholar] [CrossRef]
- Moser, C.; Lang, S.A.; Stoeltzing, O. Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 2009, 29, 2031–2042. Available online: https://pubmed.ncbi.nlm.nih.gov/19528462/ (accessed on 25 May 2022).
- Saleh, M.M.; Laughton, C.A.; Bradshaw, T.D.; Moody, C.J. Development of a series of bis-triazoles as G-quadruplex ligands. RSC Adv. 2017, 7, 47297–47308. [Google Scholar] [CrossRef]
- Mergny, J.-L. Fluorescence energy transfer as a probe for tetraplex formation: I-motif. Biochemistry 1999, 38, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Gavathiotis, E.; Heald, R.A.; Stevens, M.F.G.; Searle, M.S. Drug Recognition and Stabilisation of the Parallel-stranded DNA Quadruplex d(TTAGGGT)4 Containing the Human Telomeric Repeat. J. Mol. Biol. 2003, 334, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Causes of Death Statistics. Eurostat Statistics Explained. 2017. Available online: https://ec.europa.eu/eurostat/statisticsexplained/index.php/Causes_of_death_statistics#Causes_of_death_in_EU27_Member_States_in_2017 (accessed on 29 September 2020).
- CORDIS EU Research Results. European Commission. 2020. Available online: https://cordis.europa.eu/results-packs/en (accessed on 29 September 2020).
- Melanoma Skin Cancer. European Commission. 2017. Available online: https://ec.europa.eu/research/health/pdf/factsheets/melanoma_skin_cancer.pdf (accessed on 29 September 2020).
- Parker, E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Murakami, T.; Chmielowski, B.; Igarashi, K.; Kiyuna, T.; Unno, M.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; Li, Y.; et al. Vemurafenib-resistant BRAF-V600E-mutated melanoma is regressed by MEK-targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2016, 7, 71737–71743. [Google Scholar] [CrossRef]
- Saleh, M.M.; Abuirmeileh, A.N.; Al-Rousan, R.M.; Abudoleh, S.M.; Hassouneh, L.K.; Zihlif, M.A.; Taha, M.O.; Abutayeh, R.F.; Mansour, H.; Abu-Irmaileh, B. Biological Evaluation and Reverse Pharmacophore Mapping of Innovative Bis-Triazoles as Promising Anticancer Agents. Open Med. Chem. J. 2022, 16, e187410452207200. [Google Scholar] [CrossRef]
- Munshi, A.; Hobbs, M.; Meyn, R.E. Clonogenic cell survival assay. Methods Mol. Med. 2005, 110, 21–28. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Bree, C.V. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Raja, V.J.; Lim, K.-H.; Leong, C.-O.; Kam, T.-S.; Bradshaw, T.D. Novel antitumour indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Investig. New Drugs 2014, 32, 838–850. [Google Scholar] [CrossRef]
- Chen, R.; Keating, M.J.; Gandhi, V.; Plunkett, W. Transcription inhibition by flavopiridol: Mechanism of chronic lymphocytic leukemia cell death. Blood 2005, 106, 2513–2519. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.-C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some Practical Considerations and Applications of the National Cancer Institute In Vitro Anticancer Drug Discovery Screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D.; Rubinstein, L.R. Data display and analysis strategies for the NCl disease-oriented in vitro antitumor drug screen. In Cytotoxic Anticancer Drugs: Models and Concepts for Drug Discovery and Development, 1st ed.; Valeriote, F.A., Corbett, T., Baker, L., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1992; Volume 1, pp. 11–34. [Google Scholar]
- Boyd, M.K. Status of the NCI preclinical antitumor drug discovery screen. In Cancer: Principles and Practice of Oncology Updates, 1st ed.; DeVita, V.T.J., Hellman, S., Rosenberg, S.A., Eds.; J. B. Lippincot: Philadelphi, PN, USA, 1989; Volume 3, pp. 1–12. [Google Scholar]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- NCI-60 Screening Methodology. National Cancer Institute. 2015. Available online: https://dtp.cancer.gov/discovery_development/nci60/methodology.htm#:~:text=NCI%2060%20Cell%20Five%2DDose%20Screen&text=For%20a%20typical%20screening%20experiment,time%20of%20individual%20cell%20lines (accessed on 11 September 2020).
- Ali, A.A.A.; Lee, Y.-R.; Chen, T.-C.; Chen, C.-L.; Lee, C.-C.; Shiau, C.-Y.; Chiang, C.-H.; Huang, H.-S. Novel Anthra[1,2-c][1,2,5]Thiadiazole-6,11-Diones as Promising Anticancer Lead Compounds: Biological Evaluation, Characterization & Molecular Targets Determination. PLoS ONE 2016, 11, e0154278. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Al-Tarawneh, F.; Talib, W.H.; Sweidan, K.; Bardaweel, S.K.; Al-Shalabi, E.; Zhong, H.A.; Abu Sheikha, G.; Abu Khalaf, R.; Mubarak, M.S. Benzoin Schiff Bases: Design, Synthesis, and Biological Evaluation as Potential Antitumor Agents. Med. Chem. 2018, 14, 695–708. [Google Scholar] [CrossRef]
- King, K.L.; Cidlowski, J.A. Cell cycle and apoptosis: Common pathways to life and death. J. Cell. Biochem. 1995, 58, 175–180. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Bagatell, R.; Whitesell, L. Altered Hsp90 function in cancer: A unique therapeutic opportunity. Mol. Cancer Ther. 2004, 3, 1021–1030. [Google Scholar] [CrossRef]
- Solit, D.B.; Chiosis, G. Development and application of Hsp90 inhibitors. Drug Discov. Today 2008, 13, 38–43. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence–Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Pruzan, R.; Pongracz, K.; Gietzen, K.; Wallweber, G.; Gryaznov, S. Allosteric inhibitors of telomerase: Oligonucleotide N3’-P5’ phosphoramidates. Nucleic Acids Res. 2002, 30, 559–568. [Google Scholar] [CrossRef][Green Version]
- Huang, F.-C.; Chang, C.-C.; Wang, J.-M.; Chang, T.-C.; Lin, J.-J. Induction of senescence in cancer cells by the G-quadruplex stabilizer, BMVC4, is independent of its telomerase inhibitory activity. Br. J. Pharmacol. 2012, 167, 393–406. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, M.M.; Abuarqoub, D.A.; Hammad, A.M.; Hossan, M.S.; Ahmed, N.; Aslam, N.; Naser, A.Y.; Moody, C.J.; Laughton, C.A.; Bradshaw, T.D. In Vitro Anticancer Properties of Novel Bis-Triazoles. Curr. Issues Mol. Biol. 2023, 45, 175-196. https://doi.org/10.3390/cimb45010014
Saleh MM, Abuarqoub DA, Hammad AM, Hossan MS, Ahmed N, Aslam N, Naser AY, Moody CJ, Laughton CA, Bradshaw TD. In Vitro Anticancer Properties of Novel Bis-Triazoles. Current Issues in Molecular Biology. 2023; 45(1):175-196. https://doi.org/10.3390/cimb45010014
Chicago/Turabian StyleSaleh, Maysaa M., Duaa A. Abuarqoub, Alaa M. Hammad, Md Shahadat Hossan, Najneen Ahmed, Nazneen Aslam, Abdallah Y. Naser, Christopher J. Moody, Charles A. Laughton, and Tracey D. Bradshaw. 2023. "In Vitro Anticancer Properties of Novel Bis-Triazoles" Current Issues in Molecular Biology 45, no. 1: 175-196. https://doi.org/10.3390/cimb45010014
APA StyleSaleh, M. M., Abuarqoub, D. A., Hammad, A. M., Hossan, M. S., Ahmed, N., Aslam, N., Naser, A. Y., Moody, C. J., Laughton, C. A., & Bradshaw, T. D. (2023). In Vitro Anticancer Properties of Novel Bis-Triazoles. Current Issues in Molecular Biology, 45(1), 175-196. https://doi.org/10.3390/cimb45010014








