Prognostic Impact of Low-Level p53 Expression on Brain Astrocytomas Immunopositive for Epidermal Growth Factor Receptor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Protocol
2.2. Immunohistochemical Staining of p53 and EGFR
2.3. Cell Culture and Treatment with the EGFR Inhibitor (AG490)
2.4. Proliferation Assay
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Expressions of p53 and EGFR in Astrocytomas
3.3. Survival Analysis According to p53 and EGFR Expression
3.4. Expression of p53, EGFR, and IC50s after Treatment with EGFR Inhibitors in GBM Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Palmisciano, P.; Ferini, G.; Watanabe, G.; Ogasawara, C.; Lesha, E.; Bin-Alamer, O.; Umana, G.E.; Yu, K.; Cohen-Gadol, A.A.; El Ahmadieh, T.Y.; et al. Gliomas Infiltrating the Corpus Callosum: A Systematic Review of the Literature. Cancers 2022, 14, 2507. [Google Scholar] [CrossRef] [PubMed]
- Figarella-Branger, D.; Appay, R.; Metais, A.; Tauziede-Espariat, A.; Colin, C.; Rousseau, A.; Varlet, P. The 2021 WHO classification of tumours of the central nervous system. Ann. Pathol. 2021. [Google Scholar] [CrossRef]
- Bonosi, L.; Ferini, G.; Giammalva, G.R.; Benigno, U.E.; Porzio, M.; Giovannini, E.A.; Musso, S.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; et al. Liquid Biopsy in Diagnosis and Prognosis of High-Grade Gliomas; State-of-the-Art and Literature Review. Life 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Camara-Quintana, J.Q.; Nitta, R.T.; Li, G. Pathology: Commonly monitored glioblastoma markers: EFGR, EGFRvIII, PTEN, and MGMT. Neurosurg. Clin. N. Am. 2012, 23, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Bhaskara, V.K.; Babu, P.P. Implications of mitogen-activated protein kinase signaling in glioma. J. Neurosci. Res. 2016, 94, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1206–1211. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E., 2nd; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 2010, 96, 219–230. [Google Scholar] [CrossRef]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010, 12, 1300–1310. [Google Scholar] [CrossRef] [Green Version]
- Karsy, M.; Neil, J.A.; Guan, J.; Mahan, M.A.; Colman, H.; Jensen, R.L. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg. Focus 2015, 38, E4. [Google Scholar] [CrossRef]
- Siegal, T. Clinical impact of molecular biomarkers in gliomas. J. Clin. Neurosci. 2015, 22, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Benavente, S.; Armstrong, E.A.; Li, C.; Wheeler, D.L.; Harari, P.M. P53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2011, 71, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Youland, R.S.; Schomas, D.A.; Brown, P.D.; Nwachukwu, C.; Buckner, J.C.; Giannini, C.; Parney, I.F.; Laack, N.N. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013, 15, 1102–1110. [Google Scholar] [CrossRef]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Fekete, B.; Werlenius, K.; Orndal, C.; Rydenhag, B. Prognostic factors for glioblastoma patients—A clinical population-based study. Acta Neurol. Scand. 2016, 133, 434–441. [Google Scholar] [CrossRef]
- Pignatti, F.; van den Bent, M.; Curran, D.; Debruyne, C.; Sylvester, R.; Therasse, P.; Afra, D.; Cornu, P.; Bolla, M.; Vecht, C.; et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 2002, 20, 2076–2084. [Google Scholar] [CrossRef]
- Waqar, M.; Hanif, S.; Brodbelt, A.R.; Rathi, N.; Das, K.; Zakaria, R.; Walker, C.; Jenkinson, M.D. Prognostic Factors in Lobar World Health Organization Grade II Astrocytomas. World Neurosurg. 2015, 84, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: A prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, F.; Kugawa, S.; Tateno, H.; Kokubu, F.; Fukuchi, K. Analysis of EGFR, KRAS and P53 mutations in lung cancer using cells in the curette lavage fluid obtained by bronchoscopy. Lung Cancer 2012, 78, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Vadala, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.; et al. Stereotactic body radiotherapy in non-operable lung cancer patients. Clin. Transl. Oncol. 2016, 18, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Malkoun, N.; Chargari, C.; Forest, F.; Fotso, M.J.; Cartier, L.; Auberdiac, P.; Thorin, J.; Pacaut, C.; Peoc’h, M.; Nuti, C.; et al. Prolonged temozolomide for treatment of glioblastoma: Preliminary clinical results and prognostic value of p53 overexpression. J. Neurooncol. 2012, 106, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Lejeune, J.; Benouaich-Amiel, A.; Laigle-Donadey, F.; Criniere, E.; Mokhtari, K.; Thillet, J.; Delattre, J.Y.; Hoang-Xuan, K.; Sanson, M. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer 2006, 106, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Felsberg, J.; Rapp, M.; Loeser, S.; Fimmers, R.; Stummer, W.; Goeppert, M.; Steiger, H.J.; Friedensdorf, B.; Reifenberger, G.; Sabel, M.C. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin. Cancer Res. 2009, 15, 6683–6693. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- England, B.; Huang, T.; Karsy, M. Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumour. Biol. 2013, 34, 2063–2074. [Google Scholar] [CrossRef]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef]
- Ruano, Y.; Ribalta, T.; de Lope, A.R.; Campos-Martin, Y.; Fiano, C.; Perez-Magan, E.; Hernandez-Moneo, J.L.; Mollejo, M.; Melendez, B. Worse outcome in primary glioblastoma multiforme with concurrent epidermal growth factor receptor and p53 alteration. Am. J. Clin. Pathol. 2009, 131, 257–263. [Google Scholar] [CrossRef]
- Azuaje, F.; Tiemann, K.; Niclou, S.P. Therapeutic control and resistance of the EGFR-driven signaling network in glioblastoma. Cell Commun. Signal. 2015, 13, 23. [Google Scholar] [CrossRef] [Green Version]
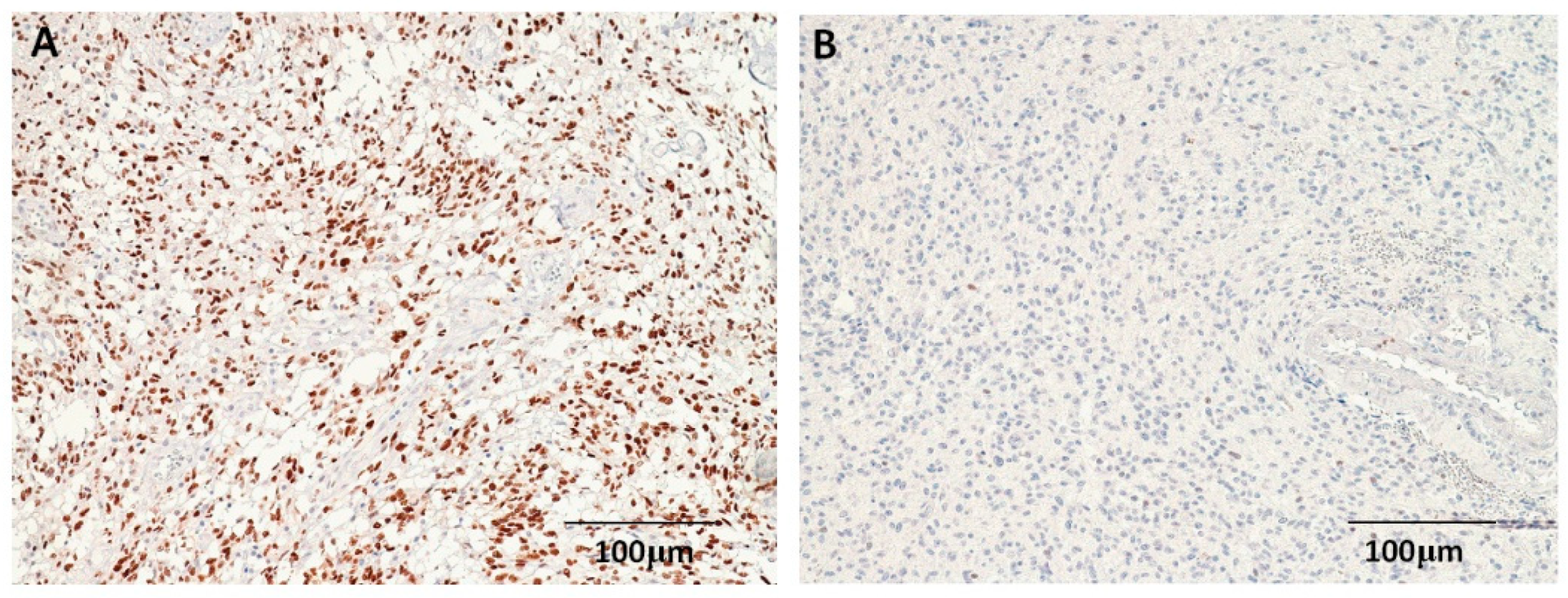
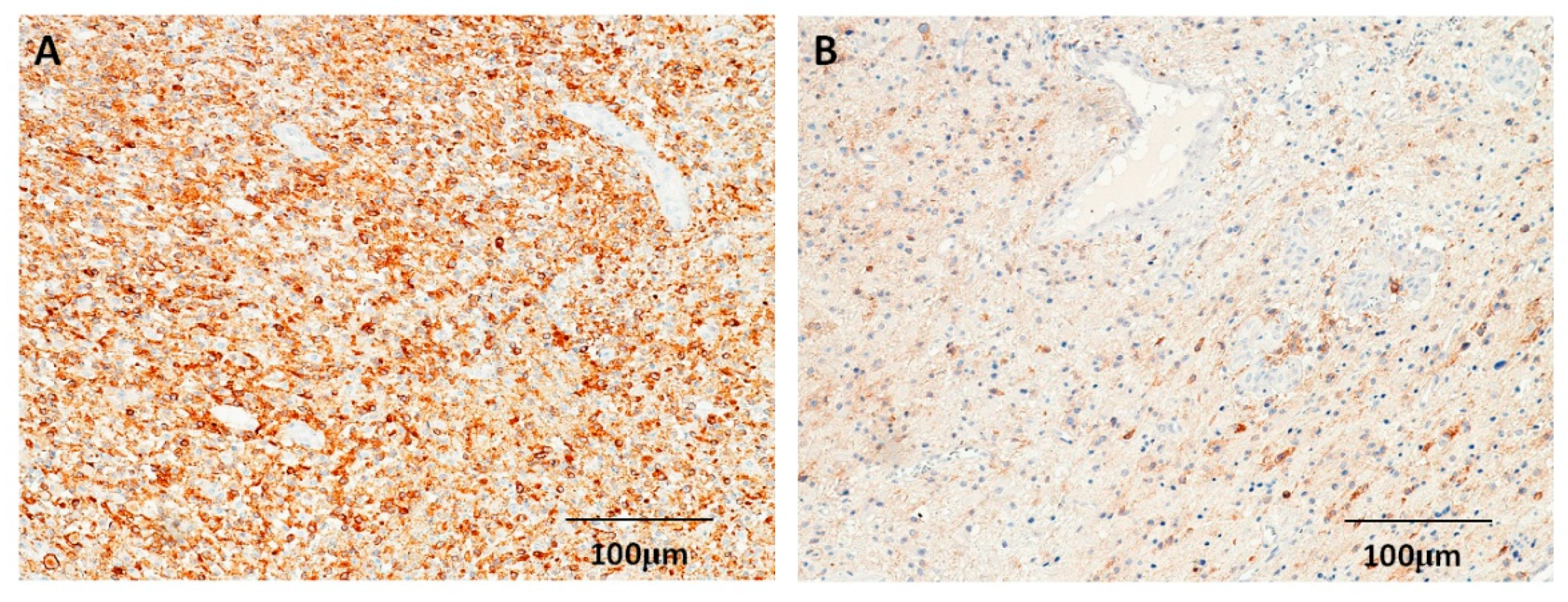

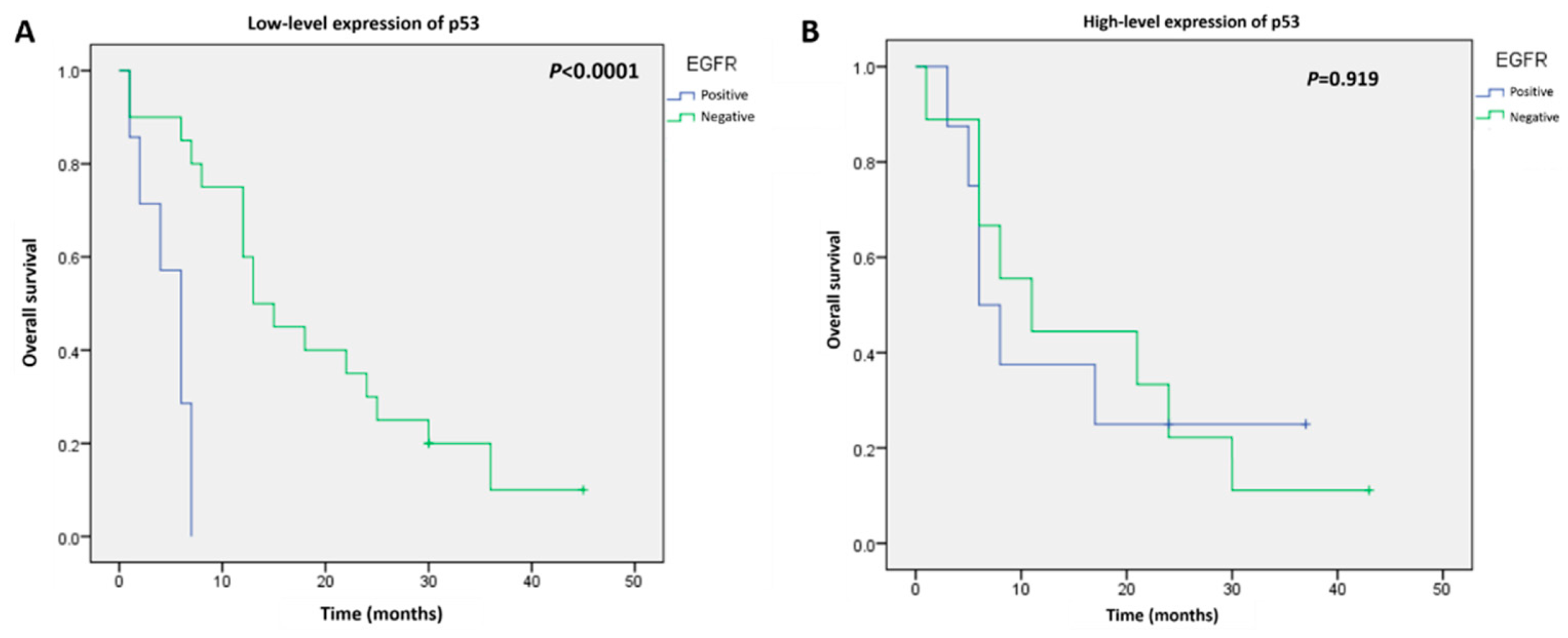

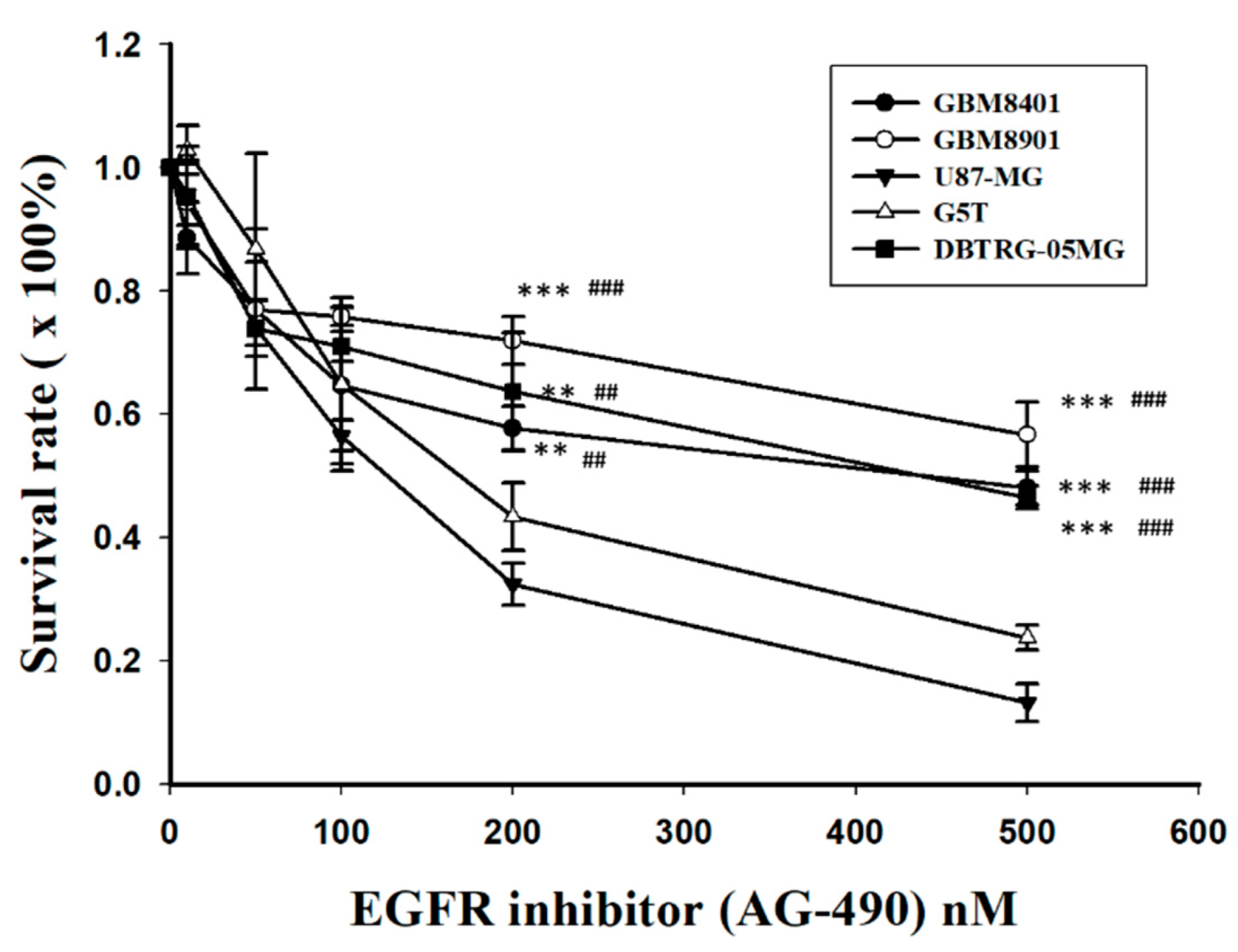
| No. | p53 Expression (n, %) | EGFR Expression (n, %) | |||||
|---|---|---|---|---|---|---|---|
| Low | High | p-Value | Negative | Positive | p-Value | ||
| Age | 0.207 | 0.425 | |||||
| ≤60 | 61 | 40 (48.8%) | 21 (25.6%) | 20 (24.4%) | 41 (50%) | ||
| >60 | 21 | 11 (13.4%) | 10 (12.2%) | 8 (9.8%) | 13 (15.9%) | ||
| Gender | 0.412 | 0.09 | |||||
| Male | 45 | 27 (32.9%) | 18 (22.0%) | 12 (14.6%) | 33 (40.2%) | ||
| Female | 37 | 24 (29.3%) | 13 (15.9%) | 16 (19.5%) | 21 (25.6%) | ||
| WHO Grade | 0.022 * | 0.329 | |||||
| I/II | 31 | 24 (29.3%) | 7 (8.5%) | 12 (14.6%) | 19 (23.2%) | ||
| III/IV | 51 | 27 (32.9%) | 24 (29.3%) | 16 (19.5%) | 35 (42.7%) | ||
| Tumor size | 0.519 | 0.150 | |||||
| ≤3 cm | 46 | 29 (35.4%) | 17 (20.7%) | 13 (15.9%) | 33 (40.2%) | ||
| >3 cm | 36 | 22 (26.8%) | 14 (17.1%) | 15 (18.3%) | 21 (25.6%) | ||
| Radiotherapy | 0.412 | 0.299 | |||||
| Yes | 37 | 24 (29.3%) | 13 (15.9%) | 11 (13.4%) | 26 (31.7%) | ||
| No | 45 | 27 (32.9%) | 18 (22.0) | 17 (20.7%) | 28 (34.1%) | ||
| Chemotherapy | 0.329 | 0.065 | |||||
| Yes | 28 | 16 (19.5%) | 12 (14.6) | 6 (7.3%) | 22 (31.7%) | ||
| No | 54 | 35 (42.7%) | 19 (23.2%) | 17 (20.7%) | 28 (34.1%) | ||
| KPS | 0.412 | 0.120 | |||||
| ≤70 | 58 | 37 (45.1%) | 21 (25.6%) | 17 (20.7%) | 41 (50%) | ||
| >70 | 24 | 14 (17.1%) | 10 (12.2%) | 11 (13.4%) | 13 (15.9%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-P.; Lin, C.-J.; Wu, C.-H.; Chen, Y.-T.; Lu, Y.-Y.; Kwan, A.-L.; Lieu, A.-S. Prognostic Impact of Low-Level p53 Expression on Brain Astrocytomas Immunopositive for Epidermal Growth Factor Receptor. Curr. Issues Mol. Biol. 2022, 44, 4142-4151. https://doi.org/10.3390/cimb44090284
Tsai H-P, Lin C-J, Wu C-H, Chen Y-T, Lu Y-Y, Kwan A-L, Lieu A-S. Prognostic Impact of Low-Level p53 Expression on Brain Astrocytomas Immunopositive for Epidermal Growth Factor Receptor. Current Issues in Molecular Biology. 2022; 44(9):4142-4151. https://doi.org/10.3390/cimb44090284
Chicago/Turabian StyleTsai, Hung-Pei, Chien-Ju Lin, Chieh-Hsin Wu, Yi-Ting Chen, Ying-Yi Lu, Aij-Lie Kwan, and Ann-Shung Lieu. 2022. "Prognostic Impact of Low-Level p53 Expression on Brain Astrocytomas Immunopositive for Epidermal Growth Factor Receptor" Current Issues in Molecular Biology 44, no. 9: 4142-4151. https://doi.org/10.3390/cimb44090284
APA StyleTsai, H.-P., Lin, C.-J., Wu, C.-H., Chen, Y.-T., Lu, Y.-Y., Kwan, A.-L., & Lieu, A.-S. (2022). Prognostic Impact of Low-Level p53 Expression on Brain Astrocytomas Immunopositive for Epidermal Growth Factor Receptor. Current Issues in Molecular Biology, 44(9), 4142-4151. https://doi.org/10.3390/cimb44090284






