Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
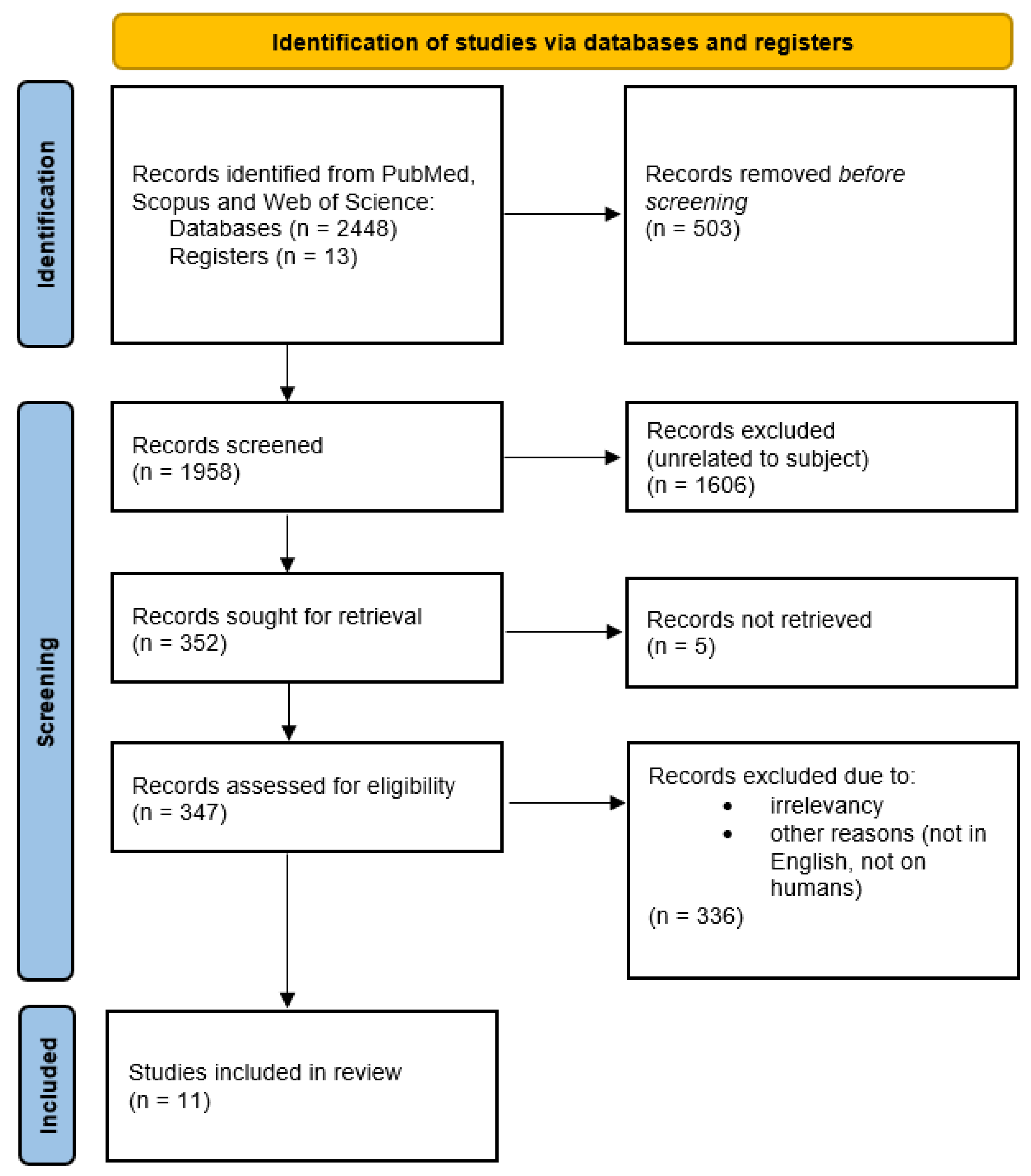
3.1. Overview of the Selected Articles
3.2. The Current State of Biomarkers in NENs and Future Perspectives
3.3. Angiogenesis in Neuroendocrine Tumors
- − Vessel co-option: preexisting vessels are “hijacked” to serve tumor cells.
- − Vasculogenic mimicry: tumor cells build blood channels similar to the endothelium [42].
3.4. The Vascular Endothelial Growth Factor Family
3.5. Placental Growth Factor
3.6. Angiopoietin-Tie-2
3.7. Other Molecules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.; Carneiro, F.; Cree, I. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–13342. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Neuroendocrine Tumor of the Gastrointestinal Tract: Introduction. Cancer.net. 2021. Available online: https://www.cancer.net/cancer-types/neuroendocrine-tumor-gastrointestinal-tract/introduction (accessed on 2 May 2022).
- Rodallec, M.; Vilgrain, V.; Couvelard, A.; Rufat, P.; O’Toole, D.; Barrau, V.; Sauvanet, A.; Ruszniewski, P.; Menu, Y. Endocrine Pancreatic tumours and helical CT: Contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology 2006, 6, 77–85. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, H.; Wang, G.; Yin, L.; Xu, W.; Peng, Y.; Wu, J.; Jiang, K.; Miao, Y. A meta-analysis of prognostic factor of pancreatic neuroendocrine neoplasms. Sci. Rep. 2018, 8, 7271. [Google Scholar] [CrossRef] [PubMed]
- Ebos, J.M.L.; Lee, C.R.; Christensen, J.G.; Mutsaers, A.J.; Kerbel, R.S. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc. Natl. Acad. Sci. USA 2007, 104, 17069–17074. [Google Scholar] [CrossRef]
- Beyens, M.; Vandamme, T.; Peeters, M.; van Camp, G.; de Beeck, K.O. Resistance to targeted treatment of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, R109–R130. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fonseca, P.; Martín, M.N.; Carmona-Bayonas, A.; Calvo, A.; Fernández-Mateos, J.; Redrado, M.; Capdevila, J.; Lago, N.M.; Lacasta, A.; Muñarrizet, J.; et al. Biomarkers and polymorphisms in pancreatic neuroendocrine tumors treated with sunitinib. Oncotarget 2018, 9, 36894–36905. [Google Scholar] [CrossRef] [PubMed]
- Zurita, A.J.; Khajavi, M.; Wu, H.K.; Tye, L.; Huang, X.; Kulke, M.H.; Lenz, H.; Meropol, N.; Carley, W.; DePrimo, S.; et al. Circulating cytokines and monocyte subpopulations as biomarkers of outcome and biological activity in sunitinib-treated patients with advanced neuroendocrine tumours. Br. J. Cancer 2015, 112, 1199–1205. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevilla, J.; De Vries, E.; et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef]
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; Sastre, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hassler, G.; Baum, U.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Circulating of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin. Endocrinol. 2005, 62, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Srirajaskanthan, R.; Dancey, G.; Hackshaw, A.; Luong, T.; Caplin, M.E.; Meyer, T. Circulating angiopoietin-2 is elevated in patients with neuroendocrine tumours and correlates with disease burden and prognosis. Endocr. Relat. Cancer 2009, 16, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Melen-Mucha, G.; Niedziela, A.; Mucha, S.; Motylewska, E.; Lawnicka, H.; Komorowski, J.; Stepien, H. Elevated peripheral blood plasma concentrations of tie-2 and angiopoietin 2 in patients with neuroendocrine tumors. Int. J. Mol. Sci. 2012, 13, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Mol. Cell Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef]
- Detjen, K.M.; Rieke, S.; Deters, A.; Schulz, P.; Rexin, A.; Vollmer, S.; Hauff, P.; Wiedenmann, B.; Pavel, M.; Scholz, A. Angiopoietin-2 promotes disease progression of neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 420–429. [Google Scholar] [CrossRef]
- Figueroa-Vega, N.; Díaz, Á.; Adrados, M.; Álvarez-Escolá, C.; Paniagua, A.; Aragonés, J.; Martín-Pérez, E.; Leskela, S.; Moreno-Otero, R.; González-Amaro, R.; et al. The association of the angiopoietin/Tie-2 system with the development of metastasis and leukocyte migration in neuroendocrine tumors. Endocr. Relat. Cancer 2010, 17, 897–908. [Google Scholar] [CrossRef]
- Hilfenhaus, G.; Göhrig, A.; Pape, U.F.; Neumann, T.; Jann, H.; Zdunek, D.; Hess, G.; Stassen, J.M.; Wiedenmann, B.; Detjen, K.; et al. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr. Relat. Cancer 2013, 20, 305–319. [Google Scholar] [CrossRef]
- Oberg, K.; Couvelard, A.; Delle Fave, G.; Gross, D.; Grossman, A.; Jensen, R.T.; Pape, U.F.; Perren, A.; Rindi, G.; Ruszniewski, P.; et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Biochemical markers. Neuroendocrinology 2017, 105, 201–211. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Li, Z.; Cheng, C.; Yang, T.; Wang, C.; Liu, L.; Liu, S. Diagnostic value of circulating chromogranin A for neuroendocrine tumors: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124884. [Google Scholar] [CrossRef] [Green Version]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yang, Q.C.; Lin, Y.; Xue, L.; Chen, M.H.; Chen, J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014, 93, e247. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, X.; Shen, B.; Jing, Y.; Li, Q.; Cai, M.C.; Gu, Z.; Yang, Q.; Zhang, Z.; Liu, J.; et al. A VEGF-dependent gene signature enriched in mesenchymal ovarian cancer predicts patient prognosis. Sci. Rep. 2016, 6, 31079. [Google Scholar] [CrossRef] [PubMed]
- D’Herbomez, M.; Coppin, L.; Bauters, C.; Rouaix-Emery, N.; Carnaille, B.; Do Cao, C. Les marqueurs biologiques des tumeurs endocrines. Ann. Biol. Clin. 2016, 74, 669–679. [Google Scholar] [CrossRef]
- Ferolla, P.; Faggiano, A.; Mansueto, G.; Avenia, N.; Cantelmi, M.G.; Giovenali, P.; Del Basso De Caro, M.L.; Milone, F.; Scarpelli, G.; Masone, F.; et al. The biological characterization of neuroendocrine tumors: The role of neuroendocrine markers. J. Endocrinol. Investig. 2008, 31, 277–286. [Google Scholar] [CrossRef]
- Nobels, F.R.; Kwekkeboom, D.J.; Coopmans, W.; Schoenmakers, C.H.; Lindemans, J.; De Herder, W.W.; Krenning, E.P.; Bouillon, R.; Lamberts, S.W.J. Chromogranin A as serum marker for neuroendocrine neoplasia: Comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J. Clin. Endocrinol. Metab. 1997, 82, 2622–2628. [Google Scholar]
- Yao, J.C.; Pavel, M.; Phan, A.T.; Kulke, M.H.; Hoosen, S.; St Peter, J.; Cherfi, A.; Öberg, K.E. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J. Clin. Endocrinol. Metab. 2011, 96, 3741–3749. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, J.; Li, J.; Shen, L.; Li, N.; Zhu, H.; Zhai, S.; Zhang, Y.; Yang, Z.; Lu, M. Clinical and prognostic value of PET/CT imaging with combination of (68)Ga-DOTATATE and (18)F-FDG in gastroenteropancreatic neuroendocrine neoplasms. Contrast Media Mol. Imaging 2018, 26, 2340389. [Google Scholar] [CrossRef]
- Drevs, J.; Schneider, V. The use of vascular biomarkers and imaging studies in the early clinical development of anti-tumour agents targeting angiogenesis. J. Intern. Med. 2006, 260, 517–529. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Boland, P.M.; Handorf, E.; Gustafson, K.S.; Gong, Y.; Cooper, H.S.; Sheriff, F.; Astsaturov, I.; Cohen, S.J.; Engstrom, P.F. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: A Fox Chase Cancer Center pilot study. Br. J. Cancer 2016, 115, 564–570. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A precision oncology knowledge base. JCO Precis. Oncol. 2017, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.; Drozdov, I.; Kidd, M. A multitranscript blood neuroendocrine tumor molecular signature to identify treatment efficacy and disease progress. J. Clin. Oncol. 2013, 31, A4137. [Google Scholar] [CrossRef]
- Malczewska, A.; Kos-Kudła, B.; Kidd, M.; Drozdov, I.; Bodei, L.; Matar, S.; Oberg, K.; Modlin, I.M. The clinical applications of a multigene liquid biopsy (NETest) in neuroendocrine tumors. Adv. Med. Sci. 2020, 65, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.; Califano, A.; Strosberg, J.R.; Ma, S.; Pape, U.; Bodei, L.; Kaltsas, G.; Toumpanakis, C.; Goldenring, J.R.; Frilling, A.; et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann. Oncol. 2020, 31, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, D.; Cho, H.; Machida, E.; Kawamura, A.; Takashima, A.; Wada, S.; Tsunoda, T.; Kohno, T.; Shiraishi, K. Update on epidemiology, diagnosis, and biomarkers in gastroenteropancreatic neuroendocrine neoplasms. Cancers 2022, 14, 1119. [Google Scholar] [CrossRef]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 365–372. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T.; Peeters, M.; Beyens, M.; Driessen, A.; Janssens, K.; Zwaenepoel, K.; Roeyen, G.; Van Camp, G.; De Beeck, K.O. Cell-free DNA from metastatic pancreatic neuroendocrine tumor patients contains tumor-specific mutations and copy number variations. Front. Oncol. 2018, 8, 467. [Google Scholar] [CrossRef]
- Roldo, C.; Missiaglia, E.; Hagan, J.P.; Falconi, M.; Capelli, P.; Bersani, S.; Calin, G.A.; Volinia, S.; Liu, C.G.; Scarpa, A.; et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 2006, 24, 4677–4684. [Google Scholar] [CrossRef]
- Schneider, B.P.; Shen, F.; Miller, K.D. Pharmacogenetic biomarkers for the prediction of response to antiangiogenic treatment. Lancet Oncol. 2012, 13, e427–e436. [Google Scholar] [CrossRef]
- Ping, Y.F.; Bian, X.W. Consice review: Contribution of cancer stem cells to neovascularization. Stem Cells 2011, 29, 888–894. [Google Scholar] [CrossRef]
- Scoazec, J.Y. Angiogenesis in neuroendocrine tumors: Therapeutic applications. Neuroendocrinology 2013, 97, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Ghayouri, M.; Morse, B.; Brelsford, M.; Black, M.; Rizzo, A.; Meeker, A.; Strosberg, J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Bart Rose, J.; Liu, Y.; Jaskular-Sztul, R.; Contreras, C.; Beck, A.; Chen, H. Heterogeneity of vascular endothelial cells, de novo arteriogenesis and therapeutic implications in pancreatic neuroendocrine tumors. J. Clin. Med. 2019, 8, 1980. [Google Scholar] [CrossRef]
- Poncet, G.; Villaume, K.; Walter, T.; Pourreyron, C.; Theillaumas, A.; Lépinasse, F.; Hervieu, V.; Cordier-Bussat, M.; Scoazec, J.Y.; Roche, C. Angiogenesis and tumor progression in neuroendocrine digestive tumors. J. Surg. Res. 2009, 154, 68–77. [Google Scholar] [CrossRef]
- Ferrara, N. From the discovery of vascular endothelial growth factor to the introduction of avastin in clinical trials—An interview with Napoleone Ferrara by Domenico Ribatti. Int. J. Dev. Biol. 2011, 55, 383–388. [Google Scholar]
- Fischer, C.; Mazzone, M.; Jonckx, B.; Carmeliet, P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat. Rev. Cancer 2008, 8, 942–956. [Google Scholar] [CrossRef]
- Mentlein, R.; Eichler, O.; Forstreuter, F.; Held-Feindt, J. Somatostatin inhibits the production of vascular endothelial growth factor in human glioma cells. Int. J. Cancer 2001, 92, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; del Ferro, E.; Ligi, M.; Staccioli, M.P.; Giordani, P.; Catalano, V.; Agostinelli, R.; Muretto, P.; Catalano, G. Inhibition of vascular endothelial growth factor by octreotide in colorectal cancer patients. Cancer Investig. 2001, 19, 8–12. [Google Scholar] [CrossRef]
- McCarron, S.L.; Edwards, S.; Evans, P.R.; Gibbs, R.; Dearnaley, D.P.; Dowe, A.; Southgate, C.; Easton, D.F.; Eeles, R.A.; Howell, W.M. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002, 62, 3369–3372. [Google Scholar]
- Yamamori, M.; Sakaeda, T.; Nakamura, T.; Okamura, N.; Tamura, T.; Aoyama, N.; Kamigaki, T.; Ohno, M.; Shirakawa, T.; Gotoh, A.; et al. Association of VEGF genotype with mRNA level in colorectal adenocarcinomas. Biochem. Biophys. Res. Commun. 2004, 325, 144–150. [Google Scholar] [CrossRef] [PubMed]
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pozas, J.; Román, M.S.; Alonso-Gordoa, T.; Pozas, M.; Caracuel, L.; Carrato, A.; Molina-Cerrillo, J. Targeting angiogenesis in pancreatic neuroendocrine tumors: Resistance mechanisms. Int. J. Mol. Sci. 2019, 20, 4949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coxon, A.; Bready, J.; Min, H.; Kaufman, S.; Leal, J.; Yu, D.; Lee, T.A.; Sun, J.R.; Estrada, J.; Bolon, B.; et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: Implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol. Cancer Ther. 2010, 9, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Sales, K.J.; Sutherland, J.R.; Jabbour, H.N.; Katz, A.A. Seminal plasma induces angiogenic chemokine expression in cervical cancer cells and regulates vascular function. Biochim. Biophys. Acta 2012, 1823, 1789–1795. [Google Scholar] [CrossRef]
- Maynard, J.P.; Ertunc, O.; Kulac, I.; Baena-Del Valle, J.A.; De Marzo, A.M.; Sfanos, K.S. IL8 expression is associated with prostate cancer aggressiveness and androgen receptor loss in primary and metastatic prostate cancer. Mol. Cancer Res. 2020, 18, 153–165. [Google Scholar] [CrossRef]
- Filimon, A.; Preda, I.A.; Boloca, A.F.; Negroiu, G. Interleukin-8 in melanoma pathogenesis, prognosis and therapy—An integrated view into other neoplasms and chemokine networks. Cells 2022, 11, 120. [Google Scholar] [CrossRef]
- Hussain, F.; Wang, J.; Ahmed, R.; Guest, S.K.; Lam, E.W.F.; Stamp, G.; El-Bahrawy, M. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine 2010, 49, 134–140. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, G.; Dai, T.; Huang, H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1α/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
| Author, Journal, Year | Country | Study Type | Enrollment Period | Follow-Up (Months) | Control Group | Sample size | Endpoints | Biomarkers | Source | Technique (for Circulating Markers) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pavel et al., Clin Endocrinol, 2005 [13] | Germany | retrospective | June 1999–July 2002 | 24 | yes | 38 | Progression and OS | VEGF, IL-8, bFGF, Angiogenin | blood | ELISA, usELISA |
| Berković et al., Mol Cell Endocrinol, 2016 [16] | Croatia | prospective | NA | NA | yes | 145 | Correlation with DP; The role of VEGF 1154 SNPs in VEGF expression | VEGF, VEGF 1154A/G polymorphism | blood tissue | ELISA |
| Srirajaskanthan et al., Endoc Rel Cancer, 2009 [14] | UK | prospective | July 2007–March 2008 | 6 | yes | 47 | Correlation with DP and PFS | Ang-1, Ang-2 | blood tissue | ELISA |
| Derjen et al., Clin Cancer Res, 2010 [17] | Germany | prospective | 1998–2005 | 59 | yes | 42 | Correlation with DP and OS; Physiological implications of Ang-2 | Ang-2 | blood tissue xenograft | ELISA |
| N Figueroa- Vega et al., Endocr Rel Cancer, 2010 [18] | Spain | retrospective | NA | NA | yes | 47 | Correlation with DP and response to treatment. Physiological implications of Ang 1 and 2 -Tie 2 axis | Tie-2, VEGF Ang-1, Ang-2, TEM | blood tissue | ELISAFACS |
| Melen-Mucha et al., Int. J. Mol. Sci, 2012 [15] | Poland | retrospective | May 2008–February 2011 | NA | yes | 36 | Correlation with DP | VEGF, Ang-1, Ang-2, Tie-2, Endostatin, osteopontin | blood | ELISA |
| Hilfenhaus et al., Endocr Rel Cancer, 2013 [19] | Germany | retrospective & prospective | Retrospective: 1998–2012 Prospective: May 2009 December 2012 | NA | yes | 175 | Expression, function, prognostic value and potential therapeutic target | PlGF | blood tissue in vitro xenograft | ELISA |
| Jiménez-Fonseca et al., Oncotarget, 2018 [9] | Spain | prospectivemulticenterphase IV clinical trial with Sunitinib | November 2012— February 2015 | 51 | No | 43 | Correlation with OS, PFS, response to treatment, adverse events | panel of 14 SNPs, HGF, IL-6, IL-8, TIMP1, sE-selectin, osteopontin | blood | multiplex bead assays |
| Zurita et al., BJC, 2015 [10] | USA | prospectivemulticenterphase II clinical trial with Sunitinib | March 2003–November 2005 | NA | No | 105 | Correlation with OS, PFS, response to treatment, adverse events | VEGF-A, VEGFR-2, VEGFR-3, IL-8, SDF-1α, Circulating myelomonocytic and endothelial cells | blood | ELISA FACS |
| Yao et al., J of Clin Oncol, 2016 “RADIANT-3” [11] (extension phase) | Multi- based | prospective, randomized, placebo-controlled, phase III, initially double-blind then open-label clinical trial with Everolimus | July 2007– March 2014 | NA | Initially double blind | Placebo: 203 Everolimus: 207 | Correlation with OS | PlGF, VEGF-A, VEGFR1, VEGFR2, bFGF | blood | ELISA |
| Grande et al., Annals of Oncology, 2015 [12] “PAZONET” | Spain | prospectiveopen-label, phase II clinical trial with Pazopanib | January 2011– March 2012 | 17 | No | 44 | Correlation with OS, PFS, response to treatment, adverse events | VEGF-A, VEGFR-2, CTCs, CECs, cytochrome P450 3A5, VEGFR3 SNPs | blood tissue | ELISA Cell Search |
| Author | Primary site | Metastatic | Previous treatment | Conclusions |
|---|---|---|---|---|
| Pavel et al., Clin Endocrinol, 2005 [13] | pNENs: 11 GI-NENs: 13 Other: 14 | 37 (97%) | pretreated: 22 treatment naïve: 16 | ↑ VEGF levels correlated with PD ↓ baseline IL-8 correlated with PD and ↓ OS |
| Berković et al., Mol Cell Endocrinol, 2016 [16] | pNENs: 65 GI-NENs: 80 | 58 (40%) | NA | ↑ VEGF in GEP-NENs, particularly in lymph node metastases and secretory status. |
| Srirajaskanthan, et al., Endoc Rel Cancer, 2009 [14] | pNENs: 17 GI-NENs: 22 Other: 8 | NA | pretreated: 43 treatment naïve: 4 | ↑ Ang-2 in NETs proportional to tumor burden and prognostic of poorer outcome |
| Detjen et al., Clin Cancer Res, 2010 [17] | pNENs: 25 GI-NENs: 15 Unknown: 2 | 28 (66%) | NA | ↑ Ang-2 in metastatic NETs and prognostic for ↓ OS |
| Figueroa-Vega et al., Endocr Rel Cancer, 2010 [18] | pNENs: 23 GI-NENs: 12 Other: 12 | 28 | NA | ↑ Ang-1, Ang-2 and Tie-2 in GEP NENs, without prognostic relevance; Ang-2 stimulates TEM recruitment at tumor site |
| Melen-Mucha et al., Int. J. Mol. Sci, 2012 [15] | pNENs: 2 GI-NENs: 12 Other: 22 | 27 (75%) | pretreated: 14 (with SSA) treatment naïve: 22 | ↑Tie-2 in NET↑ Ang-2 in metastatic disease |
| Hilfenhaus et al., Endocr Rel Cancer, 2013 [19] | pNENs: 118 GI-NENs: 57 | 155 | pretreated: 85 naïve: 90 | ↑ PlGF in NET. Correlation with grading, not metastases. ↑ VEGFR1 in metastatic disease. ↑ PlGF predicted ↓ OS in pNETs (not confirmed in multivariate analysis) and shorter time to progression in GI-NETs. In vivo: significant reduction in tumor volume after PlGF inhibition |
| Jiménez-Fonseca et al., Oncotarget, 2018 [9] | All pNEN | 42 (97%) | pretreated: 25 treatment naïve: 18 | 2 VEGFR-3 SNP (rs307826 and rs307821) associated with ↓ OS↓ IL-8 associated to better objective response |
| Zurita et al., BJC, 2015 [10] | pNENs: 66 Carcinoid: 39 | NA | NA | ↑ VEGFR-2 predicted ↑ OS in pNETs↓ IL8 predicted response to treatment in carcinoid↑ VEGFR-3 and IL-8 correlated with ↓ PFS and ↓ OS in carcinoid↑ SDF-1α predicted ↑ PFS and ↓ OS |
| Yao et al., J of Clin Oncol, 2016 [11] “RADIANT-3” (extension phase) | All pNEN | NA | NA | PlGF is an independent prognostic factor for PD |
| Grande et al., Annals of Oncology, 2015 [12] “PAZONET” | pNENs: 18 GI-NENs: 15 Other: 11 | NA | all | No significant correlation with circulant biomarkers was noted; VEGFR3rs307821 correlated with ↓ PFS in GEP NET |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandra, I.; Cazacu, I.M.; Croitoru, V.M.; Mihaila, M.; Herlea, V.; Diculescu, M.M.; Dima, S.O.; Croitoru, A.E. Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 4001-4014. https://doi.org/10.3390/cimb44090274
Sandra I, Cazacu IM, Croitoru VM, Mihaila M, Herlea V, Diculescu MM, Dima SO, Croitoru AE. Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Current Issues in Molecular Biology. 2022; 44(9):4001-4014. https://doi.org/10.3390/cimb44090274
Chicago/Turabian StyleSandra, Irina, Irina Mihaela Cazacu, Vlad Mihai Croitoru, Mariana Mihaila, Vlad Herlea, Mircea Mihai Diculescu, Simona Olimpia Dima, and Adina Emilia Croitoru. 2022. "Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review" Current Issues in Molecular Biology 44, no. 9: 4001-4014. https://doi.org/10.3390/cimb44090274
APA StyleSandra, I., Cazacu, I. M., Croitoru, V. M., Mihaila, M., Herlea, V., Diculescu, M. M., Dima, S. O., & Croitoru, A. E. (2022). Circulating Angiogenic Markers in Gastroenteropancreatic Neuroendocrine Neoplasms: A Systematic Review. Current Issues in Molecular Biology, 44(9), 4001-4014. https://doi.org/10.3390/cimb44090274







