MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Downloading and Processing
2.2. Detection of Differentially Expressed miRNAs and mRNA Combined with Clinical Information
2.3. Construction of Sample Grouping and Prognostic Module
2.4. Independent Prognostic Ability of miRNA
2.5. miRNA Target Genes Prediction and Functions Analysis
2.6. Screening Core Target Genes and Survival Analysis
3. Results
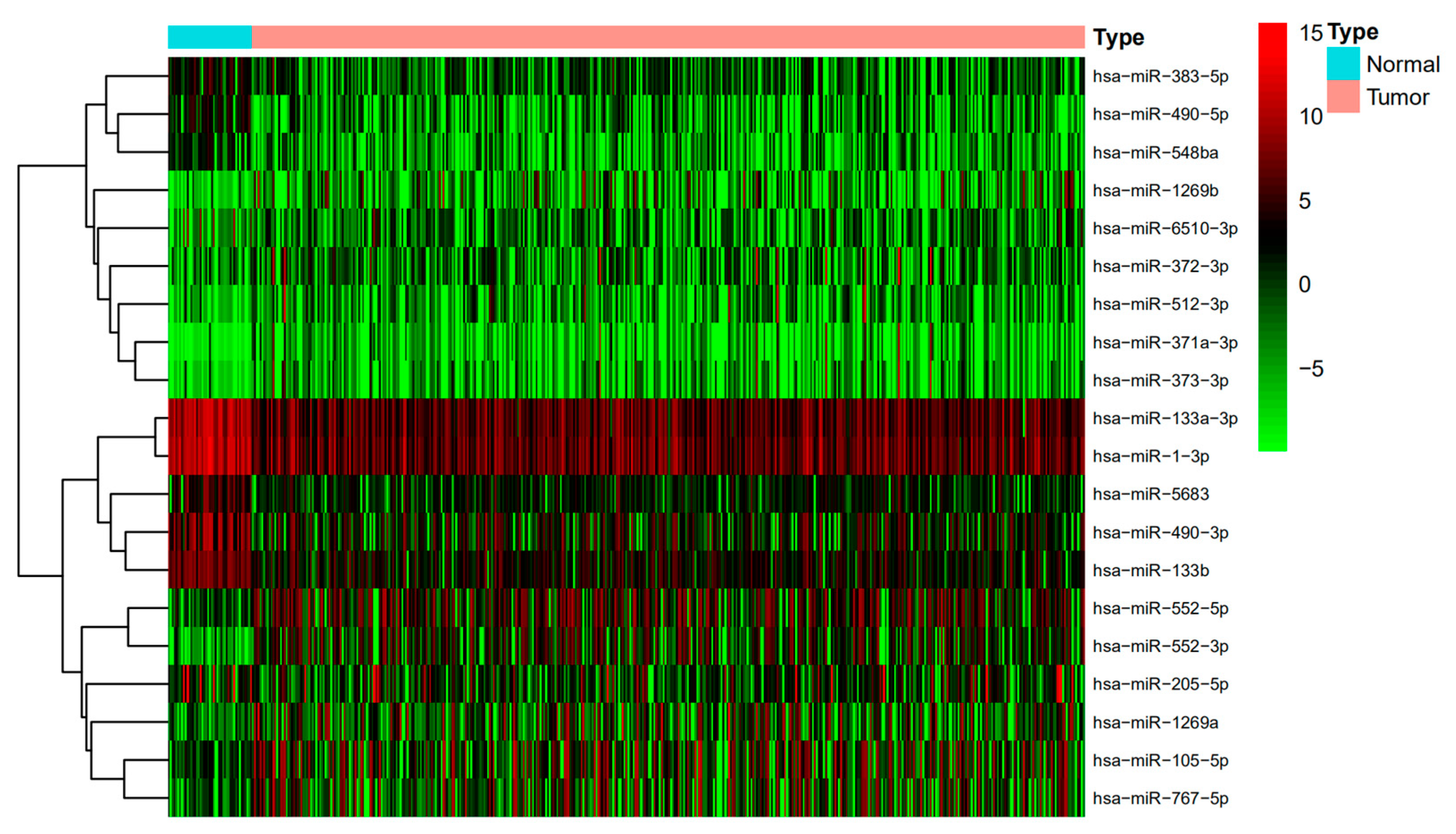
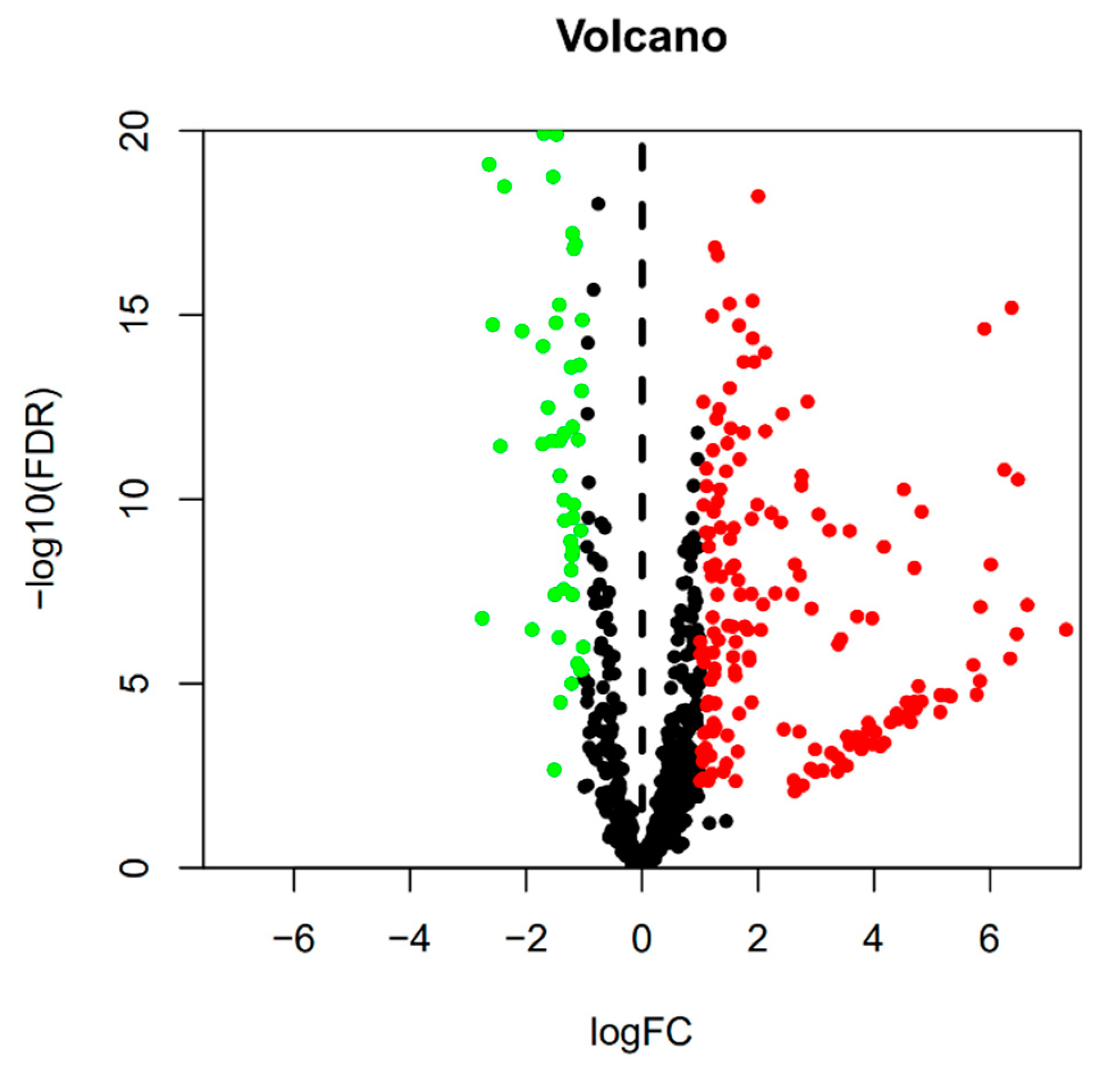
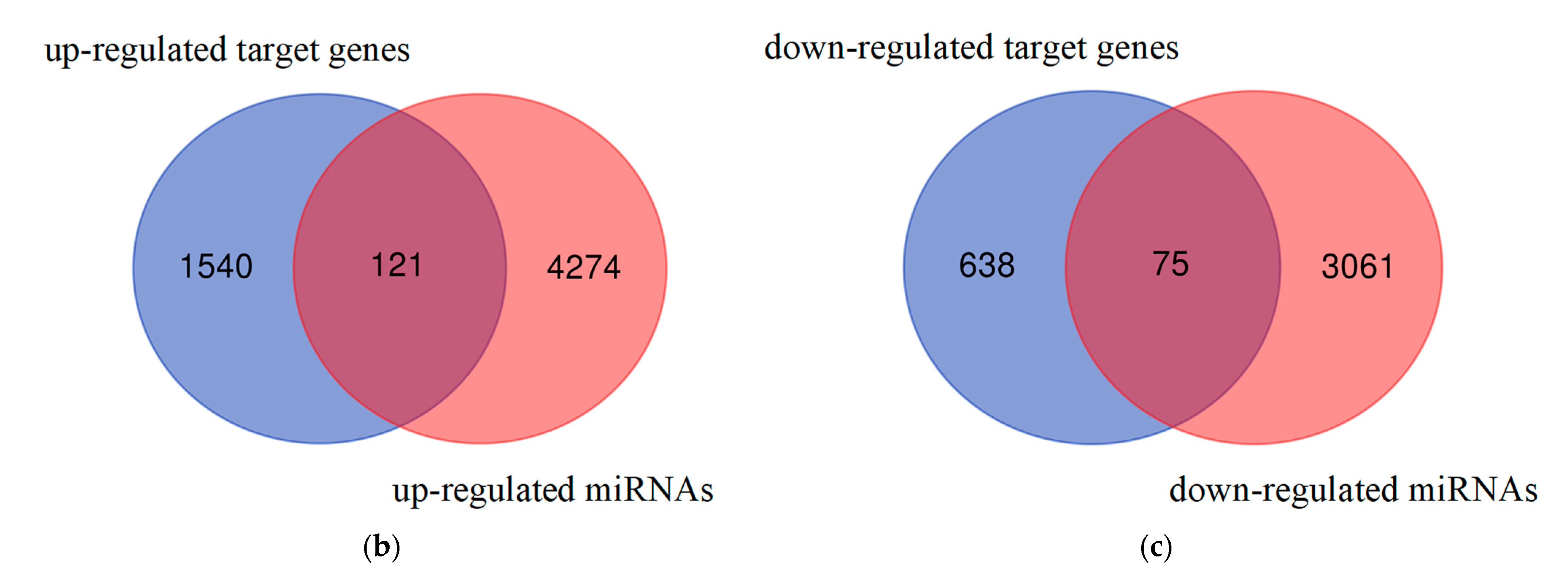
3.1. Detection of Differentially Expressed miRNAs and Differentially Expressed mRNAs
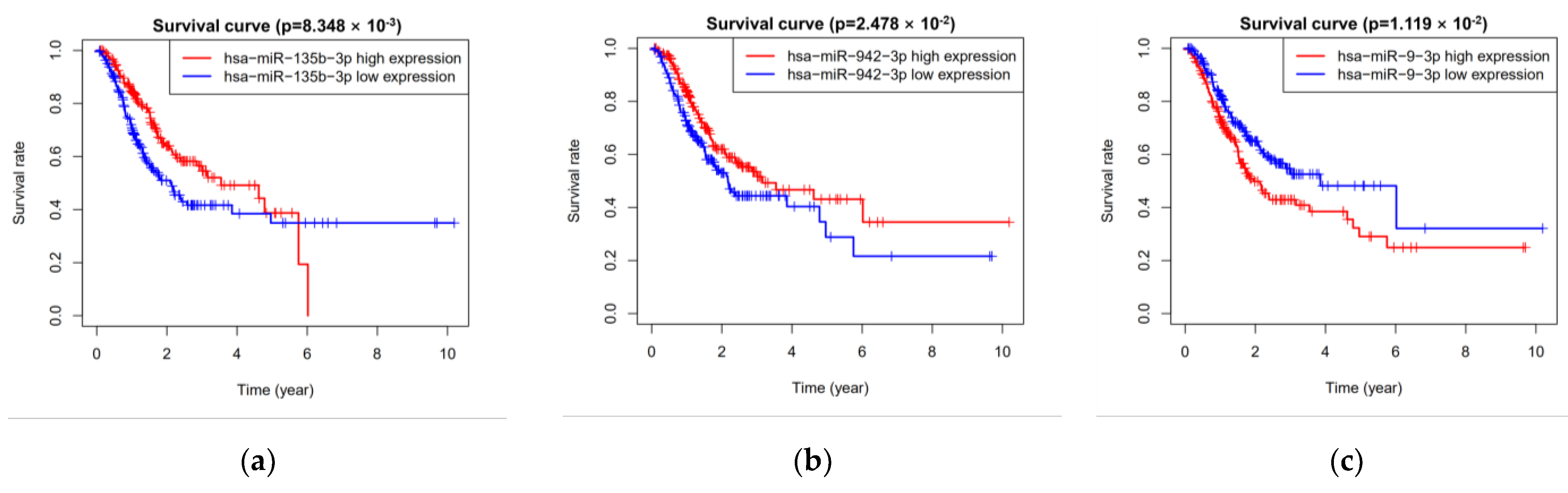
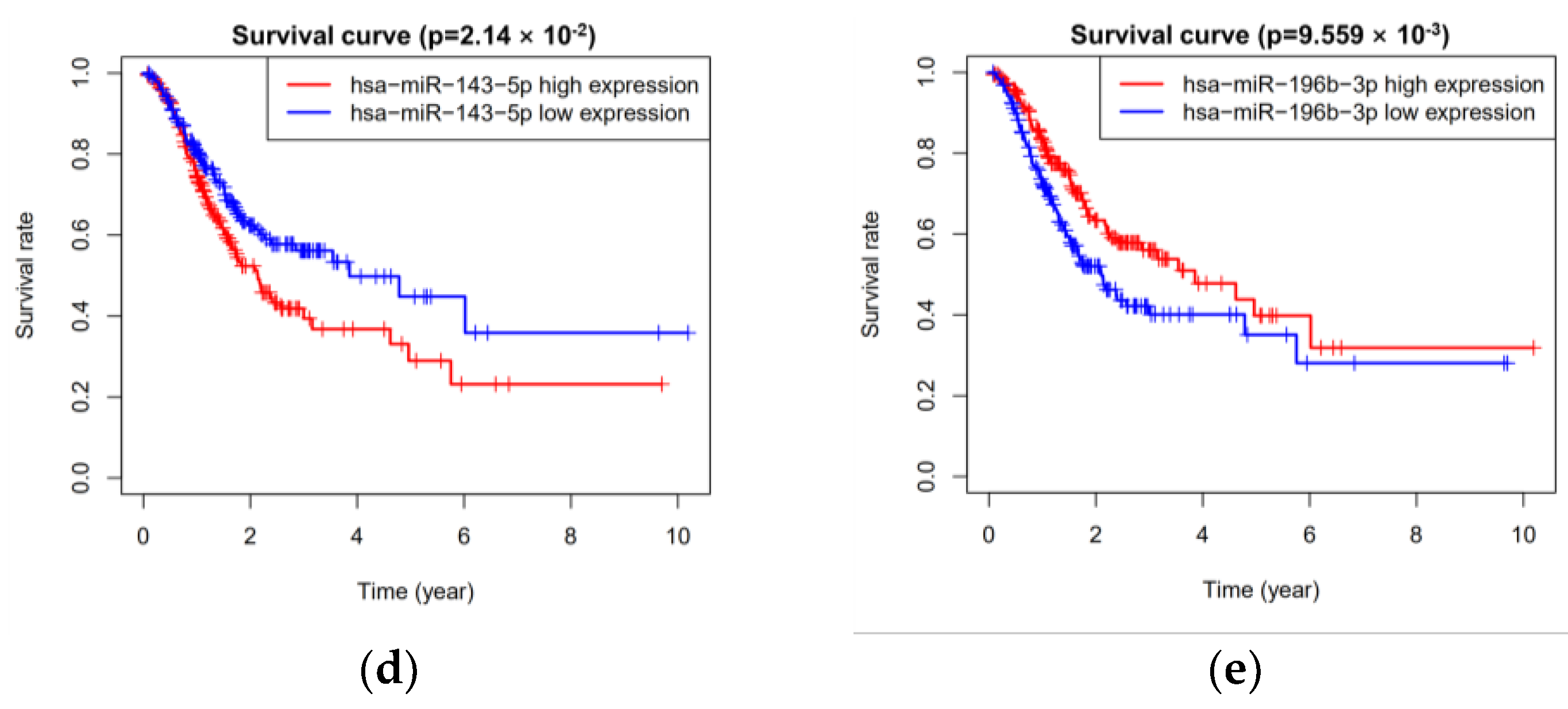
3.2. Five miRNAs Associated with Overall Survival
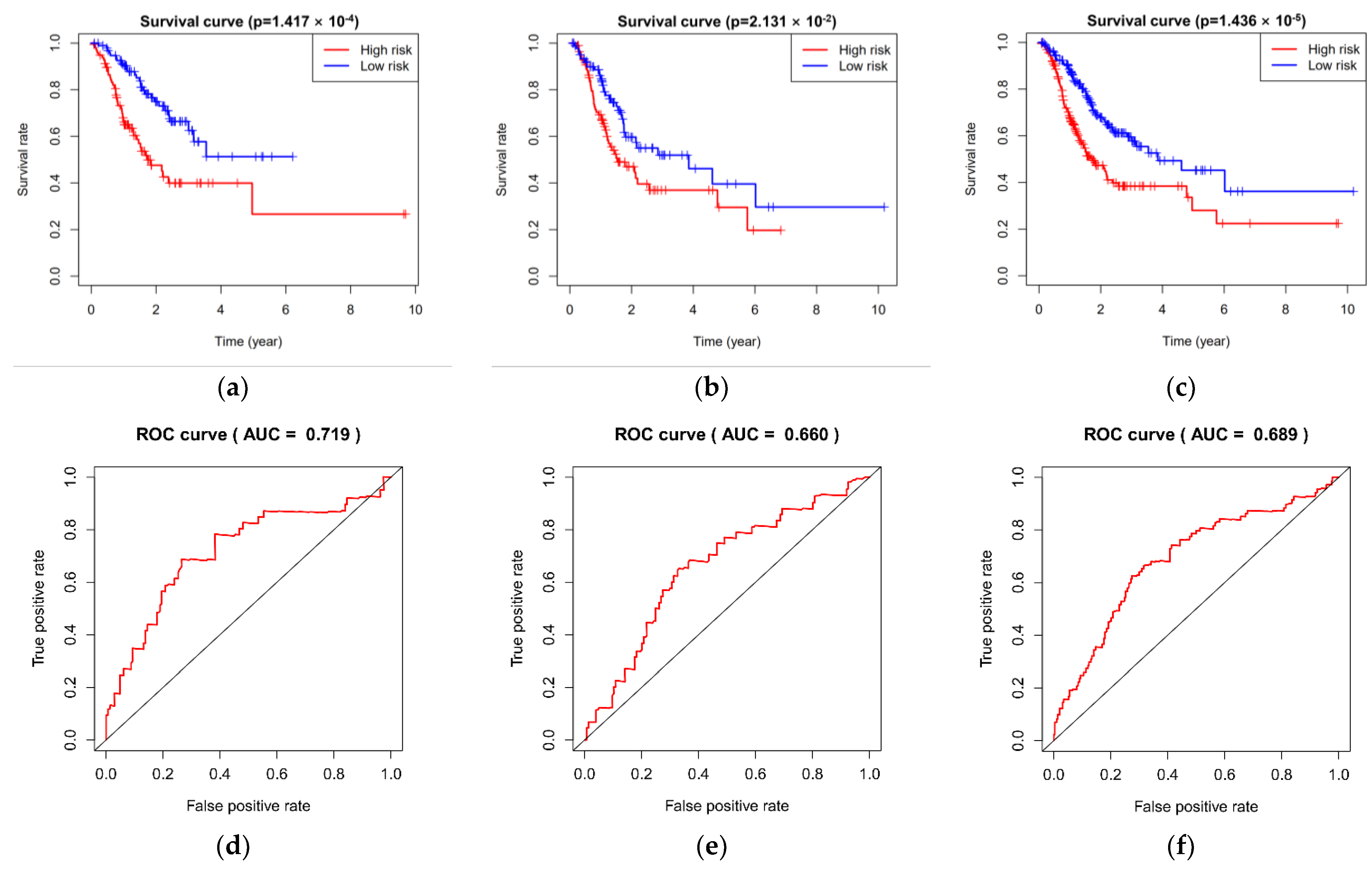
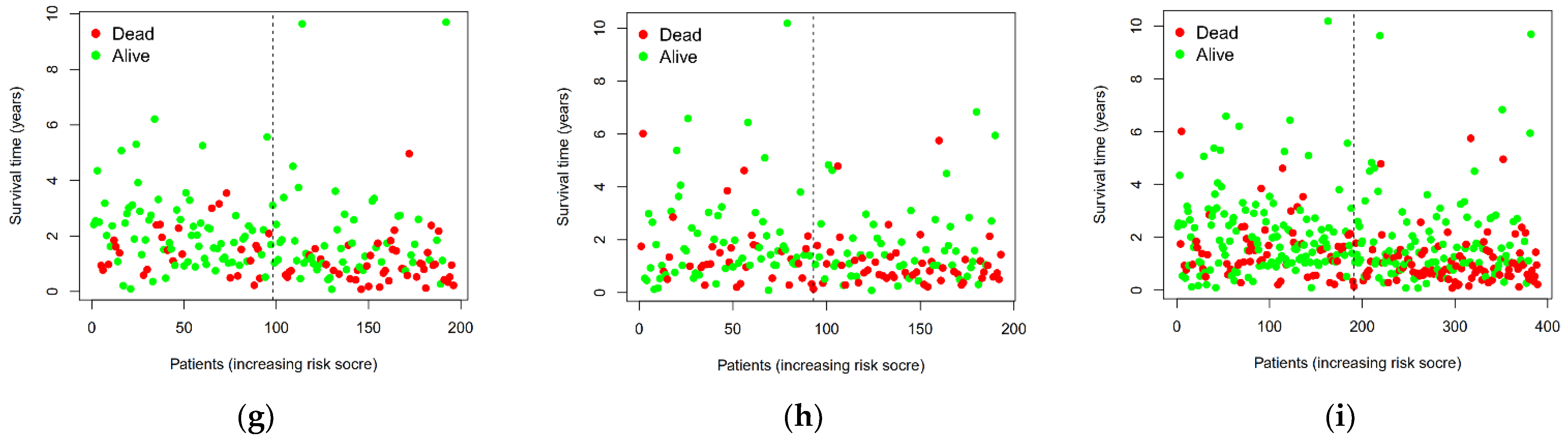
3.3. Prediction and Assessment of Five miRNAs for Overall Survival in Three Groups
3.4. Independence of the Five miRNAs
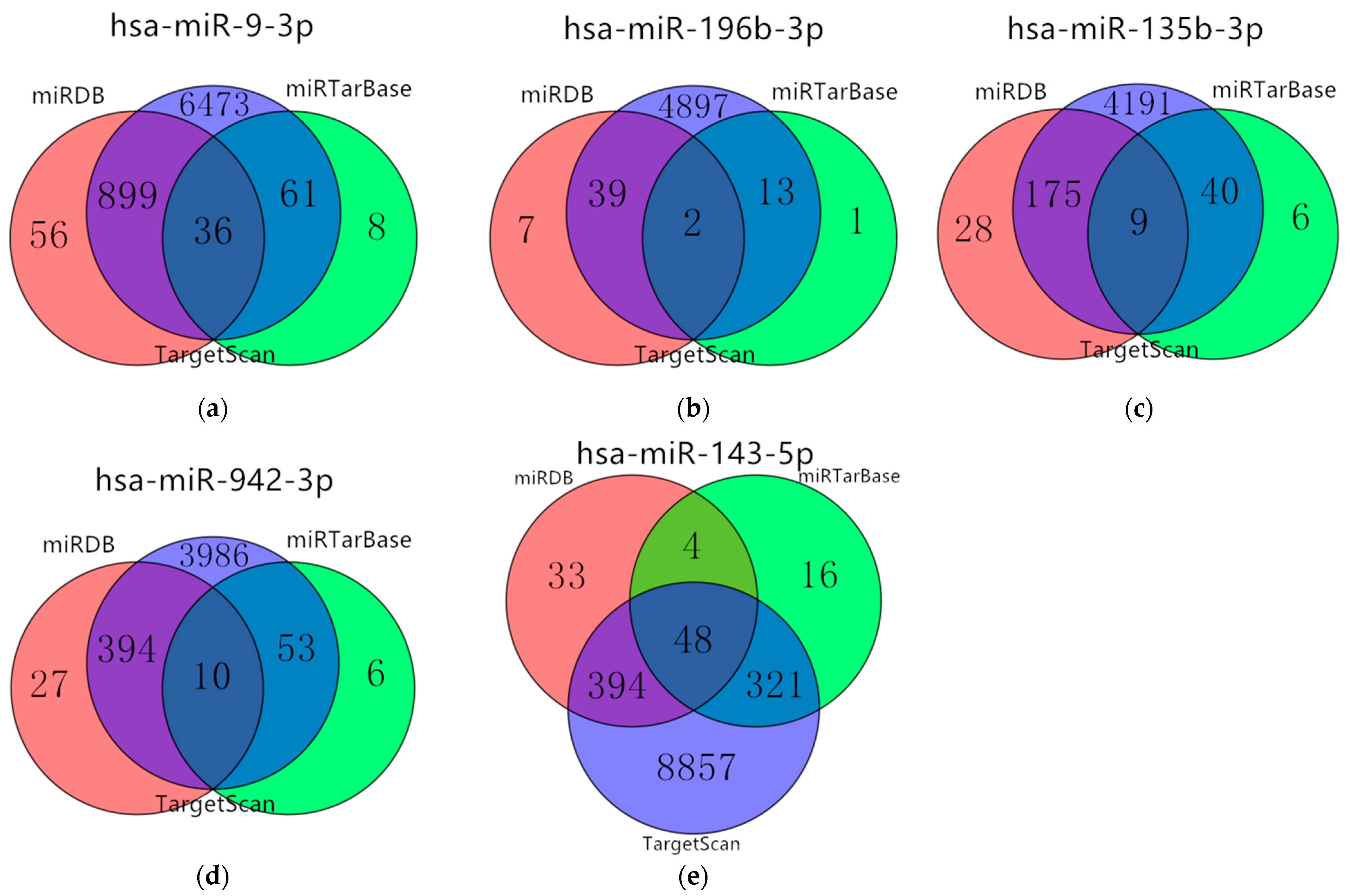
3.5. Target Genes Prediction of Five miRNAs
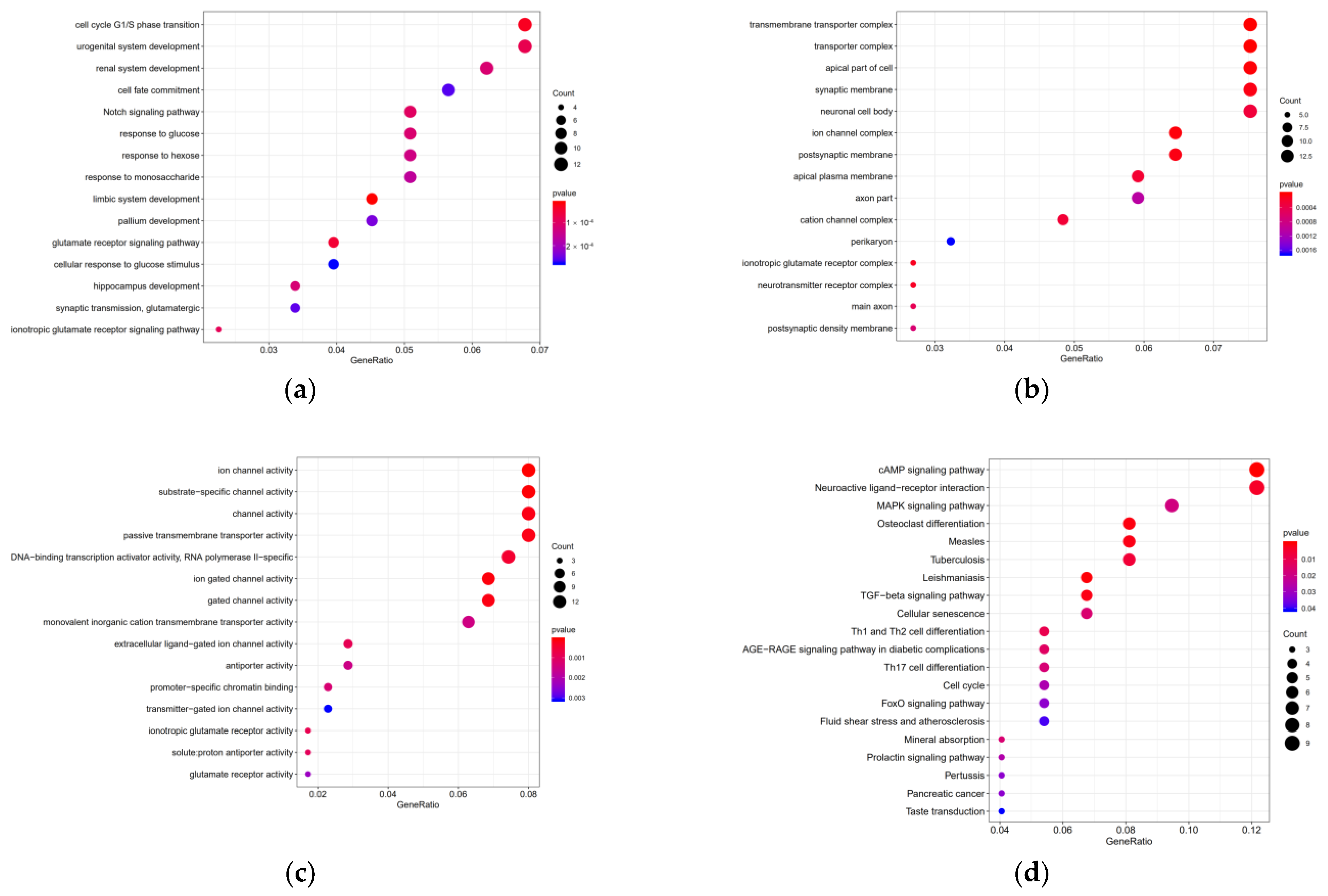
3.6. Target Genes Functional Enrichment Analysis
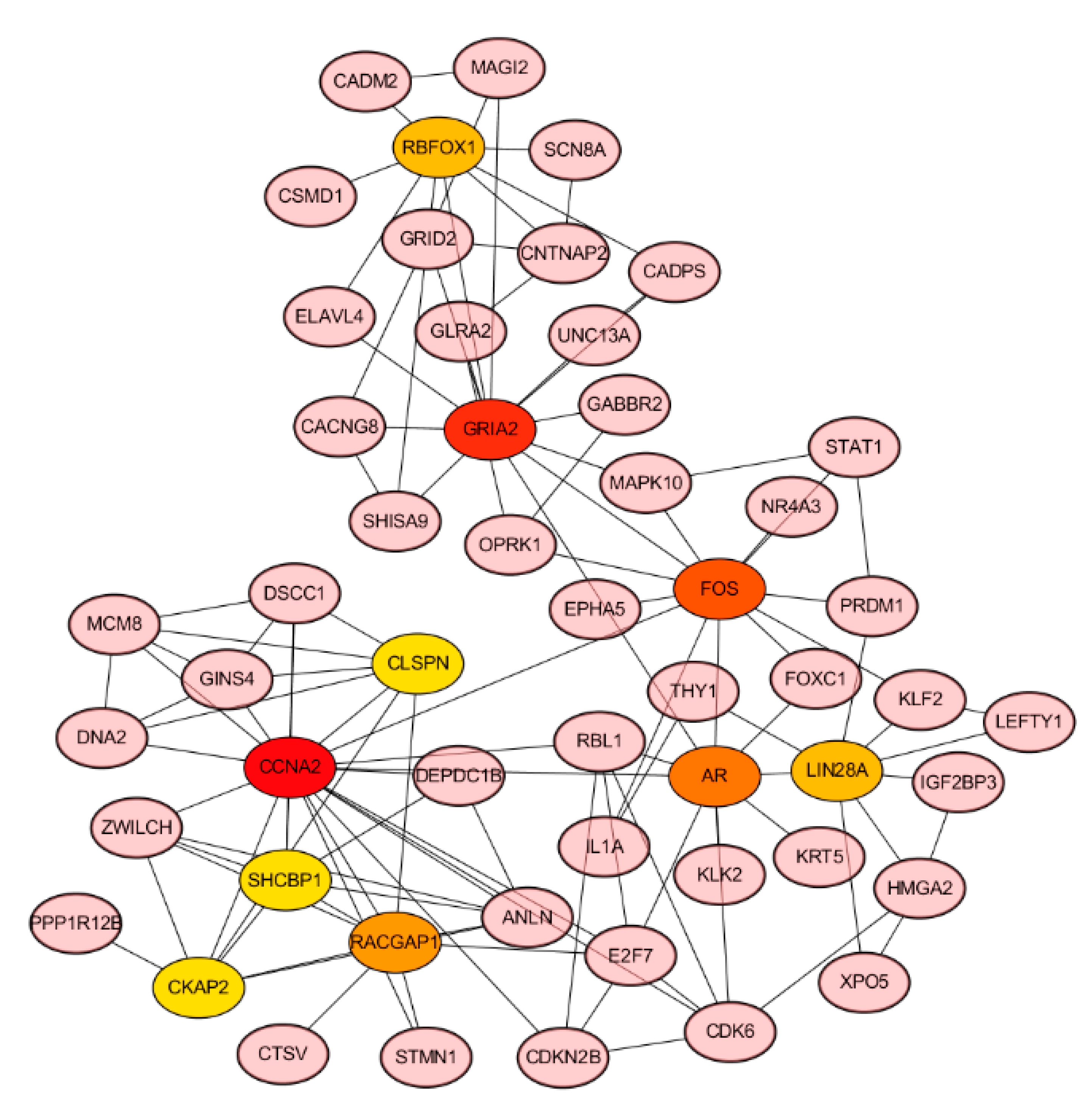
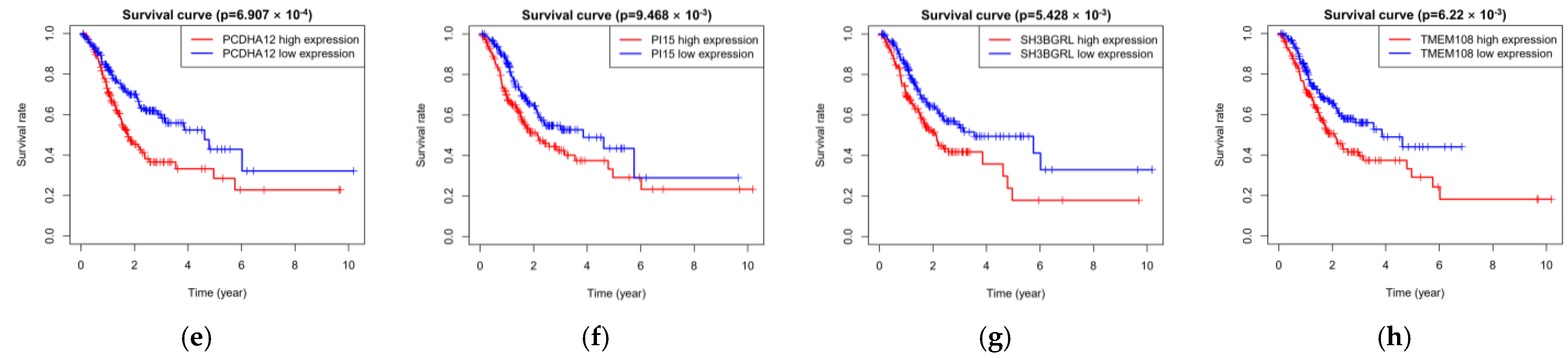
3.7. Hub Genes of PPI Network and Survival Analysis of Target Genes
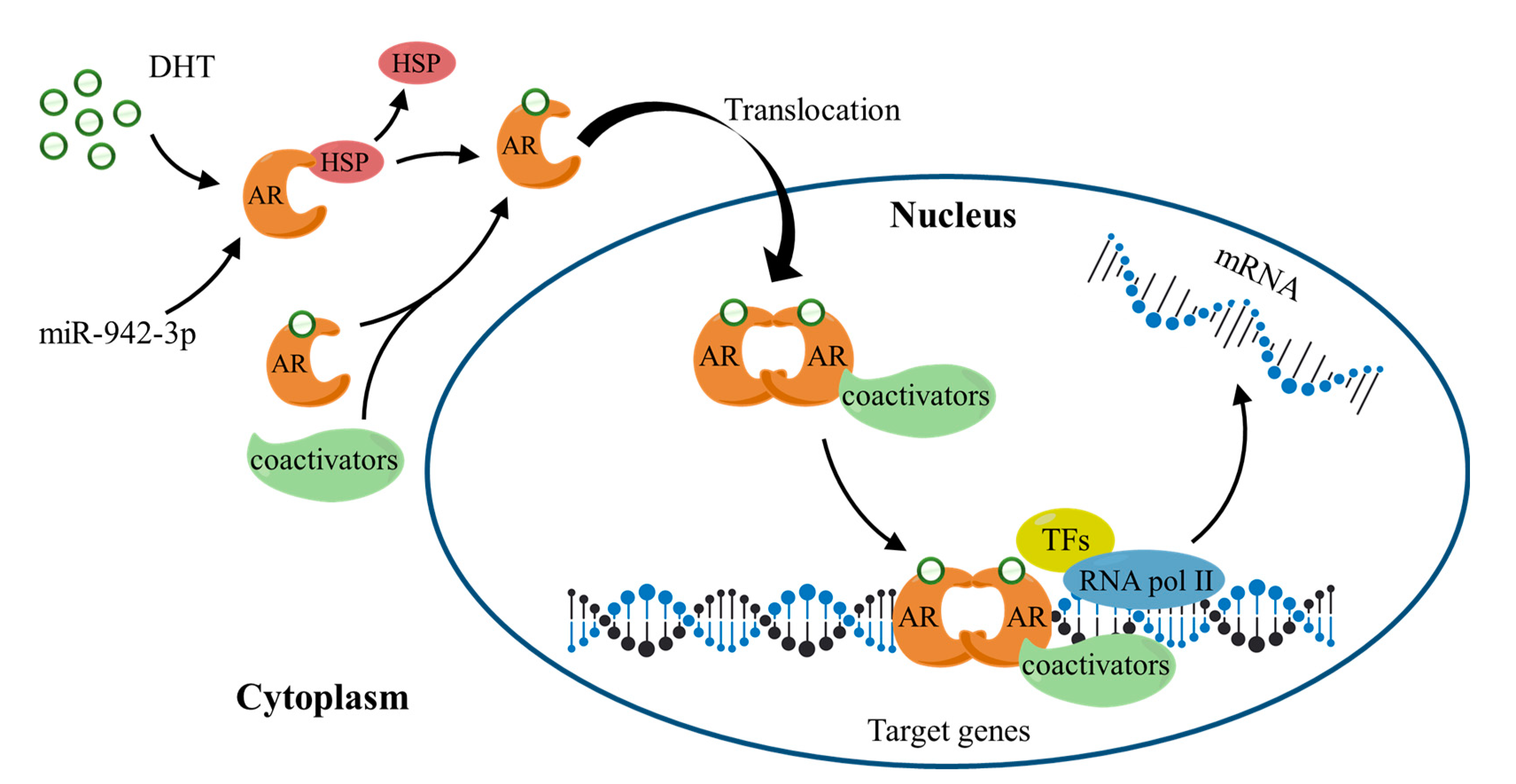
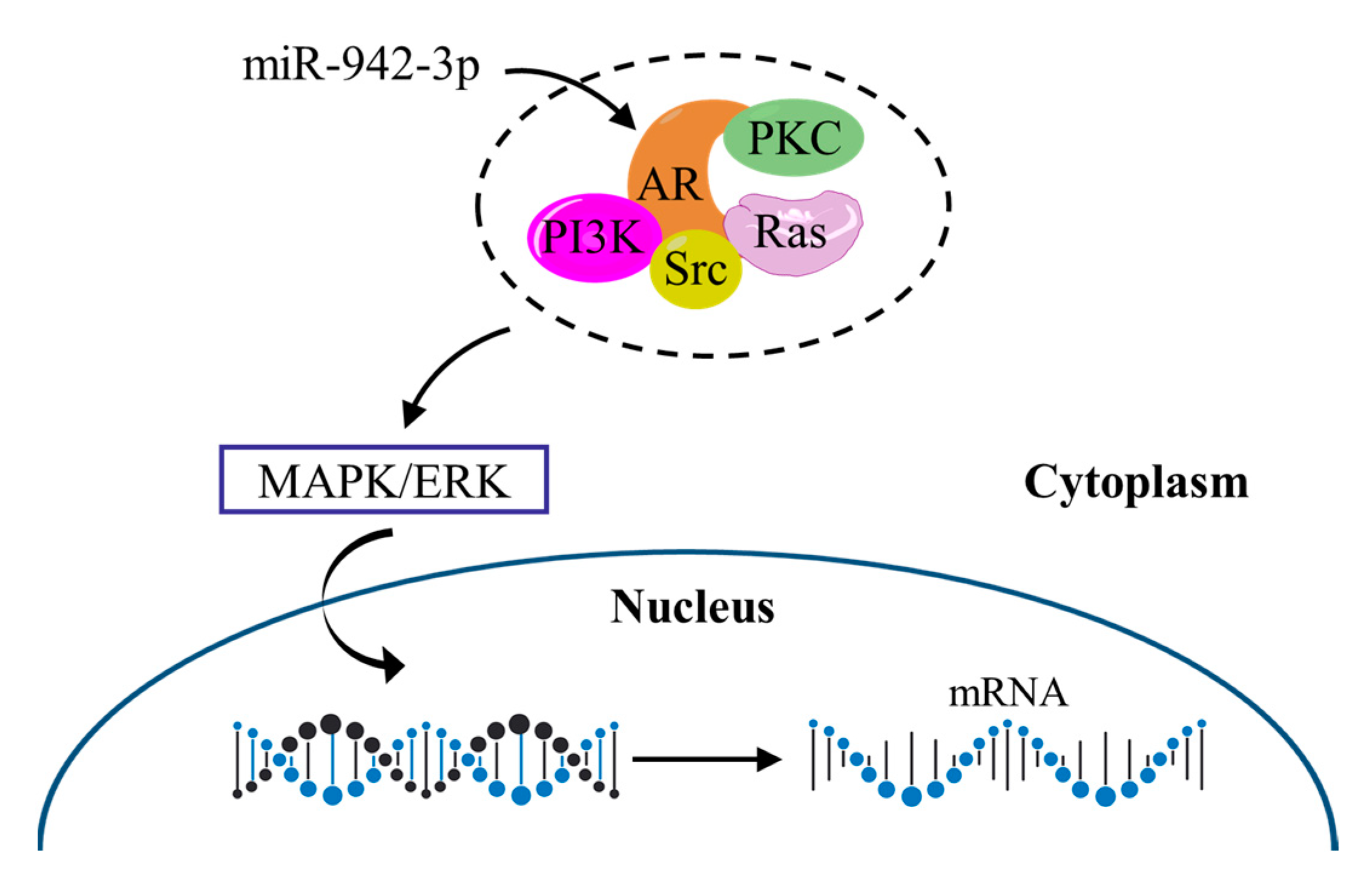
3.8. The Working Mechanism of AR and Its Potential Relationship with the MAPK/ERK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Gires, O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell. Mol. Life Sci. 2011, 68, 4009–4022. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Mu, Y.; Cui, H.; Liang, Y.; Su, X. MiR-9-3p augments apoptosis induced by H2O2 through down regulation of Herpud1 in glioma. PLoS ONE 2017, 12, e0174839. [Google Scholar]
- Chen, Z.; Gu, D.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. Regulation of miR-143-5p on cadmium-induced renal cell apoptosis and its mechanism. J. Nanjing Med. Univ. Nat. Sci. Ed. 2015, 35, 490–495. [Google Scholar]
- Ma, R.; Yan, W.; Zhang, G.; Lv, H.; Liu, Z.; Fang, F.; Zhang, W.; Zhang, J.; Tao, T.; You, Y.; et al. Upregulation of miR-196b confers a poor prognosis in glioblastoma patients via inducing a proliferative phenotype. PLoS ONE 2012, 7, e38096. [Google Scholar]
- Chen, M.; Ma, L.; Teng, Y. Research Progress of miRNA-135b in Tumor Development. Med. Recapitul. 2018, 24, 4836–4841. [Google Scholar]
- Verma, H.K.; Bhaskar, L. MicroRNA a small magic bullet for gastric cancer. Gene 2020, 753, 144801. [Google Scholar] [CrossRef]
- Lin, A.; Wang, G.; Duan, R.; Li, T. Expression of miRNA-449b in gastric cancer and lymph node metastasis. Acta Acad. Med. Wannan 2015, 34, 113–116. [Google Scholar]
- Ma, X.; Lu, K.; Lei, J.; Ji, L.; Shi, L. Relationship between the methylation and expression of microRNA-375 and its clinicopathological characteristics in gastric cancer. Prog. Mod. Biomed. 2018, 18, 1931–1935. [Google Scholar]
- Han, W.; Su, X. The mechanism of miRNA-30c in gastric cancer. In Proceedings of the National Symposium on Tumor Epidemiology and Etiology, Chengdu, China, 19 August 2015. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Yao, T.; Liu, Y.; Li, C.; Hu, L. Regression model analysis of survival data-Cox proportional hazard regression model analysis of survival data. Sichuan Ment. Health 2020, 33, 27–32. [Google Scholar]
- Zhe, W.; Ryan, M. TModel-free posterior inference on the area under the receiver operating characteristic curve. J. Stat. Plan. Inference 2020, 209, 174–186. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs: New Biomarkers for Diagnosis, prognosis, Therapy prediction and Therapeutic Tools for Breast Cancer. Theranostics 2015, 5, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xie, Z.; Wang, Z.; Cheng, K.; Liang, K.; Song, Z. Overexpression of miR-191 predicts poor prognosis and promotes proliferation and invasion in esophageal squamous cell carcinoma. Yonsei Med. J. 2017, 58, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Zheng, H. Low expression of microRNA-1908 predicts a poor prognosis for patients with ovarian cancer. Oncol. Lett. 2017, 14, 4277–4281. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.F.; Qin, C.F. MicroRNA-217 functions as a prognosis predictor and inhibits pancreatic cancer cell proliferation and invasion via targeting E2F3. Eur. Rev. Med. Pharmacol. 2017, 21, 4050–4057. [Google Scholar]
- Si, L.; Tian, H.; Yue, W.; Li, L.; Li, S.; Gao, C.; Qi, L. Potential use of microRNA-200c as a prognostic marker in non-small cell lung cancer. Oncol. Lett. 2017, 14, 4325–4330. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Zhao, J.; Wang, X. A three-microRNA signature as a diagnostic and prognostic marker in clear cell renal cancer: An in silico analysis. PLoS ONE 2017, 12, e0180660. [Google Scholar] [CrossRef]
- Shi, X.H.; Li, X.; Zhang, H.; He, R.Z.; Zhao, Y.; Zhou, M.; Pan, S.T.; Zhao, C.L.; Feng, Y.C.; Wang, M.; et al. A five-microRNA signature for survival prognosis in pancreatic adenocarcinoma based on TCGA data. Sci. Rep. 2018, 8, 7638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.D.; Ma, M.H. Three-microRNA signature identified by bioinformatics analysis predicts prognosis of gastric cancer patients. World J. Gastroenterol. 2018, 24, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Juhnn, Y.S. cAMP signaling increases histone deacetylase 8 expression via the Epac2-Rap1A-Akt pathway in H1299 lung cancer cells. Exp. Mol. Med. 2017, 49, e297. [Google Scholar] [CrossRef] [PubMed]
- Jagriti, P.; Vikas, P.; Anupam, K.; Kavneet, K.; Chitra, S.; Kumaravel, S. Genetic landscape of glioma reveals defective neuroactive ligand receptor interaction pathway as a poor prognosticator in glioblastoma patients. Cancer Res. 2017, 77, 2454. [Google Scholar] [CrossRef]
- Higa, G.M.; Fell, R.G. Sex hormone receptor repertoire in breast cancer. Int. J. Breast Cancer 2013, 2013, 284036. [Google Scholar] [CrossRef]
- Li, D.; Zhou, W.; Pang, J.; Tang, Q.; Zhong, B.; Shen, C.; Xiao, L.; Hou, T. A magic drug target: Androgen receptor. Med. Res. Rev. 2019, 39, 1485–1514. [Google Scholar] [CrossRef]
- Li, J.; Al-Azzawi, F. Mechanism of androgen receptor action. Maturitas 2009, 63, 142–148. [Google Scholar] [CrossRef]
- Salma, S.; Stephen, J.L.; Christopher, A.L.; Alan, P.L.; Maria, M. The p14ARF tumor suppressor restrains androgen receptor activity and prevents apoptosis in prostate cancer cells. Cancer Lett. 2020, 483, 12–21. [Google Scholar]
- Peng, L.; Li, Y.; Wei, S.; Li, X.; Zhang, G. LAMA4 activated by Androgen receptor induces the cisplatin resistance in gastric cancer. Biomed. Pharmacother. 2020, 124, 109667. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.S.; Ma, S.; Miao, L.; Li, R.; Yin, Y.; Raj, G.V. Androgen receptor-mediated non-genomic regulation of prostate cancer cell proliferation. Transl. Androl. Urol. 2013, 2, 187–196. [Google Scholar] [PubMed]


| Variables | miRNA Expression Profiles | mRNA Expression Profiles | |
|---|---|---|---|
| Case | Count | 436 | 380 |
| Primary Site | Stomach | stomach | |
| Program | TCGA | TCGA | |
| Project | TCGA-STAD | TCGA-STAD | |
| Files | Count | 491 | 407 |
| Data Category | Transcriptome Profiling | Transcriptome Profiling | |
| Data Type | Isoform Expression Quantification | Gene Expression Quantification | |
| Workflow Type | BCGSC miRNA Profiling | HTSeq-Counts |
| Variables | Case | Percentage (%) | |
|---|---|---|---|
| Gender | Male | 285 | 64.3 |
| Female | 158 | 35.7 | |
| Age (years) | Range | 30–90 | |
| Median | 68 | 3.1 | |
| Futime (day) | Range | 0–3720 | |
| Median | 422 | ||
| Fustat | 1 | 171 | 38.6 |
| 0 | 272 | 61.3 | |
| Clinical stage | I | 59 | 13.2 |
| II | 130 | 29.2 | |
| III | 183 | 41.1 | |
| IV | 44 | 9.9 | |
| Unknown | 27 | 6 | |
| T stage | T1 | 23 | 5 |
| T2 | 93 | 20.8 | |
| T3 | 198 | 44.6 | |
| T4 | 119 | 26.7 | |
| TX | 10 | 2.2 | |
| Lymph node stage | N0 | 132 | 29.7 |
| N1 | 119 | 26.8 | |
| N2 | 85 | 19.1 | |
| N3 | 88 | 19.7 | |
| NX | 17 | 3.8 | |
| Unknown | 2 | 0.4 | |
| Metastatic | M0 | 391 | 88.2 |
| M1 | 30 | 6.7 | |
| MX | 22 | 4.9 |
| ID | Univariate Cox Regression | Multivariate Cox Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | HR.95L | HR.95H | p-Value | Coef | HR | HR.95L | HR.95H | p-Value | |
| hsa-miR-96-5p | 0.761 | 0.642 | 0.903 | 0.002 | |||||
| hsa-miR-7-5p | 0.801 | 0.695 | 0.923 | 0.002 | |||||
| hsa-let-7e-3p | 1.379 | 1.112 | 1.71 | 0.003 | |||||
| hsa-miR-143-5p | 1.265 | 1.077 | 1.487 | 0.004 | 0.134 | 1.144 | 0.961 | 1.361 | 0.129 |
| hsa-miR-942-3p | 0.727 | 0.586 | 0.902 | 0.004 | −0.178 | 0.837 | 0.663 | 1.056 | 0.132 |
| hsa-miR-183-5p | 0.806 | 0.69 | 0.942 | 0.007 | |||||
| hsa-miR-196b-3p | 0.648 | 0.468 | 0.897 | 0.009 | −0.307 | 0.736 | 0.527 | 1.027 | 0.072 |
| hsa-miR-125a-5p | 1.401 | 1.067 | 1.839 | 0.015 | |||||
| hsa-miR-135b-3p | 0.799 | 0.665 | 0.96 | 0.017 | −0.148 | 0.862 | 0.706 | 1.052 | 0.144 |
| hsa-miR-30a-3p | 1.21 | 1.024 | 1.428 | 0.025 | |||||
| hsa-miR-652-5p | 0.784 | 0.623 | 0.986 | 0.037 | |||||
| hsa-miR-9-3p | 1.17 | 1.008 | 1.359 | 0.039 | 0.147 | 1.159 | 0.989 | 1.358 | 0.069 |
| hsa-miR-99a-3p | 1.175 | 1.007 | 1.372 | 0.040 | |||||
| hsa-miR-139-5p | 1.221 | 1.007 | 1.48 | 0.042 | |||||
| hsa-miR-137-3p | 1.16 | 1.000 | 1.346 | 0.049 | |||||
| Clinical Features | Univariate Cox Regression | Multivariate Cox Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | HR.95L | HR.95H | p-Value | HR | HR.95L | HE.95H | p-Value | |
| Age | 1.015 | 0.999 | 1.032 | 0.062 | 1.027 | 1.010 | 1.045 | 0.002 |
| Gender | 1.225 | 0.853 | 1.760 | 0.271 | 1.510 | 1.027 | 2.218 | 0.036 |
| Grade | 1.278 | 0.908 | 1.800 | 0.160 | 1.115 | 0.781 | 1.591 | 0.550 |
| Stage | 1.607 | 1.294 | 1.996 | <0.001 | 1.210 | 0.807 | 1.815 | 0.357 |
| T | 1.288 | 1.038 | 1.599 | 0.022 | 1.215 | 0.911 | 1.621 | 0.186 |
| M | 1.880 | 1.013 | 3.489 | 0.045 | 1.818 | 0.844 | 3.917 | 0.127 |
| N | 1.361 | 1.170 | 1.584 | <0.001 | 1.233 | 0.987 | 1.540 | 0.065 |
| riskScore | 1.726 | 1.395 | 2.136 | <0.001 | 1.971 | 1.557 | 2.494 | <0.001 |
| ID | Description | p-Value | Q-Value | Count | Gene |
|---|---|---|---|---|---|
| hsa04024 | cAMP signaling pathway | 0.0002 | 0.0272 | 9 | TIAM1/FOS/GRIA2/MAPK10/PLN/MC2R/ATP2B4/GABBR2/RAP1A |
| hsa05140 | Leishmaniasis | 0.0007 | 0.0603 | 5 | FCGR3A/FOS/STAT1/IL1A/FCGR2A |
| hsa04380 | Osteoclast differentiation | 0.0011 | 0.0603 | 6 | FCGR3A/FOS/MAPK10/STAT1/IL1A/FCGR2A |
| hsa05162 | Measles | 0.0017 | 0.0603 | 6 | CDK6/FOS/MAPK10/STAT1/IL1A/IL2RA |
| hsa04350 | TGF-beta signaling pathway | 0.0017 | 0.0603 | 5 | CDKN2B/RGMB/LEFTY1/BAMBI/RBL1 |
| hsa04080 | Neuroactive ligand-receptor interaction | 0.0039 | 0.1148 | 9 | GRIA2/GRID2/MC2R/F2/GLRA2/GABRP/GRIK3/GABBR2/OPRK1 |
| hsa05152 | Tuberculosis | 0.0062 | 0.1570 | 6 | FCGR3A/MAPK10/RIPK2/STAT1/IL1A/FCGR2A |
| hsa04658 | Th1 and Th2 cell differentiation | 0.0101 | 0.2256 | 4 | FOS/MAPK10/STAT1/IL2RA |
| hsa04933 | AGE-RAGE signaling pathway | 0.0135 | 0.2506 | 4 | MAPK10/STAT1/IL1A/COL4A1 |
| hsa04218 | Cellular senescence | 0.0159 | 0.2506 | 5 | CDK6/CDKN2B/IL1A/CCNA2/RBL1 |
| hsa04978 | Mineral absorption | 0.0162 | 0.2506 | 3 | SLC6A19/CYBRD1/ATP2B4 |
| hsa04659 | Th17 cell differentiation | 0.0169 | 0.2506 | 4 | FOS/MAPK10/STAT1/IL2RA |
| hsa04010 | MAPK/ERK signaling pathway | 0.0187 | 0.2554 | 7 | CACNG8/FOS/MAPK10/IL1A/STMN1/FGF5/RAP1A |
| hsa04917 | Prolactin signaling pathway | 0.0266 | 0.3244 | 3 | FOS/MAPK10/STAT1 |
| hsa04110 | Cell cycle | 0.0274 | 0.3244 | 4 | CDK6/CDKN2B/CCNA2/RBL1 |
| hsa04068 | FoxO signaling pathway | 0.0326 | 0.3247 | 4 | CDKN2B/MAPK10/KLF2/RAG2 |
| hsa05133 | Pertussis | 0.0329 | 0.3247 | 3 | FOS/MAPK10/IL1A |
| hsa05212 | Pancreatic cancer | 0.0329 | 0.3247 | 3 | CDK6/MAPK10/STAT1 |
| hsa05418 | Fluid shear stress and atherosclerosis | 0.0392 | 0.3471 | 4 | FOS/MAPK10/KLF2/IL1A |
| hsa04742 | Taste transduction | 0.0410 | 0.3471 | 3 | PDE1C/TAS2R5/GABBR2 |
| Node_Name | MCC | DMNC | MNC | Degree | EPC | Bottle Neck | Ec Centricity | Closeness | Radiality | Betweenness | Stress | Clustering Coefficient |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCNA2 | 236 | 0.264 | 18 | 18 | 35.308 | 19 | 0.097 | 43.961 | 7.799 | 2843.324 | 5374 | 0.235 |
| GRIA2 | 22 | 0.233 | 8 | 13 | 33.492 | 71 | 0.129 | 44.383 | 8.065 | 3792.232 | 8494 | 0.128 |
| FOS | 17 | 0.220 | 7 | 12 | 34.894 | 84 | 0.111 | 46.076 | 8.159 | 3863.375 | 7988 | 0.091 |
| AR | 33 | 0.329 | 7 | 10 | 35.226 | 18 | 0.111 | 43.369 | 7.987 | 2490.437 | 5478 | 0.200 |
| RACGAP1 | 131 | 0.408 | 8 | 9 | 33.683 | 2 | 0.086 | 33.073 | 7.110 | 210.833 | 484 | 0.389 |
| RBFOX1 | 12 | 0.238 | 6 | 8 | 28.267 | 4 | 0.111 | 35.586 | 7.525 | 953.058 | 2332 | 0.179 |
| LIN28A | 9 | 0.309 | 3 | 8 | 28.652 | 3 | 0.097 | 36.095 | 7.517 | 790.409 | 2030 | 0.107 |
| DSCC1 | 28 | 0.454 | 5 | 7 | 30.653 | 3 | 0.086 | 32.073 | 7.094 | 392.500 | 704 | 0.333 |
| GRID2 | 13 | 0.285 | 6 | 7 | 28.277 | 2 | 0.111 | 34.919 | 7.525 | 366.790 | 918 | 0.286 |
| OPRK1 | 10 | 0.463 | 3 | 7 | 25.901 | 13 | 0.097 | 37.251 | 7.721 | 1633.949 | 3034 | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Ying, N.; Rao, X.; Chen, X. MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway. Curr. Issues Mol. Biol. 2022, 44, 3835-3848. https://doi.org/10.3390/cimb44090263
Liu W, Ying N, Rao X, Chen X. MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway. Current Issues in Molecular Biology. 2022; 44(9):3835-3848. https://doi.org/10.3390/cimb44090263
Chicago/Turabian StyleLiu, Wenjia, Nanjiao Ying, Xin Rao, and Xiaodong Chen. 2022. "MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway" Current Issues in Molecular Biology 44, no. 9: 3835-3848. https://doi.org/10.3390/cimb44090263
APA StyleLiu, W., Ying, N., Rao, X., & Chen, X. (2022). MiR-942-3p as a Potential Prognostic Marker of Gastric Cancer Associated with AR and MAPK/ERK Signaling Pathway. Current Issues in Molecular Biology, 44(9), 3835-3848. https://doi.org/10.3390/cimb44090263








