Sonicated Bordetella bronchiseptica Bacterin Can Protect Dendritic Cells from Differential Cytotoxicity Caused by Doxorubicin and Vincristine and Enhance Their Antigen-Presenting Capability
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Preparation of DCs
2.3. Measurement of the Metabolic Activity and Viability of DCs
2.4. Flow Cytometry Analysis
2.5. Mixed Lymphocyte Reaction
2.6. Cytokine Production
2.7. Statistical Analysis
3. Results
3.1. The Effect of sBb on Cell Metabolic Activity of DCs
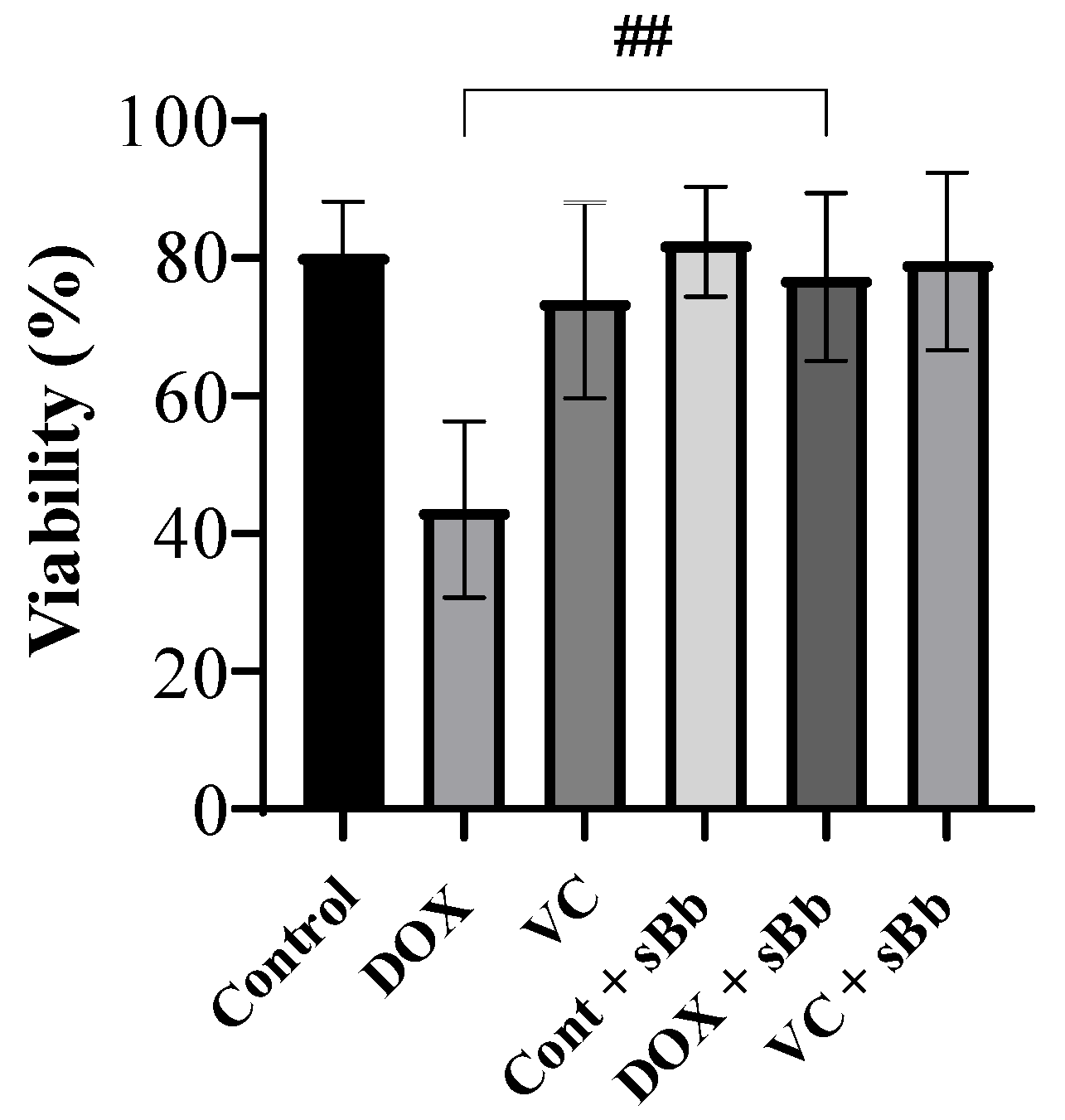
3.2. Increment of DC Viability Damaged by Anti-Cancer Drugs
3.3. sBb Increases the Mitochondrial Function of DOX-Treated DCs
3.4. sBb Differentially up-Regulates the Expression of Surface Markers on DCs
3.5. sBb Increases the Antigen-Presenting Capability of DCs
3.6. sBb increases the Cytokine Production of DCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinman, R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Kaneno, R.; Shurin, G.V.; Tourkova, I.L.; Shurin, M.R. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J. Transl. Med. 2009, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Tourkova, I.L.; Kaneno, R.; Shurin, M.R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol. 2009, 183, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Butterfield, L.H. Dendritic Cell-Based Cancer Vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef]
- Hogle, W.P. Cytoprotective agents used in the treatment of patients with cancer. Semin. Oncol. Nurs. 2007, 23, 213–224. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Candelaria, M.; Dueñas-Gonzalez, A. Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in diffuse large B-cell lymphoma. Ther. Adv. Hematol. 2021, 12, 2040620721989579. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Chao, M.W.; Lai, M.J.; Liou, J.P.; Chang, Y.L.; Wang, J.C.; Pan, S.L.; Teng, C.M. The synergic effect of vincristine and vorinostat in leukemia in vitro and in vivo. J. Hematol. Oncol. 2015, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Hallman, B.E.; Hauck, M.L.; Williams, L.E.; Hess, P.R.; Suter, S.E. Incidence and risk factors associated with development of clinical cardiotoxicity in dogs receiving doxorubicin. J. Vet. Intern. Med. 2019, 33, 783–791. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Xinyong, C.; Zhiyi, Z.; Lang, H.; Peng, Y.; Xiaocheng, W.; Ping, Z.; Liang, S. The role of toll-like receptors in myocardial toxicity induced by doxorubicin. Immunol. Lett. 2020, 217, 56–64. [Google Scholar] [CrossRef]
- Chao, D.; Bahl, P.; Houlbrook, S.; Hoy, L.; Harris, A.; Austyn, J.M. Human cultured dendritic cells show differential sensitivity to chemotherapy agents as assessed by the MTS assay. Br. J. Cancer 1999, 81, 1280–1284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Starobova, H.; Monteleone, M.; Adolphe, C.; Batoon, L.; Sandrock, C.J.; Tay, B.; Deuis, J.R.; Smith, A.V.; Mueller, A.; Nadar, E.I.; et al. Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release. J. Exp. Med. 2021, 218, e20201452. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, Y.; Joo, H.G. Bordetella bronchiseptica is a potent and safe adjuvant that enhances the antigen-presenting capability of dendritic cells. Korean J. Physiol. Pharmacol. 2020, 24, 47–52. [Google Scholar] [CrossRef]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Jonuleit, H.; Knop, J.; Enk, A.H. Cytokines and their effects on maturation, differentiation and migration of dendritic cells. Arch. Dermatol. Res. 1996, 289, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytok.kines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Konduri, V.; Halpert, M.M.; Baig, Y.C.; Coronado, R.; Rodgers, J.R.; Levitt, J.M.; Cerroni, B.; Piscoya, S.; Wilson, N.; DiBernardi, L.; et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther. 2019, 26, 282–291. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.Y.; Joo, H.-G. Sonicated Bordetella bronchiseptica Bacterin Can Protect Dendritic Cells from Differential Cytotoxicity Caused by Doxorubicin and Vincristine and Enhance Their Antigen-Presenting Capability. Curr. Issues Mol. Biol. 2022, 44, 3089-3099. https://doi.org/10.3390/cimb44070213
Sung JY, Joo H-G. Sonicated Bordetella bronchiseptica Bacterin Can Protect Dendritic Cells from Differential Cytotoxicity Caused by Doxorubicin and Vincristine and Enhance Their Antigen-Presenting Capability. Current Issues in Molecular Biology. 2022; 44(7):3089-3099. https://doi.org/10.3390/cimb44070213
Chicago/Turabian StyleSung, Ji Yun, and Hong-Gu Joo. 2022. "Sonicated Bordetella bronchiseptica Bacterin Can Protect Dendritic Cells from Differential Cytotoxicity Caused by Doxorubicin and Vincristine and Enhance Their Antigen-Presenting Capability" Current Issues in Molecular Biology 44, no. 7: 3089-3099. https://doi.org/10.3390/cimb44070213
APA StyleSung, J. Y., & Joo, H.-G. (2022). Sonicated Bordetella bronchiseptica Bacterin Can Protect Dendritic Cells from Differential Cytotoxicity Caused by Doxorubicin and Vincristine and Enhance Their Antigen-Presenting Capability. Current Issues in Molecular Biology, 44(7), 3089-3099. https://doi.org/10.3390/cimb44070213






