Rice Lesion Mimic Gene Cloning and Association Analysis for Disease Resistance
Abstract
:1. Introduction
2. Characteristics and Nomenclature of Lesion Mimic Mutants
3. The Pathogenesis of Plant Lesion Mimic Mutants
3.1. Gene Mutation
3.2. Hormone Imbalance
3.3. Metabolic Disorder
3.4. Disorder of PCD
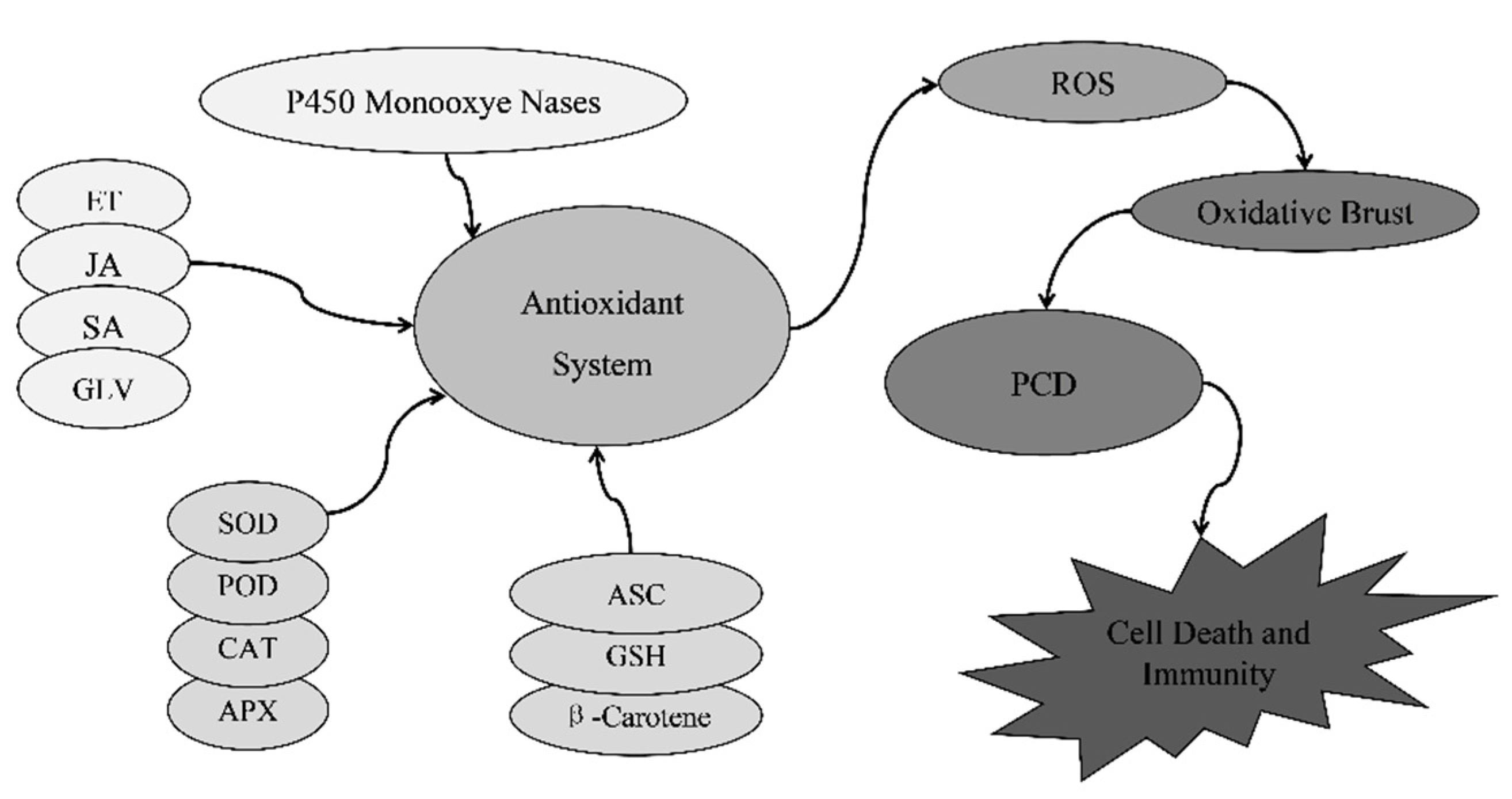
3.5. Accumulation of Reactive Oxygen Species
3.6. Abiotic Factors
4. Cloning of Rice Lesion Mimic Gene
| Name | Gene ID | Gene Function | References |
|---|---|---|---|
| SPL7 | LOC_Os05g45410 | heat shock transcription factor | [30] |
| SPL11 | LOC_Os12g38210 | U-box E3 ubiquitin ligase | [24] |
| OsNPR1 | LOC_Os01g09800 | disease resistance factor | [41] |
| OsLSD1 | LOC_Os08g06280 | zinc finger protein | [29] |
| SPL18 | LOC_Os10g11980 | acyltransferase | [42] |
| OsPti1a | LOC_Os05g04520 | receptor-like protein kinase | [31] |
| OsSSI2 | LOC_Os01g69080 | fatty acid desaturase | [18] |
| OsACDR1 | LOC_Os03g06410 | mitogen kinase kinase kinase | [22] |
| OsSL | LOC_Os12g16720 | P450 monooxygenase | [43] |
| SPL28 | LOC_Os01g50770 | grid-associated adaptor protein complex | [33] |
| GF14e | LOC_Os02g36974 | 14-3-3 protein | [23] |
| RLIN1 | LOC_Os04g52130 | coproporphyrinogen III oxidase | [20] |
| NLS1 | LOC_Os11g14380 | CC-NB-LRR protein | [16] |
| RLS1 | LOC_Os02g10900 | Novel protein containing NB-ARM domain | [44] |
| OsCATC | LOC_Os03g03910 | catalase C | [26] |
| OsHPL3 | LOC_Os02g02000 | Lipid hydroperoxide lyase | [45] |
| OsLMS | LOC_Os02g42600 | RNA binding protein | [34] |
| OsCs1F6 | LOC_Os08g06380 | cellulose synthase | [46] |
| OsNPR1 | LOC_Os01g09800 | transcriptional coactivator | [47] |
| FGL | LOC_Os10g35370 | protochlorophyllate oxidoreductase B | [21] |
| LMR | LOC_Os06g03940 | AAA-type ATPase | [36] |
| SPL5 | LOC_Os02g08070 | SF3b3-type splicing factor | [48] |
| SPL29 | LOC_Os08g10600 | UAP1 | [35] |
| OsWAK25 | LOC_Os03g12470 | wall-associated receptor-like kinase 25 | [49] |
| LLB | LOC_Os07g14350 | leucine carboxyl methyltransferase | [50] |
| OsPLS1 | LOC_Os06g45120 | vacuolar-type H+-ATPase subunit A1 | [51] |
| EBR1 | LOC_Os05g19970 | Ring-type E3 ubiquitin ligase | [52] |
| OsCUL3a | LOC_Os02g51180 | cullin-RING-like ubiquitin ligase complex | [25] |
| SPL32 | LOC_Os07g46460 | Fd-GOGAT | [53] |
| SPL33 | LOC_Os01g02720 | translation elongation factor | [27] |
| SDS2 | LOC_Os01g57480 | S-domain receptor-like kinase | [54] |
| OsPELOTA | LOC_Os04g56480 | eukaryotic translation release factor | [55] |
| SPL35 | LOC_Os03g10750 | CUEDC protein | [10] |
| SPL30 | LOC_Os12g37870 | ATP-citrate lyase A2 subunit | [37] |
| OsJAZ13 | LOC_Os10g25230 | jasmonate ZIM-domain protein | [56] |
| ELL1 | LOC_Os12g16720 | Cytochrome P450 Monooxygenase | [38] |
| OsNBL3 | LOC_Os03g06370 | pentatricopeptide repeat protein | [39] |
| OsSCYL2 | LOC_Os01g42950 | A conserved clathrin-coated vesicle component | [40] |
5. Disease Resistance of Lesion Mimic Mutants
6. Discussion and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.D.; Liu, J.L.; Triplett, L.; Leach, J.E.; Wang, G.L. Novel Insights into Rice Innate Immunity Against Bacterial and Fungal Pathogens. Annu. Rev. Phytopathol. 2013, 52, 213–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, E.; Kato, N.; Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Dickman, M. Plant programmed cell death: Can’t live with it; can’t live without it. Mol. Plant Pathol. 2008, 9, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.S.; Cui, Y.; Li, F.F.; Tan, J.; Xie, Y.H.; Sang, X.C.; Ling, Y.H. Phenotypic characterizing and gene mapping of a lesion mimic premature senescence 1 (lmps1) mutant in rice (Oryza sativa L.). Acta Agron. Sin. 2019, 45, 46–54. [Google Scholar] [CrossRef]
- Wu, C.J.; Bordoes, A.; Madamda, M.R.S.; Baraoidan, M.; Ramos, M.; Wang, G.L.; Leach, J.E.; Leung, H. Rice lesion mimic mutants with enhanced resistance to diseases. Mol. Genet. Genom. 2008, 279, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Kiyosawa, S. Inheritance of a particular sensitivity of the rice variety, Sekiguchi Asahi, to pathogens and chemicals, and linkage relationship with blast resistance genes. Tokyo Natl. Inst. Agric. Sci. Bull. Search 1970, 21, 61–72. [Google Scholar]
- Liu, G.; Wang, L.; Zhou, Z.; Leung, H.; Wang, G.L.; He, C. Physical mapping of a rice lesion mimic gene, Spll, to a 70-kb segment of rice chromosome 12. Mol. Genet. Genom. 2004, 272, 108–115. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.F.; Ma, X.D.; Meng, L.Z.; Jing, R.N.; Fan, W.; Zhang, X.; Jiang, L.; Wang, J.L.; Wang, J.; et al. Disruption of gene SPL35, encoding a novel CUE domain containing protein, leads to cell death and enhanced disease response in rice. Plant Biotechnol. J. 2019, 17, 1679–1693. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Jin, X.Y.; Huang, L.; Hong, Y.B.; Zhang, Y.F.; Ouyang, Z.G.; Li, X.H.; Song, F.M.; Li, D.Y. Molecular characterization of rice sphingosine-l-phosphate lyase gene OsSPLl and functional analysis of its role in disease resistance response. Plant Cell Rep. 2014, 33, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Chen, S.L.; Zhang, J.H.; Dong, Y.J.; Teng, S. Identification and genetic mapping of a lesion mimic mutant rice. Rice Sci. 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, L.X.; Wang, L.Y.; Zhang, L.H.; Zhu, C.N.; He, Z.H.; Jin, Q.S.; Fan, H.H.; Yu, X. Response to Illumination Induction and Effect of Temperature on Lesion formation of lrd (Lesion Resembling Disease) in Rice. Sci. Agric. Sin. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Walbot, V.; Hoisington, D.A.; Neuffer, M.G. Disease lesion mimic mutations. Genet. Eng. Plants 1983, 26, 431–442. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, J.H.; Agrawal, G.K.; Rakwal, R.; Kim, J.A.; Shim, J.K.; Lee, S.K.; Jeon, J.S.; Koh, H.J.; Lee, Y.H.; et al. The rice (Oryza sativa) blast lesion mimic mutant, blm, may confer resistance to blast pathogens by triggering multiple defense-associated signaling pathways. Plant Physiol. Biochem. 2005, 43, 397–406. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zhu, X.D.; Wang, Y.Q.; Liu, L.C.; Xu, B.; Li, F.; Fang, J.; Chu, C.C. Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J. 2011, 66, 996–1007. [Google Scholar] [CrossRef]
- Jiang, C.J.; Shimono, M.; Maeda, S.; Inoue, H.; Mori, M.; Hasegawa, M.; Sugano, S.; Takatsuji, H. Suppression of the rice fatty-acid desaturase gene OsSSI2 enhances resistance to blast and leaf blight diseases in rice. Mol. Plant-Microbe Interact. 2009, 22, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.L.; Liu, H.B.; Yuan, B.; Li, X.H.; Xu, C.G.; Wang, S.P. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant Cell Environ. 2011, 34, 179–191. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, F.; Tang, J.Y.; Wang, W.H.; Zhang, F.X.; Wang, G.D.; Chu, J.F.; Yan, C.Y.; Wang, T.Q.; Chu, C.; et al. Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice. PLoS ONE 2012, 7, e50089. [Google Scholar] [CrossRef]
- Sun, C.h.; Liu, L.c.; Tang, J.y.; Lin, A.h.; Zhang, F.T.; Fang, J.; Zhang, G.F.; Chu, C.C. RLIN1, encoding a putative coproporphyrinogen III oxidase, is involved in lesion initiation in rice. J. Genet. Genom. 2011, 38, 29–37. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Rahman, M.L.; Cho, S.H.; Kim, Y.S.; Koh, H.J.; Yoo, S.C.; Paek, N.C. The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J. 2013, 74, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Cho, K.; Singh, R.; Jung, Y.H.; Jeong, S.H.; Kim, S.H.; Lee, J.E.; Cho, Y.S.; Agrawal, G.K.; Rakwal, R.; et al. Rice OsACDR1 (Oryza sativaaccelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance. Mol. Cells 2009, 28, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, P.M.; Bruce, M.; Leach, J.E. Rice 14-3-3 protein (GF14e) negatively affects cell death and disease resistance. Plant J. 2011, 68, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.R.; Qu, S.h.; Bordeos, A.; Yang, C.W.; Baraoidan, M.; Yan, H.Y.; Xie, Q.; Nahm, B.H.; Leung, H.; Wang, G.L. Spotted leaf 11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 2004, 16, 2795–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.E.; Ning, Y.S.; Zhang, Y.X.; Yu, N.; Zhao, C.D.; Zhan, X.D.; Wu, W.X.; Chen, D.B.; Wei, X.J.; Wang, G.L.; et al. OsCUL3a Negatively regulates cell death and immunity by degrading OsNPR1 in rice. Plant Cell 2017, 29, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.H.; Wang, Y.Q.; Tang, J.Y.; Xue, P.; Li, C.L.; Liu, L.C.; Hu, B.; Yang, F.Q.; Loake, G.J.; Chu, C.C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Lei, C.L.; Wang, J.L.; Ma, J.; Tang, S.; Wang, C.L.; Zhao, K.J.; Tian, P.; Zhang, H.; Qi, C.Y.; et al. SPL33, encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice. J. Exp. Bot. 2017, 68, 899–913. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, X.D.; Wang, L.Y.; Zhang, L.H.; Xue, Q.Z.; He, Z.H. Physiological and genetic analysis of rice lesion mimic mutants. J. Plant Physiol. Mol. Biol. 2004, 30, 331–338. [Google Scholar] [CrossRef]
- Wang, L.J.; Pei, Z.Y.; Tian, Y.C.; He, C.Z. OsLSD1, a rice zinc finger protein, regulates programmed cell death and callus differentiation. Mol. Plant-Microbe Interact. 2005, 18, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Yamanouchi, U.; Yano, M.; Lin, H.X.; Ashikari, M.; Yamada, K. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA 2002, 99, 7530–7535. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Agrawal, G.K.; Yamazaki, M.; Onosato, K.; Miyao, A.; Kawasaki, T.; Shimamoto, K.; Hirochika, H. Rice Pti1a negatively regulates RAR1-dependent defense responses. Plant Cell 2007, 19, 2940–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, H.; Miyao, A.; Takahashi, A.; Hirochika, H. Pdk1 Kinase Regulates Basal Disease Resistance Through the OsOxi1-OsPti1a Phosphorylation Cascade in Rice. Plant Cell Physiol. 2010, 51, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.L.; Jiang, W.Z.; Lee, J.H.; Park, B.; Choi, M.S.; Piao, R.H.; Woo, M.O.; Roh, J.H.; Han, L.Z.; Paek, N.C.; et al. SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit micro 1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa). New Phytol. 2010, 185, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Undan, J.R.; Tamiru, M.; Abe, A.; Yoshida, K.; Kosugi, S.; Takagi, H.; Yoshida, K.; Kanzaki, H.; Saitoh, H.; Fekih, R.; et al. Mutation in OsLMS, a gene encoding a protein with two double-stranded RNA binding motifs, causes lesion mimic phenotype and early senescence in rice (Oryza sativa L.). Genes Genet. Syst. 2012, 87, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.H.; Wang, Y.; Hong, X.; Hu, D.H.; Liu, C.X.; Yang, J.; Li, Y.; Huang, Y.Q.; Feng, Y.Q.; Gong, H.Y.; et al. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice. J. Exp. Bot. 2015, 66, 973–987. [Google Scholar] [CrossRef] [Green Version]
- Fekih, R.; Tamiru, M.; Kanzaki, H.; Abe, A.; Yoshida, K.; Kanzaki, E.; Saitoh, H.; Takagi, H.; Natsume, S.; Undan, J.R.; et al. The rice (Oryza sativa L.) LESION MIMIC RESEMBLING, which encodes an AAA-type ATPase, is implicated in defense response. Mol. Genet. Genom. 2015, 290, 611–622. [Google Scholar] [CrossRef]
- Ruan, B.P.; Hua, Z.H.; Zhao, J.; Zhang, B.; Ren, D.Y.; Liu, C.L.; Yang, S.L.; Zhang, A.P.; Jiang, H.Z.; Yu, H.P.; et al. OsACL-A2 negatively regulates cell death and disease resistance in rice. Plant Biotechnol. J. 2019, 17, 1344–1356. [Google Scholar] [CrossRef]
- Cui, Y.J.; Peng, Y.L.; Zhang, Q.; Xia, S.S.; Ruan, B.P.; Xu, Q.K.; Yu, X.Q.; Zhou, T.T.; Liu, H.; Zeng, D.L.; et al. Disruption of EARLY LESION LEAF 1, encoding a cytochrome P450 monooxygenase, induces ROS accumulation and cell death in rice. Plant J. 2021, 105, 942–956. [Google Scholar] [CrossRef]
- Qiu, T.C.; Zhao, X.S.; Feng, H.J.; Qi, L.L.; Yang, J.; Peng, Y.L.; Zhao, W.S. OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol. J. 2021, 19, 2277–2290. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.H.; Cheng, C.; Niu, F.A.; Zhang, A.P.; Sun, B.; Tu, R.J.; Wan, J.N.; Li, Y.; Huang, Y.W.; et al. A conserved clathrin-coated vesicle component, OsSCYL2, regulates plant innate immunity in rice. Plant Cell Environ. 2022, 45, 542–555. [Google Scholar] [CrossRef]
- Chern, M.; Fitzgerald, H.A.; Canlas, P.E.; Navarre, D.A.; Ronald, P.C. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant Microbe Interact. 2005, 18, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.; Tomita, C.; Sugimoto, K.; Hasegawa, M.; Hayashi, N.; Dubouzet, J.G.; Ochiai, H.; Sekimoto, H.; Hirochika, H.; Kikuchi, S. Isolation and molecular characterization of a Spotted leaf 18 mutant by modified activation-tagging in rice. Plant Mol. Biol. 2007, 63, 847–860. [Google Scholar] [CrossRef]
- Fujiwara, T.; Maisonneuve, S.; Isshiki, M.; Mizutani, M.; Chen, L.; Wong, H.L.; Kawasaki, T.; Shimamoto, K. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J. Biol. Chem. 2010, 285, 11308–11313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, B.B.; Wang, J.J.; Zhu, X.D.; Zeng, L.J.; Li, Q.; He, Z.H. A Novel Protein RLS1 with NB–ARM Domains Is Involved in Chloroplast Degradation during Leaf Senescence in Rice. Mol. Plant 2012, 5, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.H.; Qi, J.F.; Zhu, X.D.; Mao, B.Z.; Zeng, L.J.; Wang, B.H.; Li, Q.; Zhou, G.X.; Xu, X.J.; Lou, Y.G.; et al. The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. Plant J. 2012, 71, 763–775. [Google Scholar] [CrossRef]
- Vegasánchez, M.E.; Verhertbruggen, Y.; Christensen, U.; Chen, X.W.; Sharma, V.; Varanasi, P.; Jobling, S.A.; Talbot, M.; White, R.G.; Joo, M.; et al. Loss of cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiol. 2012, 159, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Afsheen, S.; Xin, Z.J.; Han, X.; Lou, Y.G. OsNPR1 negatively regulates herbivore-induced JA and ethylene signaling and plant resistance to a chewing herbivore in rice. Physiol. Plant. 2013, 147, 340–351. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, X.R.; Jiang, B.L.; Gu, Z.M.; Zhang, P.H.; Qian, Q.; Chen, X.F.; Ma, B.J. Transcriptome profiling of the spl5 mutant reveals that SPL5 has a negative role in the biosynthesis of serotonin for rice disease resistance. Rice 2015, 8, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Harkenrider, M.; Sharma, R.; Vleesschauwer, D.D.; Tsao, L.; Zhang, X.T.; Chern, M.; Canlas, P.; Zuo, S.M.; Ronald, P.C. Overexpression of Rice Wall-Associated Kinase 25 (OsWAK25) Alters Resistance to Bacterial and Fungal Pathogens. PLoS ONE 2016, 11, e0147310. [Google Scholar] [CrossRef] [Green Version]
- Tamiru, M.; Takagi, H.; Abe, A.; Yokota, T.; Kanzaki, H.; Okamoto, H.; Saitoh, H.; Takahashi, H.; Fujisaki, K.; Oikawa, K.; et al. A chloroplast-localized protein LESION AND LAMINA BENDING affects defence and growth responses in rice. New Phytol. 2016, 210, 1282–1297. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Gong, P.; Li, K.Y.; Huang, F.D.; Cheng, F.M.; Pan, G. A single cytosine deletion in the OsPLS1 gene encoding vacuolar-type H+-ATPase subunit A1 leads to premature leaf senescence and seed dormancy in rice. J. Exp. Bot. 2016, 67, 2761–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Q.Y.; Zhai, K.R.; Yang, D.L.; Yang, W.B.; Wu, J.N.; Liu, J.Z.; Pan, W.B.; Wang, J.J.; Zhu, X.D.; Jian, Y.K.; et al. An E3 Ubiquitin Ligase-BAG Protein Module Controls Plant Innate Immunity and Broad-Spectrum Disease Resistance. Cell Host Microbe 2016, 20, 758–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.T.; Wang, Y.H.; Liu, L.L.; Wang, C.M.; Gan, T.; Zhang, Z.Y.; Wang, Y.L.; Wang, D.; Niu, M.; Long, W.H.; et al. Isolation and characterization of a spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice. Sci. Rep. 2017, 7, 41846–41858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.B.; Bai, P.F.; Ning, Y.S.; Wang, J.Y.; Shi, X.T.; Xiong, Y.H.; Zhang, K.; He, F.; Zhang, C.Y.; Wang, R.Y.; et al. The Monocot-Specific Receptor-like Kinase SDS2 Controls Cell Death and Immunity in Rice. Cell Host Microbe 2018, 23, 498–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.B.; Feng, B.H.; Wang, H.M.; Xu, X.; Shi, Y.F.; He, Y.; Chen, Z.; Sathe, A.P.; Shi, L.; Wu, J. A substitution mutation in OsPELOTA confers bacterial blight resistance by activating the salicylic acid pathway. J. Integr. Plant Biol. 2018, 60, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.J.; Zhang, L.; Wei, X.L.; Zhou, Y.; Dai, Y.; Zhu, Z. OsJAZ13 Negatively Regulates Jasmonate Signaling and Activates Hypersensitive Cell Death Response in Rice. Int. J. Mol. Sci. 2020, 21, 4379. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.G.; Lee, K.E.; Singh, M.; Kumar, P.; Matin, M.N. Rice lesion mimic mutants (LMM): The current understanding of genetic mutations in the failure of ROS scavenging during lesion formation. Plant 2021, 10, 1598. [Google Scholar] [CrossRef]
- Mizobuchi, R.; Hirabayashi, H.; Kaji, R.; Nishizawa, Y.; Satoh, H.; Ogawa, T.; Okamotoet, M. Differential expression of disease resistance in rice lesion-mimic mutants. Plant Cell Rep. 2002, 21, 390–396. [Google Scholar] [CrossRef]
- Zhu, X.B.; Ze, M.; Chern, M.; Chen, X.W.; Wang, J. Deciphering rice lesion mimic mutants to understand molecular network governing plant immunity and growth. Rice Sci. 2020, 27, 278–288. [Google Scholar] [CrossRef]
- Wang, D.F.; Wang, H.; Liu, Q.N.; Tu, R.R.; Zhou, X.P.; Zhang, Y.X.; Wu, W.X.; Yu, P.; Chen, D.B.; Zhan, X.D.; et al. Reduction of OsMPK6 activity by a R89K mutation induces cell death and bacterial blight resistance in rice. Plant Cell Rep. 2021, 40, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.R.; Wang, H.; Liu, Q.E.; Wang, D.F.; Zhou, X.P.; Xu, P.; Zhang, Y.X.; Wu, W.X.; Chen, D.B.; Cao, L.Y.; et al. Characterization and genetic analysis of the oshpl3 rice lesion mimic mutant showing spontaneous cell death and enhanced bacterial blight resistance. Plant Physiol. Biochem. 2020, 154, 94–104. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Jiang, H.; Chu, H.; Cao, L.; Chen, J. Rice Lesion Mimic Gene Cloning and Association Analysis for Disease Resistance. Curr. Issues Mol. Biol. 2022, 44, 2350-2361. https://doi.org/10.3390/cimb44050160
Zhang A, Jiang H, Chu H, Cao L, Chen J. Rice Lesion Mimic Gene Cloning and Association Analysis for Disease Resistance. Current Issues in Molecular Biology. 2022; 44(5):2350-2361. https://doi.org/10.3390/cimb44050160
Chicago/Turabian StyleZhang, Anpeng, Hongzhen Jiang, Huangwei Chu, Liming Cao, and Jingguang Chen. 2022. "Rice Lesion Mimic Gene Cloning and Association Analysis for Disease Resistance" Current Issues in Molecular Biology 44, no. 5: 2350-2361. https://doi.org/10.3390/cimb44050160
APA StyleZhang, A., Jiang, H., Chu, H., Cao, L., & Chen, J. (2022). Rice Lesion Mimic Gene Cloning and Association Analysis for Disease Resistance. Current Issues in Molecular Biology, 44(5), 2350-2361. https://doi.org/10.3390/cimb44050160







